Published online Aug 21, 2011. doi: 10.3748/wjg.v17.i31.3652
Revised: April 27, 2011
Accepted: May 4, 2011
Published online: August 21, 2011
AIM: To observe the hepatic injury induced by carbon dioxide pneumoperitoneum (CDP) in rabbits, compare the effects of low- and high-pressure pneumoperitoneum, and to determine the degree of hepatic injury induced by these two clinically relevant CDP pressures.
METHODS: Thirty healthy male New Zealand rabbits weighing 3.0 to 3.5 kg were randomly divided into three groups (n = 10 for each group) and subjected to the following to CDP pressures: no gas control, 10 mmHg, or 15 mmHg. Histological changes in liver tissues were observed with hematoxylin and eosin staining and transmission electron microscopy. Liver function was evaluated using an automatic biochemical analyzer. Adenine nucleotide translocator (ANT) activity in liver tissue was detected with the atractyloside-inhibitor stop technique. Bax and Bcl-2 expression levels were detected by western blotting.
RESULTS: Liver functions in the 10 mmHg and 15 mmHg experimental groups were significantly disturbed compared with the control group. After CDP, the levels of alanine transaminase and aspartate transaminase were 77.3 ± 14.5 IU/L and 60.1 ± 11.4 IU/L, respectively, in the 10 mmHg experimental group and 165.1 ± 19.4 IU/L and 103.8 ± 12.3 IU/L, respectively, in the 15 mmHg experimental group, which were all higher than those of the control group (P < 0.05). There was no difference in pre-albumin concentration between the 10 mmHg experimental group and the control group, but the pre-albumin level of the 15 mmHg experimental group was significantly lower than that of the control group (P < 0.05). No significant differences were observed in the levels of total bilirubin or albumin among the three groups. After 30 and 60 min of CDP, pH was reduced (P < 0.05) and PaCO2 was elevated (P < 0.05) in the 10 mmHg group compared with controls, and these changes were more pronounced in the 15 mmHg group. Hematoxylin and eosin staining showed no significant change in liver morphology, except for mild hyperemia in the two experimental groups. Transmission electron microscopy showed mild mitochondrial swelling in hepatocytes of the 10 mmHg group, and this was more pronounced in the 15 mmHg group. No significant difference in ANT levels was found between the control and 10 mmHg groups. However, ANT concentration was significantly lower in the 15 mmHg group compared with the control group. The expression of hepatic Bax was significantly increased in the two experimental groups compared with the controls, but there were no differences in Bcl-2 levels among the three groups. Twelve hours after CDP induction, the expression of hepatic Bax was more significant in the 15 mmHg group than in the 10 mmHg group.
CONCLUSION: A CDP pressure of 15 mmHg caused more substantial hepatic injury, such as increased levels of acidosis, mitochondrial damage, and apoptosis; therefore, 10 mmHg CDP is preferable for laparoscopic operations.
- Citation: Li J, Liu YH, Ye ZY, Liu HN, Ou S, Tian FZ. Two clinically relevant pressures of carbon dioxide pneumoperitoneum cause hepatic injury in a rabbit model. World J Gastroenterol 2011; 17(31): 3652-3658
- URL: https://www.wjgnet.com/1007-9327/full/v17/i31/3652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i31.3652
Developments in technology and medicine and improvements in anesthetic and surgical techniques have led to the extensive use of laparoscopic procedures for different patient groups, including high-risk patients[1-4]. The advantages of laparoscopic surgery compared with open surgery include a shorter hospital stay, early return to work, and decreased cost. Other advantages of laparoscopic surgery include less perioperative blood loss, a reduction in, or absence of, postoperative pain, and better cosmetic healing[5].
The creation and maintenance of a pneumoperitoneum to create space for dissection is one of the basic requirements for laparoscopic procedures, and carbon dioxide is the most commonly used gas for inducing a pneumoperitoneum. However, insufflation of carbon dioxide into the abdominal cavity for a short time may significantly and adversely impact respiration, circulation, and the acid-base balance in patients because of carbon dioxide absorption and persistently high intra-abdominal pressure[6-7]. Increased intra-abdominal pressure has some potential side effects, such as impairments of liver, kidney, and heart functions. The severity of such impairments is directly related to the degree of intra-abdominal pressure. Uncomplicated laparoscopic cholecystectomy can be performed reasonably safely with a low-pressure pneumoperitoneum. However, if available space is needed for extended resections or complicated reconstructive operations, such as laparoscopic colorectal surgery, a high-pressure pneumoperitoneum is induced. To maintain sufficient intra-abdominal space for surgical procedures, 15 mmHg carbon dioxide pneumoperitoneum (CDP) is routinely used instead of 10 mmHg to 12 mmHg. An increasing number of cases presenting hepatic injury after laparoscopic surgery have been reported, but the number of studies assessing this complication under clinical levels of intra-abdominal pressure is very limited[8-14]. Therefore, in this study we investigated hepatic injury in response to these two clinically relevant levels of intra-abdominal pressure to investigate the safety of CDP.
