Published online Aug 7, 2011. doi: 10.3748/wjg.v17.i29.3448
Revised: November 8, 2010
Accepted: November 15, 2010
Published online: August 7, 2011
AIM: To investigate the survival rates and prognostic factors in patients with hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF).
METHODS: Clinical data in hospitalized patients with HBV-ACLF admitted from 2006 to 2009 were retrospectively analyzed. Their general conditions and survival were analyzed by survival analysis and Cox regression analysis.
RESULTS: A total of 190 patients were included in this study. The overall 1-year survival rate was 57.6%. Patients not treated with antiviral drugs had a significantly higher mortality [relative risk (RR) = 0.609, P = 0.014]. The highest risk of death in patients with ACLF was associated with hepatorenal syndrome (HRS) (RR = 2.084, P =0.026), while other significant factors were electrolyte disturbances (RR = 2.062, P = 0.010), and hepatic encephalopathy (HE) (RR = 1.879, P < 0.001).
CONCLUSION: Antiviral therapy has a strong effect on the prognosis of the patients with HBV-ACLF by improving their 1-year survival rate. HRS, electrolyte disturbances, and HE also affect patient survival.
- Citation: Huang K, Hu JH, Wang HF, He WP, Chen J, Duan XZ, Zhang AM, Liu XY. Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol 2011; 17(29): 3448-3452
- URL: https://www.wjgnet.com/1007-9327/full/v17/i29/3448.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i29.3448
Hepatitis B virus (HBV) infection is an established cause of liver-related morbidity and mortality, and has been described as a global public health problem. Some patients had abnormal liver functions or even liver failure during the lengthy course of chronic HBV infection[1]. Liver failure is characterized by severe deterioration in liver function. This clinical syndrome involves the development of hepatic encephalopathy (HE), jaundice, coagulopathy, and hepatorenal syndrome (HRS). Acute-on-chronic liver failure (ACLF) refers to acute deterioration in liver function in a patient with previously well-compensated chronic liver disease, as a result of a precipitating event such as sepsis or upper gastrointestinal bleeding. HBV infection is the most common cause of ACLF in China[2,3]. If the relevant prognostic factors for HBV-related ACLF could be clarified in the course of chronic hepatitis B, corresponding measures could be adopted to reduce the associated mortality.
In the present study, we analyzed the outcomes of the patients with HBV-ACLF using survival analysis, and the factors affecting mortality using a Cox regression model. The results of this study will further the understanding of the prognosis of the patients with HBV-ACLF, thus helping in the management of the condition.
One hundred and ninety patients admitted to the 302 Military Hospital from September 2006 to January 2009 and diagnosed with HBV-ACLF were included in this retrospective study. Exclusion criteria included concurrent viral infections (e.g. HAV, HCV, HDV, HEV, human immunodeficiency virus, cytomegalovirus, Epstein-Barr virus); liver failure by other causes (including autoimmune, alcohol- or drug-related diseases); malignant tumors; and patients with other severe systematic or mental diseases. The research protocol was approved by the Ethics Committee of the 302 Military Hospital.
HBV-ACLF was defined as acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 wk with ascites and/or HE in a patient with previously diagnosed or undiagnosed chronic hepatitis B, which includes compensated liver cirrhosis, with total bilirubin ≥ 171 μmol/L or an increase ≥ 17.1 μmol/L, and prothrombin activity (PTA) ≤ 40%[4].
The diagnosis of liver cirrhosis was based on medical history, physical examination, biochemical parameters, ultrasound findings and/or liver biopsy. The diagnostic criteria included the presence of hepatic stigmata, spider angioma or splenomegaly on physical examination, together with laboratory abnormalities (e.g. low serum albumin, high serum globulin, high serum γ-globulin, low platelet count) and esophagogastroduodenal endoscopic signs of portal hypertension. The occurrence of liver cirrhosis during follow-up was also defined by ultrasonographic features suggestive of cirrhosis, based on a quantitative scoring system derived from the appearance of the liver margins, parenchymal echotexture, portal vein caliber, and spleen diameter[5,6], supplemented with the presence of esophageal varices or ascites.