Thirty healthy male New Zealand rabbits weighing 3.0 to 3.5 kg were randomly divided into three groups (n = 10 in each group) and submitted to different CDP pressures: a control group (no gas), 10 mmHg group (carbon dioxide pressure was 10 mmHg), and 15 mmHg group (carbon dioxide pressure was 15 mmHg). Rabbits were given no water or food for 8 h prior to the experiments. They received 1 mg/kg midazolam and 20 mg/kg ketamine before surgery. A tracheal incision was made, and a 4.5 F canal was inserted for mechanical ventilation. The tidal volume was maintained at 10 mL/kg at a frequency of 30 times per minute. Anesthesia was sustained with injections of ketamine (5 μg/kg per minute) and vecuronium bromide (0.1 mg/kg per minute). All chemical treatments were halted 10 min before CDP induction. The rabbits in the experimental groups underwent CDP for 1 h. Mechanical ventilation was stopped once the rabbits recovered from CDP. All operations were approved by the animal care guidelines of the General Hospital of Chengdu Military Command.
3H-ADP and atractyloside were obtained from Sigma Corporation (United States). Rabbit anti-Bax polyclonal IgG and anti-Bcl-2 polyclonal IgG were purchased from Santa Cruz Biotechnology (United States). The goat anti-rabbit horseradish peroxidase-conjugated antibody was purchased from Zhongshan Golden Bridge Biotechnology Co. (China).
Blood samples were collected from ear-edge veins 12 h after the commencement of CDP and allowed to clot, and sera were isolated by centrifugation at 1000 r/min for 10 min and stored at -20°C until the assay. Serum levels of alanine transaminase (ALT), aspartate transaminase (AST), and albumin were determined by routine laboratory methods using a Hitachi Automatic Analyzer (Hitachi, Inc., Japan).
Femoral artery blood samples were collected at 0 min, 30 min, 60 min, and 12 h after the CDP operation and analyzed using a blood gas analyzer (AVL 995). When the samples were collected at 0 min, 30 min, and 60 min, the rabbits underwent mechanical ventilation.
Liver biopsies were collected 12 h after the beginning of CDP, and the specimens were fixed in 10% formaldehyde for 12 h to 24 h, embedded in paraffin, sliced into 5-μm-thick sections, and stained with hematoxylin and eosin. Histological changes in the liver tissues were observed using a micrographic system (Olympus).
Liver tissues were fixed using 3% glutaraldehyde, postfixed in 1% osmium tetroxide in 0.1 mol/L cacodylate buffer, dehydrated with acetone, and embedded in EPON 812. After location by semi-thin sectioning, the samples were sectioned to a thickness of 50-80 nm and poststained with 2% aqueous uranyl acetate. All samples were examined and photographed by transmission electron microscopy (PHILIPS TECNAI 10, Netherlands) at an accelerating voltage of 100 kV.
Mitochondria in the liver tissues were isolated by centrifugation. The activity of adenine nucleotide translocator (ANT) in the liver tissue was detected using the atractyloside-inhibitor stop technique. Mitochondrial function was initiated by adding 3H-ADP and terminated after 12 s by adding adriamycin. The radioactivity in each group was measured, and ANT activity was expressed as 10-9 mol/min per g protein.
Liver tissue samples (100 mg) were homogenized in a liquid nitrogen-cooled grinding bowl and lysed in cold RIPA buffer (25 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) supplemented with Halt™ Protease Inhibitor Cocktail. Whole cell lysates were obtained by subsequent centrifugation at 15 000 g for 10 min at 4°C. Protein concentrations were determined using a Bradford Protein Assay Kit with bovine serum albumin as a standard. Protein extracts (40 μg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a Protran nitrocellulose membrane. This membrane was incubated with rabbit anti-Bax polyclonal antibody or rabbit anti-Bcl-2 polyclonal antibody at 4 °C overnight after being blocked with a 10% bovine serum albumin solution. The membrane was washed with TBST buffer (20 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, and 0.1% Tween-20), incubated with a secondary goat anti-rabbit horseradish peroxidase-conjugated antibody for 2 h at room temperature, and finally detected with a DAB Kit. Beta-actin was used as an internal control for data analysis.
All experimental data are expressed as the means ± SD and were analyzed by a t-test using SPSS 10.0 statistical software. Probability values of < 0.05 were considered to be statistically significant.
Liver function in both CDP groups was disturbed compared with the control group (Table 1). After the CDP operation, the ALT and AST levels were 77.3 ± 14.5 and 60.1 ± 11.4 IU/L, respectively, in the 10 mmHg group and 165.1 ± 19.4 and 103.8 ± 12.3 IU/L, respectively, in the 15 mmHg group; each of these were higher than the control group (P < 0.05). Compared with the control group, there was no significant difference in the serum concentration of pre-albumin in the 10 mmHg group; however, it was significantly lower in the 15 mmHg group compared to the control group (P < 0.05). No significant difference was observed in the levels of total bilirubin or albumin among the three groups.
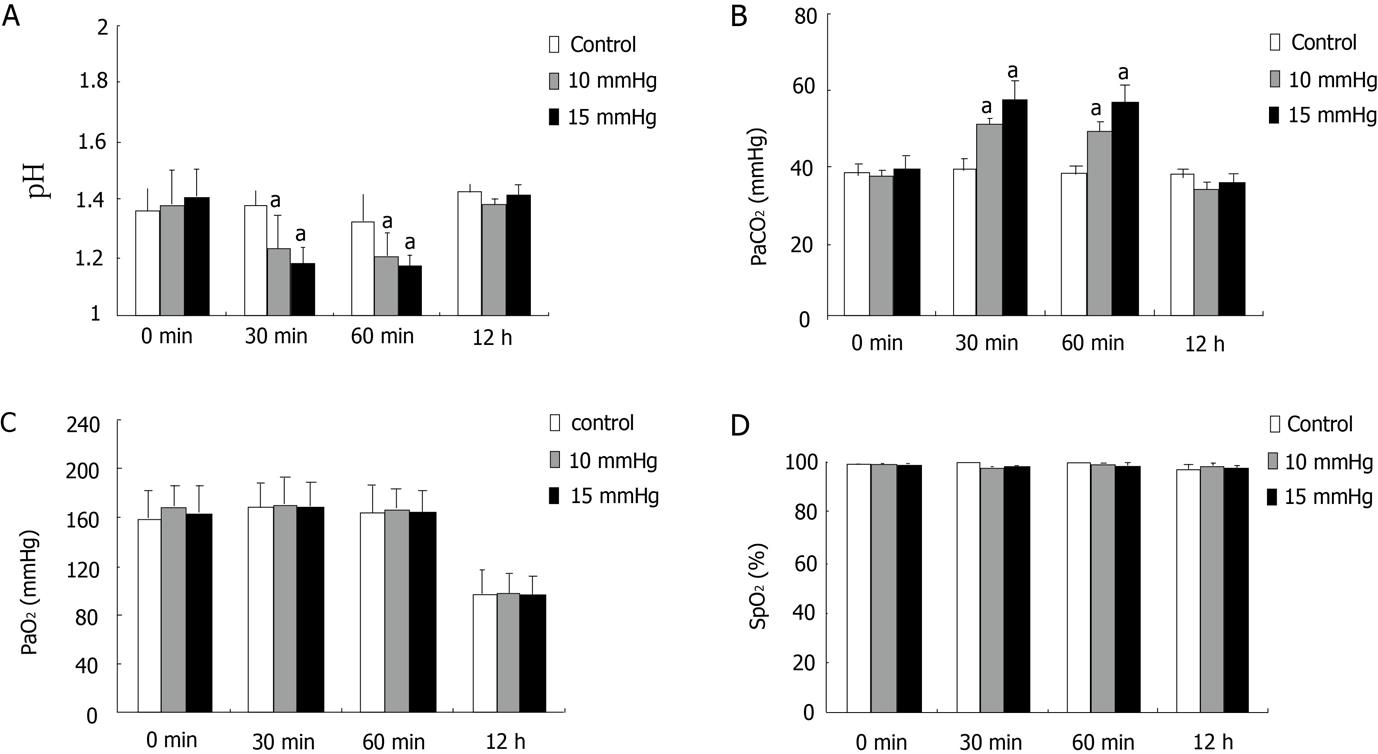
Blood pH in the 10 mmHg group was significantly decreased compared with the control group at 30 min and 60 min after CDP induction (Figure 1A), and PaCO2 was significantly increased (Figure 1B). Blood pH and PaCO2 levels were much higher in the 15 mmHg group than in the controls at these time points (Figure 1A and B). However, there were no significant differences in PaO2, SpO2, or pH levels for the experimental groups at 12 h post-CDP induction compared with the control group (Figures 1C and D).