The severity of HE was graded as: (1) slowness of mentation, mildly disturbed sleep-awake cycle; (2) drowsiness, confusion, inappropriate behavior, disorientation, apathy, mood swings, anxiety, restlessness; (3) very sleepy but rousable, unresponsive to verbal commands, markedly confused, combative and hyper-reflexic; and (4) unconscious, decerebrate or decorticate posturing[7].
HRS is a potentially reversible syndrome that occurs in patients with cirrhosis, ascites, and liver failure. It is diagnosed by presence of cirrhosis with ascites; absence of shock; serum creatinine > 133 mmol/L (1.5 mg/dL); no improvement in serum creatinine (decrease to a level of ≤ 133 mmol/L) at least 2 d after diuretic withdrawal and volume expansion with albumin, and no current or recent treatment with nephrotoxic drugs; absence of parenchymal kidney disease as indicated by proteinuria > 500 mg/d; microhematuria (> 50 red blood cells per high-power field); and/or abnormal renal ultrasonography[8].
Abnormal serum levels of sodium or potassium were considered as electrolyte disturbances. The normal serum levels of sodium and potassium were 136-145 mmol/L and 3.5-5.2 mmol/L, respectively.
All patients with HBV-ACLF were followed up for at least 12 mo. The outcome (recovery, bridging to liver transplantation, or death) was recorded for each patient. Data for patients who underwent liver transplantation or death were considered as censored data.
Statistical analyses were performed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). Patients’ clinical and biochemical indices were expressed as mean ± SD. Analysis of variance was used for comparisons between different treatment groups, and significant differences were assessed using F-tests and Student-Newman-Keuls tests. χ2 tests were used for comparisons between the rates of liver cirrhosis, complications, and sex. The Kaplan-Meier method was used to estimate the overall survival rates. The log-rank test was used to compare the death rate between groups.
We performed univariate and multivariate analyses of the associations between the 12-mo mortality (dependent variable) and independent variables, and then multivariate Cox regression analysis and calculated the relative risk ratios and 95% CI. P value < 0.05 was considered statistically significant.
The clinical and biochemical characteristics of the 190 patients (159 men and 31 women, mean ± SD age, 45.7 ± 11.1 years) with HBV-ACLF are presented in Table 1. Of the 190 patients, 141 had received antiviral therapy (nucleoside analogues: lamivudine 100 mg/d or entecavir 0.5 mg/d), and 11 patients had undergone liver transplantation. There were no significant differences in clinical or biochemical characteristics among the three patients who received entecavir, lamivudine, or no antiviral treatment). One hundred and twenty patients had liver cirrhosis (Child-Turcotte-Pugh score < 7), 64 had HE, 16 had HRS, 63 had spontaneous peritonitis (SBP), and 144 had electrolyte disturbances. The overall 12-mo survival rate was 57.6%.
| Complications and biochemical characteristics | Entecavir group | Lamivudine group | Non-antiviral group |
| No. of patients | 77 | 64 | 49 |
| Gender (M/F) | 66/11 | 56/8 | 37/12 |
| Age (yr) | 45.90 ± 11.33 | 44.38 ± 10.39 | 47.20 ± 11 |
| Ascites | 64 (83.1) | 53 (82.8) | 35 (71.4) |
| HRS | 7 (9.1) | 6 (9.4) | 3 (6.1) |
| HE | 23 (29.9) | 22 (34.4) | 19 (38.8) |
| I-II | 19 (24.7) | 18 (28.1) | 16 (32.7) |
| III-IV | 4 (5.2) | 4 (6.3) | 3 (6.1) |
| SBP | 23 (29.9) | 21 (32.8) | 19 (38.8) |
| Liver cirrhosis | 48 (62.3) | 38 (59.4) | 34 (69.4) |
| Electrolyte disturbances | 54 (70.1) | 49 (76.6) | 41 (83.7) |
| Alanine transaminase (U/L) | 412.84 ± 605.62 | 271.14 ± 304.24 | 180.96 ± 222.50 |
| Total bilirubin (μmol/L) | 311.34 ± 120.99 | 340.81 ± 123.38 | 265.51 ± 121.88 |
| Albumin (g/L) | 37.25 ± 11.84 | 34.09 ± 10.90 | 35.76 ± 12.34 |
| Prothrombin activity (%) | 32.16 ± 9.21 | 31.36 ± 10.16 | 31.64 ± 10.59 |
| International normalized ratio | 2.46 ± 0.69 | 2.47 ± 0.61 | 2.58 ± 0.86 |
| Creatinine (μmol/L) | 97.78 ± 55.42 | 88.85 ± 25.08 | 96.94 ± 38.16 |
| Sodium (mmol/L) | 133.57 ± 13.37 | 133.52 ± 6.47 | 132.57 ± 5.61 |
| HBeAg (+/-) | 33/44 | 27/37 | 22/27 |
| HBV DNA (IU/mL) | (1.00 ± 8.51) × 108 | (4.00 ± 26.69) × 107 | (4.41 ± 1.08) × 107 |
| HBV DNA level (< 103/103-107/> 107) | 4/61/11 | 3/52/10 | 4/39/6 |