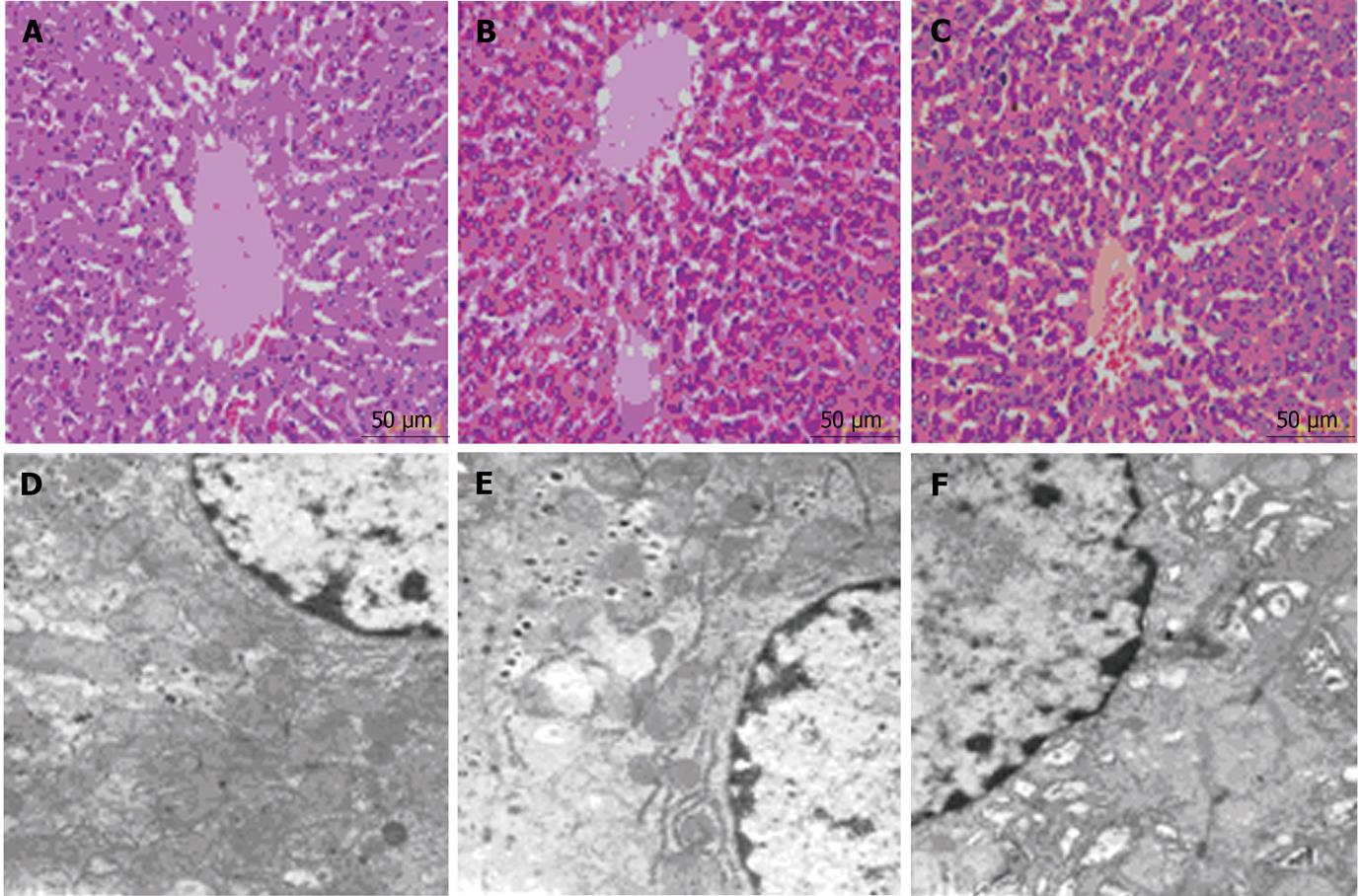
Hematoxylin and eosin staining and transmission electron microscopic images were analyzed for each group. The morphological changes of liver tissues in the 10 mmHg and 15 mmHg groups were similar and included mild hyperemia and mitochondrial swelling (Figures 2B, C, E and F). The hyperemia was more severe in the 15 mmHg group than in the 10 mmHg group, and the mitochondrial swelling was more apparent in the 15 mmHg group. In addition, the rough endoplasmic reticulum was slightly expanded in cells of the 15 mmHg group (Figure 2F).