| MELD score | 29.07 ± 4.02 | 28.69 ± 4.43 | 28.61 ± 3.49 |
| MELD score (< 30/≥ 30) | 47/30 | 41/23 | 34/15 |
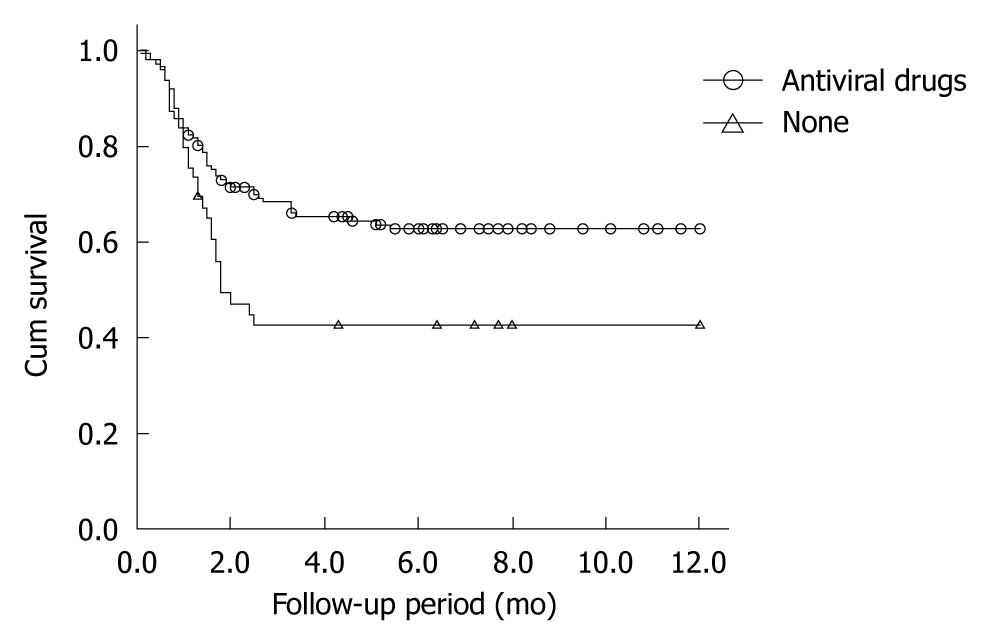
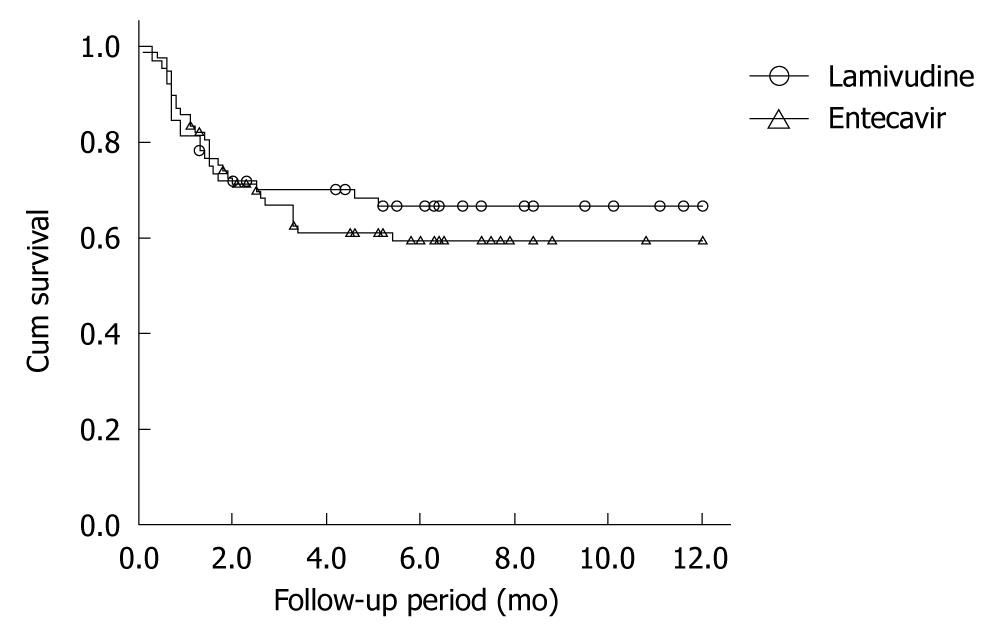
Patients not taking antiviral drugs had significantly higher mortality than those taking antivirals (χ2 = 8.050, P = 0.005). The mean (SE) 1-year survival rates of patients who did and did not receive antiviral treatment were 62.7% (0.042) and 42.5% (0.073), respectively (Figure 1). There was no significant difference in survival rates between the entecavir group ( = 77) and the lamivudine group (n = 64) (χ2 = 0.399, P = 0.527) (Figure 2).
Univariate analysis identified eight factors significantly associated with long-term patient survival: age, cirrhosis, electrolyte disturbances, HE, SBP, HRS, PTA, and the use of antiviral drugs (Table 2). However, the levels of HBV DNA before treatment and model for end-stage liver disease (MELD) score had no significant effects on the survival rate, and were not significantly different among the three groups (P = 0.383 and P = 0.053).
| Cases | Median time (mo) | 1-yr survival rate (%) | χ2 value | P value | |
| Age (yr) | |||||
| 20-45 | 88 | 6.7 | 69.9 | 9.047 | 0.003 |
| > 45 | 102 | 2.5 | 46.5 | ||
| Fundamental disease | |||||
| Cirrhosis | 120 | 2.6 | 49.1 | 8.026 | 0.005 |
| No cirrhosis | 69 | 8.4 | 71.8 | ||
| Antiviral therapy | |||||
| No | 49 | 1.8 | 42.5 | 8.050 | 0.005 |
| Yes | 141 | 5.5 | 62.7 | ||
| Electrolyte disturbances | |||||
| No | 46 | 11.8 | 84.1 | 14.397 | < 0.001 |
| Yes | 144 | 2.6 | 49.3 | ||
| HE grade | |||||
| No | 126 | 7.1 | 70.7 | 38.596 | < 0.001 |
| I-II | 53 | 1.6 | 35.1 | ||
| III-IV | 11 | 1.5 | 9.1 | ||
| SBP | |||||
| No | 127 | 6.0 | 64.3 | 5.345 | 0.021 |
| Yes | 63 | 2.5 | 44.3 | ||
| HRS | |||||
| No | 174 | 5.7 | 60.9 | 18.403 | < 0.001 |
| Yes | 16 | 0.9 | 20.8 | ||
| PTA groups (%) | |||||
| < 20 | 18 | 1.2 | 22.2 | 22.619 | < 0.001 |
| 20-30 | 77 | 5.1 | 58.6 | ||
| 30-40 | 66 | 6.7 | 70.6 | ||
| > 40 | 29 | 3.3 | 47.1 |
Forward Cox regression analysis identified antiviral therapy, HRS, HE, and electrolyte disturbances to be independently associated with the mortality (Table 3).
| Variables | Relative risk ratio | 95% CI | Wald value | P value |
| Age | 1.322 | 0.792-2.208 | 1.139 | 0.286 |
| Cirrhosis | 1.144 | 0.648-2.021 | 0.215 | 0.643 |
| Antiviral therapy | 0.609 | 0.430-1.067 | 6.809 | 0.014 |
| Electrolyte disturbances | 2.062 | 1.282-6.433 | 6.571 | 0.010 |
| HE | 1.879 | 1.335-2.646 | 13.065 | < 0.001 |
| SBP | 1.295 | 0.798-2.103 | 1.095 | 0.295 |
| HRS | 2.084 | 1.090-3.984 | 4.928 | 0.026 |
| PTA | 0.940 | 0.699-1.264 | 0.168 | 0.682 |
HBV-ACLF is associated with a high mortality[9-11], although liver transplantation can significantly improve the survival rate[12,13]. However, liver transplantation is limited by many factors, especially donor shortages; only 11 patients in the current study received a transplant. Improved medical treatment is the key to prolonging the survival of patients with HBV-ACLF. The effects of antiviral treatment with nucleoside analogs on hepatitis B related liver failure is currently a focus of clinical research, but their efficacy remains controversial. Several reports have suggested that lamivudine could significantly improve the prognosis of patients with liver failure[14-17], but Kumar et al[18] reported that, although lamivudine significantly decreased the levels of HBV DNA in patients with acute hepatitis B, it did not result in any significant biochemical or clinical improvement, compared with patients receiving a placebo. In the current study, survival analysis showed that the mortality of patients who received nucleoside analog (entecavir or lamivudine) therapy was significantly lower than that of patients who did not receive antiviral drugs. This indicates that treatment with nucleoside analogs (lamivudine/entecavir) could improve the prognosis of patients with HBV-ACLF, and suggests that nucleoside analog therapy should be implemented in these patients as soon as possible.
In addition to antiviral therapy, other factors were found to significantly influence the prognosis, including HE, electrolyte imbalance, and HRS. Methods for treating and preventing the complications of HBV-ACLF remain important research topics. Yu et al[19] found that, in HBV-ACLF patients treated with lamivudine and plasma exchange, multivariate analysis identified a MELD score of 30-40 or > 40 to be a good predictor of treatment outcome. The present study, however, found no significant effect of MELD score on prognosis. However, the P value of 0.053 suggests that a MELD score > 30 might predict a poorer prognosis in patients with HBV-ACLF if a larger sample size was analyzed. Thus, although pretreatment HBV DNA levels and MELD score had no significant effects on patient survival in this study, further studies using larger samples, or multicenter trials, are required to confirm these results.
HBV-ACLF, although rare, remains a rapidly progressive and frequently fatal condition. Traditional treatment is generally supportive, and HE, HRS and electrolyte disturbances remain the leading causes of death. In China, liver injury is caused mostly by hepaciviruses (especially HBV)[20] and may therefore be preventable. Clinicians should be aware of the rapid evolution of liver failure, and the possible risks for patients who develop any degree of coagulopathy and encephalopathy. Because the outcome is unpredictable, early transfer to a transplantation facility should be considered before the onset of advanced grades of coma, after which transfer becomes impossible. Further understanding of the pathophysiologic characteristics of this multisystemic condition and the development of better supportive therapies should improve the outcome of patients with HBV-ACLF.
Hepatitis B virus related acute-on-chronic liver failure (HBV-ACLF) is associated with a high mortality, although liver transplantation can significantly improve the survival rate. However, liver transplantation is limited by many factors, especially donor shortages. Improved medical treatment is the key to prolonging the survival of patients with HBV-ACLF. If the related prognostic factors are clarified in the course of HBV-ACLF, corresponding measures can be taken to reduce the mortality.
Although antiviral therapy using lamivudine or entecavir have shown promising results in chronic hepatitis B and liver cirrhosis, the effects of antiviral treatment with nucleoside analogs on hepatitis B related liver failure remains controversial. And it has become currently a focus of clinical research.
The present study demonstrates that antiviral therapy had a strong effect on the prognosis of patients with HBV-ACLF by improving their 1-year survival rate. Hepatorenal syndrome, electrolyte disturbances, and hepatic encephalopathy also affected patient survival.
The results of study indicate that antiviral therapy and preventing complications [especially hepatic encephalopathy (HE), hepatorenal syndrome and electrolyte disturbances] may serve as a favorable alternative to reduce the mortality of the patients with HBV-ACLF.