In the control group, ANT activity was 10.83 ± 1.11 (10-9 mol ADP/min per g protein), while the activity of ANT was 9.03 ± 0.89 in the 10 mmHg group; there was no significant difference between these two groups. In the 15 mmHg group, ANT activity was only 6.64 ± 0.77, which was significantly lower than the control group (P < 0.05), indicating that the energy metabolism of liver mitochondria was damaged by CDP (Table 2).
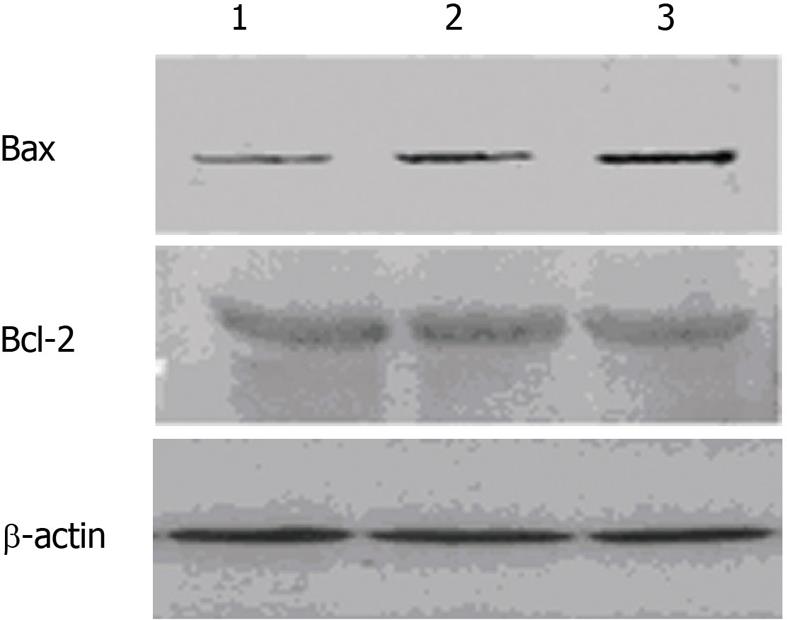
Bax and Bcl-2 protein levels were analyzed by western blot assay. The expression of Bax was significantly elevated in the 10 mmHg and 15 mmHg groups, but there was no significant change in Bcl-2 expression among the three groups. Compared with the 10 mmHg group, hepatic Bax expression in the 15 mmHg group was more significantly increased 12 h after the initiation of CDP (Figure 3).
Laparoscopic procedures are favored by both surgeons and patients because of convenience, the minor degree of trauma, rapid healing, and a good cosmetic prognosis[5]. A pneumoperitoneum is a necessary requirement during laparoscopic operations, and carbon dioxide is the most frequently used gas to create a pneumoperitoneum. Previous studies have noted that the increased abdominal pressure caused by CDP in a short time span can impact the hemodynamics, hemoperfusion, and function of critical abdominal organs, such as the liver[15-17]. Technical advancements have led to laparoscopic surgery becoming more complicated, and a lower-pressure CDP (e.g., 10-12 mmHg) is no longer sufficient. To create a sufficient operation space and decrease complications during laparoscopic procedures, many surgeons have increased the CDP abdominal pressure to 15 mmHg. However, the safety and effects of this pressure on abdominal organs have not been fully elucidated. The liver is one of the most important abdominal organs, and it is quite sensitive to harm. Therefore, in this study we investigated the effects of two clinically relevant CDP pressures on liver function, hepatocyte morphology, and protein expression.