HBV-ACLF is defined as acute hepatic insult manifesting as jaundice and coagulopathy, complicated by ascites and/or HE in a patient with previously diagnosed or undiagnosed chronic hepatitis B, which includes compensated liver cirrhosis, with total bilirubin (TBiL) ≥ 171 μmol/L or an increase in TBiL ≥ 17.1 μmol/L, and prothrombin activity ≤ 40%.
The definition of ACLF should be clarified in no uncertain terms so that it is clear to the reader of the article. Language also needs to be taken care of.
Peer reviewer: Sri P Misra, Professor, Gastroenterology, Moti Lal Nehru Medical College, Allahabad 211001, India
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Merle P, Trépo C, Zoulim F. Current management strategies for hepatitis B in the elderly. Drugs Aging. 2001;18:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Li XM, Ma L, Yang YB, Shi ZJ, Zhou SS. Analyses of prognostic indices of chronic liver failure caused by hepatitis virus. World J Gastroenterol. 2005;11:2841-2843. [PubMed] |
| 3. | Liu Q, Liu Z, Wang T, Wang Q, Shi X, Dao W. Characteristics of acute and sub-acute liver failure in China: nomination, classification and interval. J Gastroenterol Hepatol. 2007;22:2101-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Diagnostic and treatment guidelines for liver failure]. Zhonghua Gan Zang Bing Za Zhi. 2006;14:643-646. [PubMed] |
| 5. | Bambha K, Kim WR, Kremers WK, Therneau TM, Kamath PS, Wiesner R, Rosen CB, Thostenson J, Benson JT, Dickson ER. Predicting survival among patients listed for liver transplantation: an assessment of serial MELD measurements. Am J Transplant. 2004;4:1798-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Munro BH. Logistic regression; in Munro BH. Statistical Methods for Health Care Research. Philadelphia, PA: Lippincott Williams & Wilkins 2001; 287. |
| 7. | Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 257] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J. 2008;84:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Sanyal AJ, Stravitz RT. Acute liver failure. Hepatology: A textbook of liver disease. 5th ed. Philadelphia: Saunders Elsevier 2006; 383-415. |
| 10. | Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954. [PubMed] |
| 11. | Rutherford A, Davern T, Hay JE, Murray NG, Hassanein T, Lee WM, Chung RT. Influence of high body mass index on outcome in acute liver failure. Clin Gastroenterol Hepatol. 2006;4:1544-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Bui Han SH, Martin P. Liver transplantation for hepatitis B. Hepatol Res. 2004;29:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Bernal W, Wendon J. Liver transplantation in adults with acute liver failure. J Hepatol. 2004;40:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Chien RN, Lin CH, Liaw YF. The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol. 2003;38:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Schmilovitz-Weiss H, Ben-Ari Z, Sikuler E, Zuckerman E, Sbeit W, Ackerman Z, Safadi R, Lurie Y, Rosner G, Tur-Kaspa R. Lamivudine treatment for acute severe hepatitis B: a pilot study. Liver Int. 2004;24:547-551. [PubMed] [DOI] [Full Text] |
| 16. | Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, Graziadei I, Encke J, Schmidt H, Vogel W. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat. 2006;13:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Tsubota A, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Chayama K, Murashima N, Ikeda K, Kobayashi M. Lamivudine therapy for spontaneously occurring severe acute exacerbation in chronic hepatitis B virus infection: a preliminary study. Am J Gastroenterol. 2001;96:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kumar M, Satapathy S, Monga R, Das K, Hissar S, Pande C, Sharma BC, Sarin SK. A randomized controlled trial of lamivudine to treat acute hepatitis B. Hepatology. 2007;45:97-101. [PubMed] |
| 19. | Yu JW, Sun LJ, Zhao YH, Li SC. Prediction value of model for end-stage liver disease scoring system on prognosis in patients with acute-on-chronic hepatitis B liver failure after plasma exchange and lamivudine treatment. J Gastroenterol Hepatol. 2008;23:1242-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Liu XY, Hu JH, Wang HF, Chen JM. Etiological analysis of 1977 patients with acute liver failure, subacute liver failure and acute-on-chronic liver failure]. Zhonghua Gan Zang Bing Za Zhi. 2008;16:772-775. [PubMed] |