Serum ALT and AST are two most commonly used markers of hepatocyte damage. In our study, a 1-h CDP operation resulted in hepatocyte injury at both 10 mmHg and 15 mmHg CDP. Increased ALT and AST levels were observed, suggesting that hepatocytes were damaged by both carbon dioxide pressures. More pronounced changes were detected in the 15 mmHg CDP group, indicating a more severe level of hepatocyte injury. Albumin and pre-albumin levels are markers of hepatocyte protein synthesis. Our data show that 10 mmHg CDP did not impact serum albumin levels, suggesting that 10 mmHg does not affect the rate of hepatocyte protein synthesis, despite its influence on ALT and AST levels. However, 15 mmHg CDP resulted in a decrease in pre-albumin, indicating that this CDP pressure could disturb hepatocyte activity as well as liver function. No significant changes in albumin levels were observed in the two CDP groups compared with the control group. The relatively long half-life of albumin might have been responsible for this phenomenon. In this experiment, we only measured albumin levels up to 12 h after the operation, which is quite short given the 14-d half-life of albumin.
The mechanisms underlying the influence of CDP on liver function might be related to hemodynamic changes and imbalanced acid-base levels. Many studies have shown diminished portal venous flow during increased intra-abdominal pressures, which possibly leads to decreased liver blood supply and impaired hepatic function[9,15,18-21]. Hepatic perfusion is characterized by a unique autoregulatory mechanism known as the hepatic arterial buffer response. Under physiological and pathological conditions, alterations in portal venous flow are counteracted by changes in hepatic arterial flow, thereby maintaining total hepatic blood flow and preserving a sufficient supply of oxygen to the liver[22,25,26]. However, several studies have demonstrated that during CDP, hepatic arterial blood flow does not compensate for the reduction in portal venous inflow[9]. Furthermore, there is a linear relationship between intra-abdominal pressure and portal venous pressure as well as a reciprocal correlation between increased intra-abdominal pressure and decreased portal venous flow[9,20,21,27]. In this study, CDP resulted in increased PaCO2 levels and decreased blood pH, and this effect was more pronounced at 15 mmHg than at 10 mmHg. These results indicate that increased abdominal pressure leads to more severe acidosis. These changes are thought to result from the absorption of insufflated carbon dioxide or ventilation-perfusion mismatching during the procedure[28,29]. Absorption of carbon dioxide through the peritoneum may result in an accumulation of carbon dioxide and subsequent acidosis. However, increased abdominal pressure during CDP could reduce abdominal blood flow and result in local hypoxia, which is another cause of acidosis[21,30]. Some researchers have adopted this view, which has been further discussed in studies demonstrating splanchnic hypoperfusion, regardless of whether intra-abdominal pressure was increased without the use of gas[20,21] or insufflation of CO2, N2O, helium, or argon was used to induce pneumoperitoneum[17,19,31-33]. Neither PaO2 nor SpO2 was affected by the two CDP pressures, possibly because of the use of intermittent positive-pressure mechanical ventilation.
In addition to influencing hemodynamics and the acid-base balance, CDP operations resulted in changes in hepatocyte morphology. Neither pressure led to apparent tissue damage (based on hematoxylin and eosin staining), but both pressures resulted in mild liver hyperemia, the severity of which was related to CDP pressure. CDP also impacted hepatocyte ultrastructure, including mitochondrial swelling and expanded rough endoplasmic reticulum. The activity of ANT, a marker of mitochondrial energy metabolism[34], was reduced after 15 mmHg CDP, but not by 10 mmHg CDP. This suggests that a relatively lower pressure might not impact hepatocyte energy metabolism, despite mitochondrial swelling.
Bcl-2 and Bax are 2 important apoptotic regulatory genes. They are widely distributed in tissues and cells, and they coordinate with each other to regulate apoptosis. When Bax expression is upregulated, Bax/Bax homodimers are formed to induce apoptosis. However, increased Bcl-2 expression results in isodimers of Bcl-2 and Bax that inhibit apoptosis[35]. In this study, neither 10 mmHg nor 15 mmHg CDP resulted in increased Bcl-2 expression. However, both pressures led to elevated Bax expression, especially in the 15 mmHg group, suggesting that CDP procedures promote hepatocyte apoptosis in a pressure-dependent manner. Elevated levels of hepatocyte apoptosis might be responsible for the disturbed liver function caused by CDP.
To summarize, we investigated the presence and mechanisms underlying liver damage caused by two clinically relevant CDP pressures. Liver injury has been shown to be pressure-dependent[11]. Although a relatively high CDP pressure is required for laparoscopic procedures, such as the 15 mmHg pressure used in this study, we must bear in mind that this pressure can cause serious damage to liver function. The liver damage resulting from CDP may not cause severe complications, but the potential for such damage in patients with liver diseases is of particular importance. Some reports have shown that a shorter duration of carbon dioxide pressure during pneumoperitoneum might help to alleviate liver injury[8]. Stepwise increases in carbon dioxide insufflation might also be an ischemic preconditioning method to reduce liver injury[36]. Future studies are needed to elucidate the mechanisms underlying CDP-induced liver function damage, and the safety of CDP under different surgical conditions should be carefully evaluated[1-4,37].
Laparoscopic surgery is widely used, and the traditional low carbon dioxide pneumoperitoneum (CDP) pressure no longer meets the requirements of complicated operations. The safety of high CDP pressure in clinical practice is the subject of much attention.
Some studies have shown that liver injury is pressure-dependant, and hepatocyte apoptosis was observed in the present study.
The liver is a critical organ that is sensitive to many harmful factors. Liver changes during CDP operation were evaluated in this study by assaying several different markers. The data suggest that the different pressures cause hepatic injury.
As indicated by the experimental data, although higher pressure provides more space for CDP operation, the resulting hepatic injury must be considered. Therefore, the appropriate CDP pressure should be carefully chosen for laparoscopy.
CDP is the abdominal space created by insufflating carbon dioxide to provide operation space for laparoscopy.
This is an interesting study even if on a well studied subject.
Peer reviewer: Antonio Basoli, Professor, General Surgery “Paride Stefanini”, Università di Roma-Sapienza, Viale del Policlinico 155, 00161 Roma, Italy
S- Editor Tian L L- Editor Stewart G E- Editor Li JY
| 1. | McCloskey CA, Wilson MA, Hughes SJ, Eid GM. Laparoscopic colorectal surgery is safe in the high-risk patient: a NSQIP risk-adjusted analysis. Surgery. 2007;142:594-57; discussion 594-57;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Salihoglu Z, Baca B, Koksal S, Hakki Hamzaoglu I, Karahasanoglu T, Avci S, Ozben V. Analysis of laparoscopic colorectal surgery in high-risk patients. Surg Laparosc Endosc Percutan Tech. 2009;19:397-400. [PubMed] |
| 3. | Salihoglu Z, Demiroluk S, Demirkiran O, Cakmakkaya S, Aydogan F, Carkman S, Kose Y. The effects of pneumothorax on the respiratory mechanics during laparoscopic surgery. J Laparoendosc Adv Surg Tech A. 2008;18:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Struthers AD, Cuschieri A. Cardiovascular consequences of laparoscopic surgery. Lancet. 1998;352:568-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Gutt CN, Oniu T, Mehrabi A, Schemmer P, Kashfi A, Kraus T, Büchler MW. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004;21:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Xu GS, Liu HN, Li J, Wu XL, Dai XM, Liu YH. Hepatic injury induced by carbon dioxide pneumoperitoneum in experimental rats. World J Gastroenterol. 2009;15:3060-3064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Richter S, Olinger A, Hildebrandt U, Menger MD, Vollmar B. Loss of physiologic hepatic blood flow control ("hepatic arterial buffer response") during CO2-pneumoperitoneum in the rat. Anesth Analg. 2001;93:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Mujicić E, Durić A, Radovanovioć J. [Influence of CO2 pneumoperitoneum on liver function]. Med Arh. 2006;60:87-89. [PubMed] |
| 9. | Szold A, Weinbroum AA. Carbon dioxide pneumoperitoneum-related liver injury is pressure dependent: A study in an isolated-perfused organ model. Surg Endosc. 2008;22:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Leister I, Schüler P, Vollmar B, Füzesi L, Kahler E, Becker H, Markus PM. Microcirculation and excretory function of the liver under conditions of carbon dioxide pneumoperitoneum. Surg Endosc. 2004;18:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Tan M, Xu FF, Peng JS, Li DM, Chen LH, Lv BJ, Zhao ZX, Huang C, Zheng CX. Changes in the level of serum liver enzymes after laparoscopic surgery. World J Gastroenterol. 2003;9:364-367. [PubMed] |
| 12. | Omari A, Bani-Hani KE. Effect of carbon dioxide pneumoperitoneum on liver function following laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2007;17:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kleinhaus S, Sammartano R, Boley SJ. Effects of laparoscopy on mesenteric blood flow. Arch Surg. 1978;113:867-869. [PubMed] |
| 14. | Hashikura Y, Kawasaki S, Munakata Y, Hashimoto S, Hayashi K, Makuuchi M. Effects of peritoneal insufflation on hepatic and renal blood flow. Surg Endosc. 1994;8:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Junghans T, Böhm B, Gründel K, Schwenk W, Müller JM. Does pneumoperitoneum with different gases, body positions, and intraperitoneal pressures influence renal and hepatic blood flow? Surgery. 1997;121:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Gutt CN, Schmandra TC. Portal venous flow during CO(2) pneumoperitoneum in the rat. Surg Endosc. 1999;13:902-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Schmandra TC, Kim ZG, Gutt CN. Effect of insufflation gas and intraabdominal pressure on portal venous flow during pneumoperitoneum in the rat. Surg Endosc. 2001;15:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Masey SA, Koehler RC, Ruck JR, Pepple JM, Rogers MC, Traystman RJ. Effect of abdominal distension on central and regional hemodynamics in neonatal lambs. Pediatr Res. 1985;19:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992;33:279-282; discussion 282-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 246] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol. 1985;249:G549-G556. [PubMed] |
| 21. | Ezzat WR, Lautt WW. Hepatic arterial pressure-flow autoregulation is adenosine mediated. Am J Physiol. 1987;252:H836-H845. [PubMed] |
| 22. | Lautt WW, Legare DJ, Ezzat WR. Quantitation of the hepatic arterial buffer response to graded changes in portal blood flow. Gastroenterology. 1990;98:1024-1028. [PubMed] |
| 23. | Richter S, Mücke I, Menger MD, Vollmar B. Impact of intrinsic blood flow regulation in cirrhosis: maintenance of hepatic arterial buffer response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G454-G462. [PubMed] |
| 24. | Mücke I, Richter S, Menger MD, Vollmar B. Significance of hepatic arterial responsiveness for adequate tissue oxygenation upon portal vein occlusion in cirrhotic livers. Int J Colorectal Dis. 2000;15:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Jakimowicz J, Stultiëns G, Smulders F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | McMahon AJ, Baxter JN, Kenny G, O'Dwyer PJ. Ventilatory and blood gas changes during laparoscopic and open cholecystectomy. Br J Surg. 1993;80:1252-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Lindberg F, Bergqvist D, Rasmussen I, Haglund U. Hemodynamic changes in the inferior caval vein during pneumoperitoneum. An experimental study in pigs. Surg Endosc. 1997;11:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Takagi S. Hepatic and portal vein blood flow during carbon dioxide pneumoperitoneum for laparoscopic hepatectomy. Surg Endosc. 1998;12:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Gründel K, Böhm B, Bauwens K, Junghans T, Scheiba R. Influence of acute hemorrhage and pneumoperitoneum on hemodynamic and respiratory parameters. Surg Endosc. 1998;12:809-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Rademaker BM, Odoom JA, de Wit LT, Kalkman CJ, ten Brink SA, Ringers J. Haemodynamic effects of pneumoperitoneum for laparoscopic surgery: a comparison of CO2 with N2O insufflation. Eur J Anaesthesiol. 1994;11:301-306. [PubMed] |
| 31. | Sala-Blanch X, Fontanals J, Martínez-Palli G, Taurá P, Delgado S, Bosch J, Lacy AM, Visa J. Effects of carbon dioxide vs helium pneumoperitoneum on hepatic blood flow. Surg Endosc. 1998;12:1121-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Chen LF, Liu JZ, Li B. [Characteristics of adenine nucleotide translocator in mitochondria of rat cerebral cortex during hypobaric hypoxia exposure.]. Sheng Li Xue Bao. 2006;58:29-33. [PubMed] |
| 33. | Nomura M, Shimizu S, Ito T, Narita M, Matsuda H, Tsujimoto Y. Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res. 1999;59:5542-5548. [PubMed] |
| 34. | Sahin DA, Haliloglu B, Sahin FK, Akbulut G, Fidan H, Koken G, Buyukbas S, Aktepe F, Arikan Y, Dilek ON. Stepwise rising CO2 insufflation as an ischemic preconditioning method. J Laparoendosc Adv Surg Tech A. 2007;17:723-729. [PubMed] |
| 35. | Hao YX, Zhong H, Zhang C, Zeng DZ, Shi Y, Tang B, Yu PW. Effects of simulated carbon dioxide and helium peumoperitoneum on proliferation and apoptosis of gastric cancer cells. World J Gastroenterol. 2008;14:2241-2245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |