Published online Aug 7, 2011. doi: 10.3748/wjg.v17.i29.3390
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: August 7, 2011
AIM: To investigate the trend in gastric cancer surgery in the context of rapid therapeutic advancement in Japan and East Asia.
METHODS: A retrospective analysis was performed on 4163 patients who underwent gastric resection for gastric cancer with histological confirmation between 1971 and 2007 at the surgical unit in Kitasato University Hospital, to determine the trend in gastric cancer requiring surgery.
RESULTS: Gastric cancer requiring surgical resection increased in our hospital, but the incidence adjusted for population was constant during the observed period. Interestingly, the ratio of diffuse type/intestinal type gastric cancer was unexpectedly unchanged, and that of advanced/early gastric cancer (EGC) was, however, markedly reduced, while the actual incidence of potentially curative advanced gastric cancer tended to decrease. The incidence of EGC requiring surgery tended to increase as a whole, which is consistent with increased prevalence of endoscopic surveillance. As a result, overall survival and mortality of gastric cancer requiring gastric resection has recently markedly improved.
CONCLUSION: In Japan, planned interventions may improve surgical gastric cancer mortality, but an unexpected trend of persistent existence of intestinal type cancer suggests the need for more robust medical intervention.
- Citation: Yamashita K, Sakuramoto S, Nemoto M, Shibata T, Mieno H, Katada N, Kikuchi S, Watanabe M. Trend in gastric cancer: 35 years of surgical experience in Japan. World J Gastroenterol 2011; 17(29): 3390-3397
- URL: https://www.wjgnet.com/1007-9327/full/v17/i29/3390.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i29.3390
Gastric cancer was newly diagnosed in 934 000 people worldwide in 2002, accounting for 8.6% of new cancers. It was the fourth leading cancer, following lung, breast, and colorectal cancer. In addition, it was the second leading cause of cancer deaths worldwide, with approximately 700 000 deaths reported in 2002, indicating that gastric cancer had a poorer prognosis than breast and colorectal cancer. Because of improvements in food storage methods putatively associated with infection-related gastric cancer, gastric cancer incidence and mortality have fallen dramatically over the past 70 years worldwide. Although its prevalence is being reduced in most advanced countries, the number of patients is expected to increase up to 1 100 000 in 2010 as the population ages worldwide[1].
Demographic trends in gastric cancer differ by tumor location and histology. While there has been a marked decline in distal, intestinal type gastric cancers, the incidence of proximal, diffuse type adenocarcinomas of the gastric cardia has been increasing, particularly in Western countries. For example, in the USA, gastric cancer was the leading cause of death in the 1930s, but mortality rates have fallen dramatically despite no planned prevention, while diffuse type, proximal gastric cancer is sharply increasing[2]. Diverging trends in the incidence of gastric cancer by tumor location or histology suggest that they may represent two diseases with different etiologies. The main risk factors for distal gastric cancer include Helicobacter pylori (H. pylori) infection and dietary factors, whereas gastroesophageal reflux disease and obesity play important roles in the development of proximal stomach cancer. Intestinal type tumors predominate in high risk geographical areas, such as East Asia including Japan, and a decline of the intestinal type gastric cancer in the distal stomach is believed to account for most of the decrease in gastric cancer rates worldwide[3]. Detailed epidemiological data will help guide future cancer control strategies, and we herein investigate the recent Japanese trend in gastric cancer requiring surgery to provide useful cancer information.
The current study was conducted as a retrospective analysis of 4163 gastric cancer patients who underwent gastrectomy at the Kitasato University Hospital between September 1971 and March 2007 and had proven histology of adenocarcinoma of the stomach. The patients who underwent surgery without removal of stomach tissue, and had no histology were initially ruled out (almost stage IV disease or any stage with invasion of the pancreas head). Histological information was collected from the formal documents of the pathological division in the Kitasato University Hospital (the East and Main hospital). Incidence and mortality corrected for the population in the Sagamihara city were calculated between 1972 and 2006 (35 years), because the years 1971 and 2007 did not have full annual data. Histology was shown as pap, tub1, tub2, por1, por2, sci, and muc for adenocarcinoma. Intestinal type gastric cancer was defined as pap, tub1, and tub2, and diffuse type as por1, por2, sci, and muc. Among the cases, some cancers were included in both cancers, and such cases were defined as mixed type. Early gastric cancer (EGC) was defined as mucosal or submucosal cancers irrespective of lymph node metastasis status. Advanced gastric cancer (AGC) was defined as cancers invading beyond the muscularis proprior.
For all EGC, curative operations were successfully performed, while lymph node dissection was predominant for D2 during the early phase. According to the guideline for gastric cancer in 2001, lymph node dissection had gradually transitioned from D2 to D1+α (No. 7) or β (No. 7, 8, 9) dissection. For AGC stages I to III, D2 dissection had been the standard option for surgery, if severe complications were not present or in very elderly patients. R1 or R2 surgery was performed in 3% of stage I to III patients, who largely had positive margins of the esophagus or duodenum. For stage IV AGC, various operative procedures were selected according to the individual surgeons. As a whole, the earlier operations tended to be extended to include D2, D3 or even D4 lymph node dissection, while more recently D0 or D1 lymph node dissection became prevalent for cases with peritoneal dissemination and/or positive cytology (CY1) or distant metastasis. The staging system used was the 13th Japanese Classification of Gastric Cancer in all recent cases, but before 2000, there was no consistent information on CY status. CY1 was the initial staging, which could affect operation performance, and we also have to allow for changes in staging classification before and after 2000.
The population of Sagamihara city was determined from published city data (http://www.city.sagamihara.kanagawa.jp/toukei/gaiyo/001902.html). The incidence of disease was calculated by the following formula: number of gastric cancer patients requiring surgery in the surgical unit of Kitasato University/population in Sagamihara city × 100 000.
Statistical computations were performed using SAS software package (SAS Institute, Cary, NC), Stat View version 5.0. The Kaplan-Meier method was used to estimate cumulative survival rates, and differences in survival rates were assessed using the log rank test. A P value < 0.05 was considered statistically significant. The time of follow-up was calculated from the date of surgery. Mortality rates were calculated by the following formula: total deaths during the observed term/sum of the population in Sagamihara city during the same term. Cases were censored within 5 years, although these were below 1%, and were not judged as deaths.
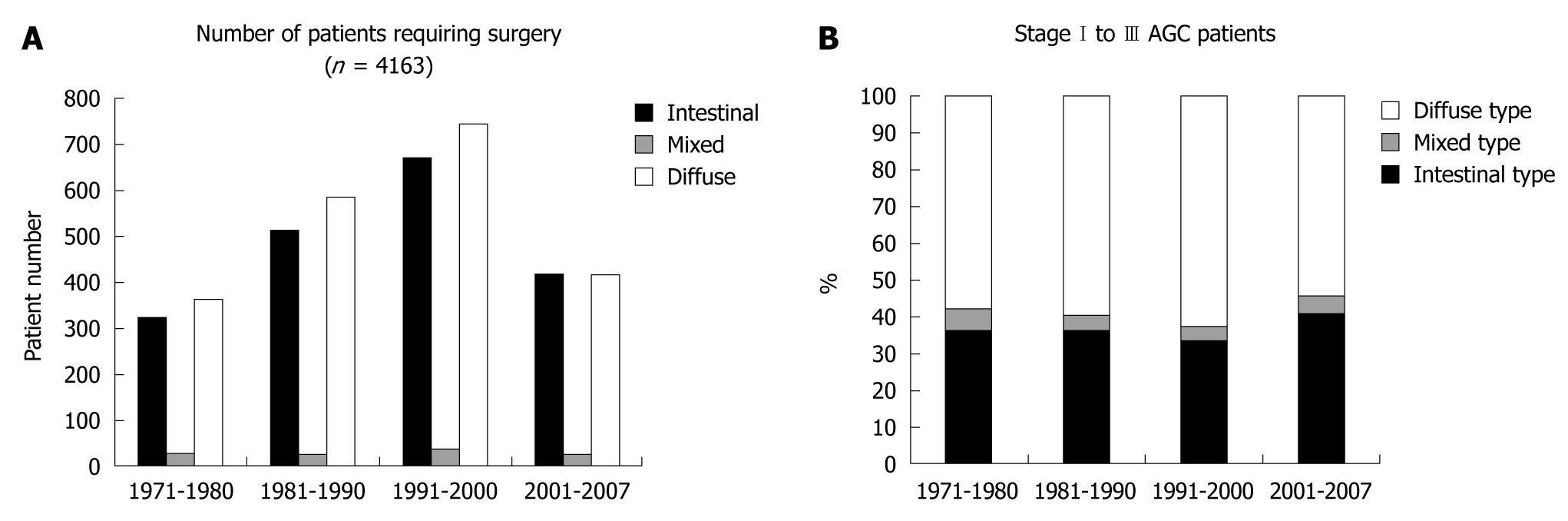
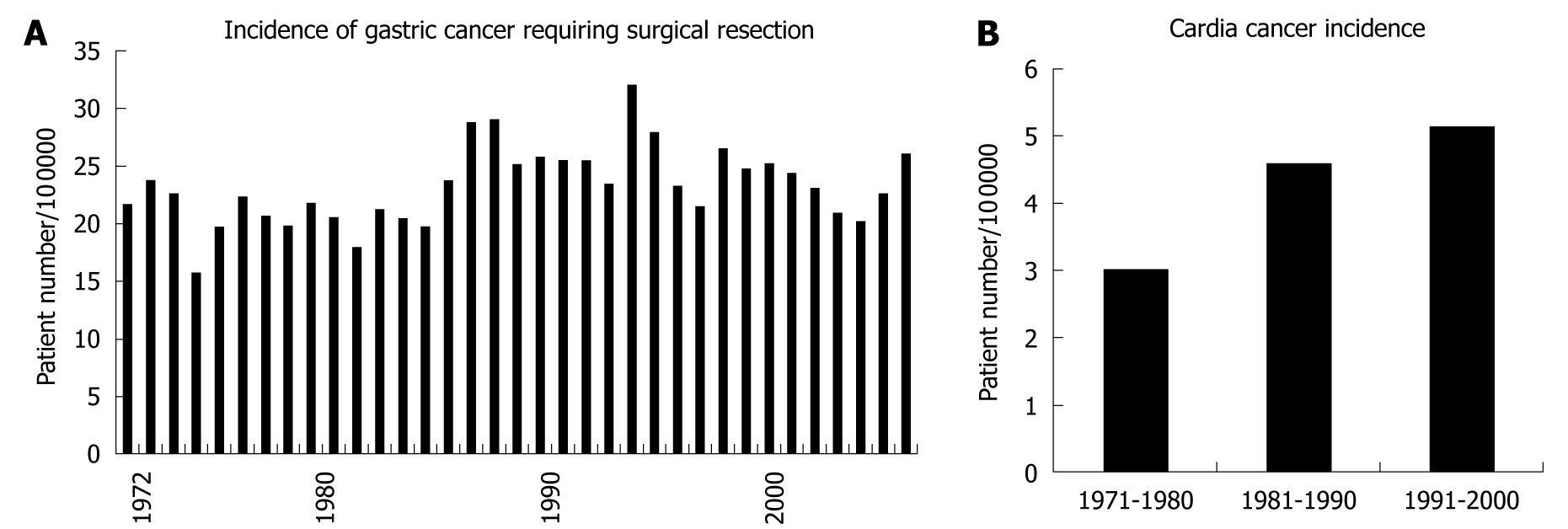
The surgical unit of Kitasato University Hospital was opened in 1971, and the number of gastric cancer patients requiring surgical resection has been increasing until recently as shown in Figure 1A. Intriguingly, the ratio of intestinal type gastric cancer to diffuse type gastric cancer has not declined like the trend worldwide or in Western countries (Figure 1A and B). The majority of patients requiring surgery had been referred by neighboring clinics or middle-scale hospitals or the Gastrointestinal Division of Internal Medicine at Kitasato University. The hospital is located in Sagamihara city (population about 700 000 in 2010), Kanagawa Prefecture, Japan, and is a dormitory town for the Tokyo and Yokohama area, with a markedly increasing population in recent years. The incidence of gastric cancer requiring surgery largely ranged between 20 and 30 per 100 000 population, and it was unexpectedly constant during the nearly 35 years of the data (Figure 2A), which is consistent with the Japanese trend as a whole. The incidence of gastric cancer in Japan is the highest in the world, and it was calculated to be 69.2 in men and 28.6 in women per 100 000 population[4]. Allowing for inoperable patients or those who were treated by endoscopic resection, a considerable proportion of patients requiring surgery in the Sagamihara city were sent to our hospital, putatively representing the Japanese trend of gastric cancer requiring surgical resection. This trend is very different from that worldwide or in other developed countries.
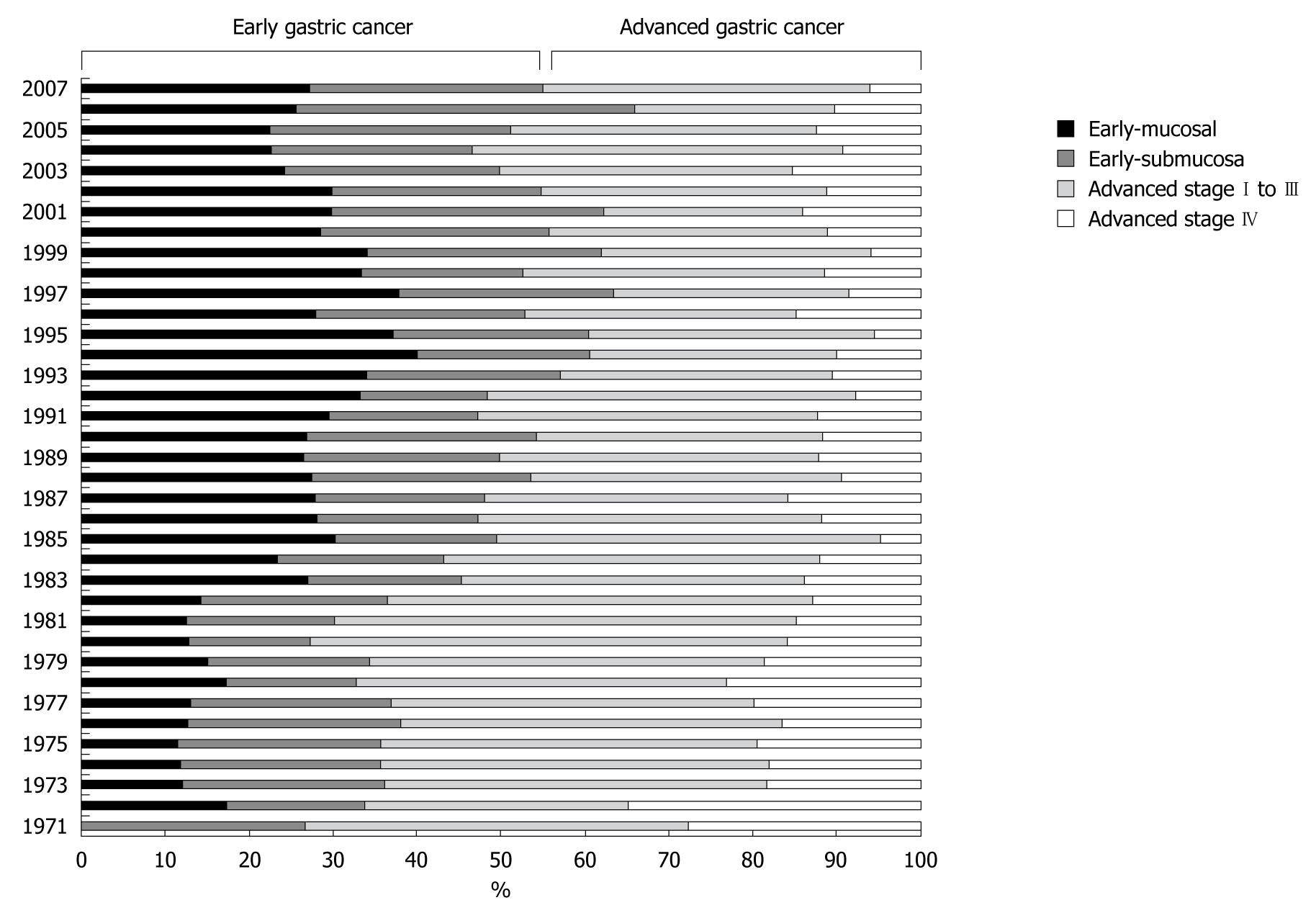
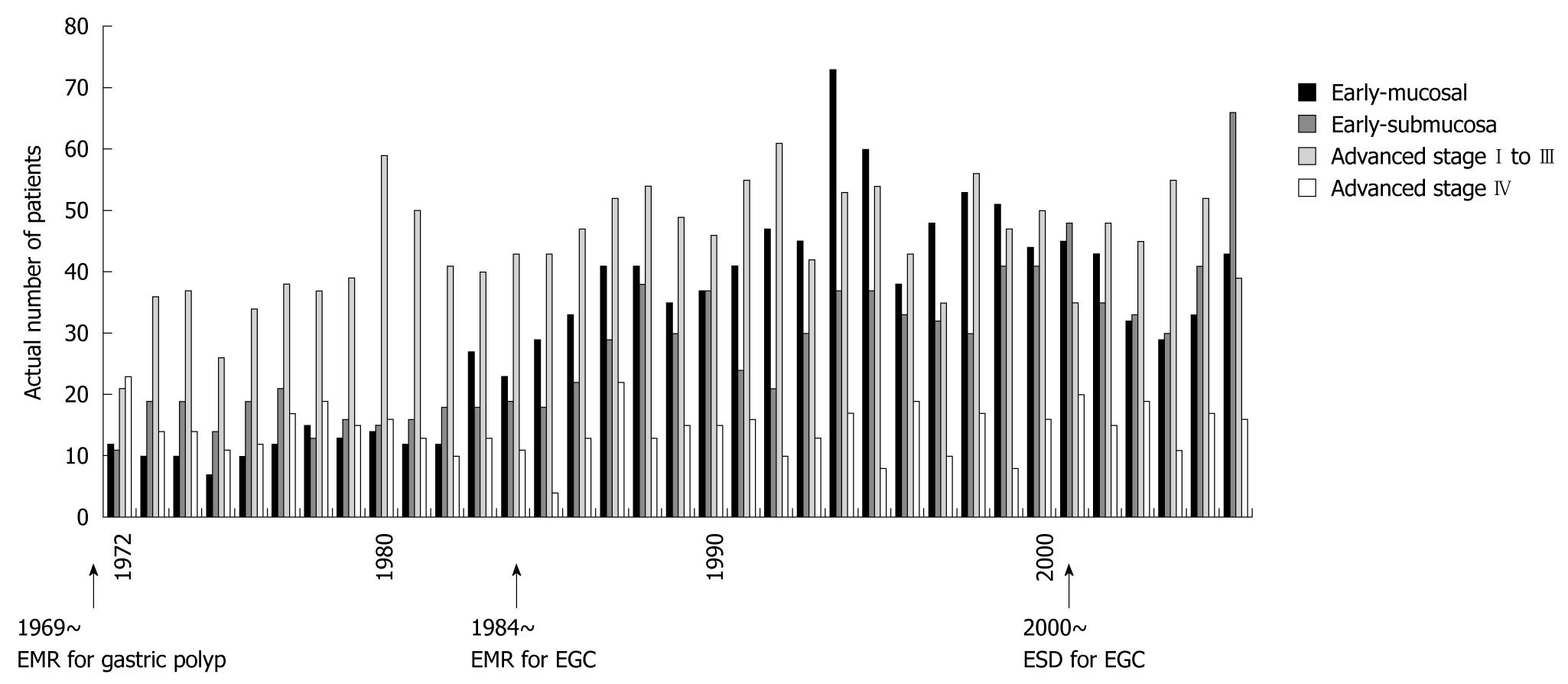
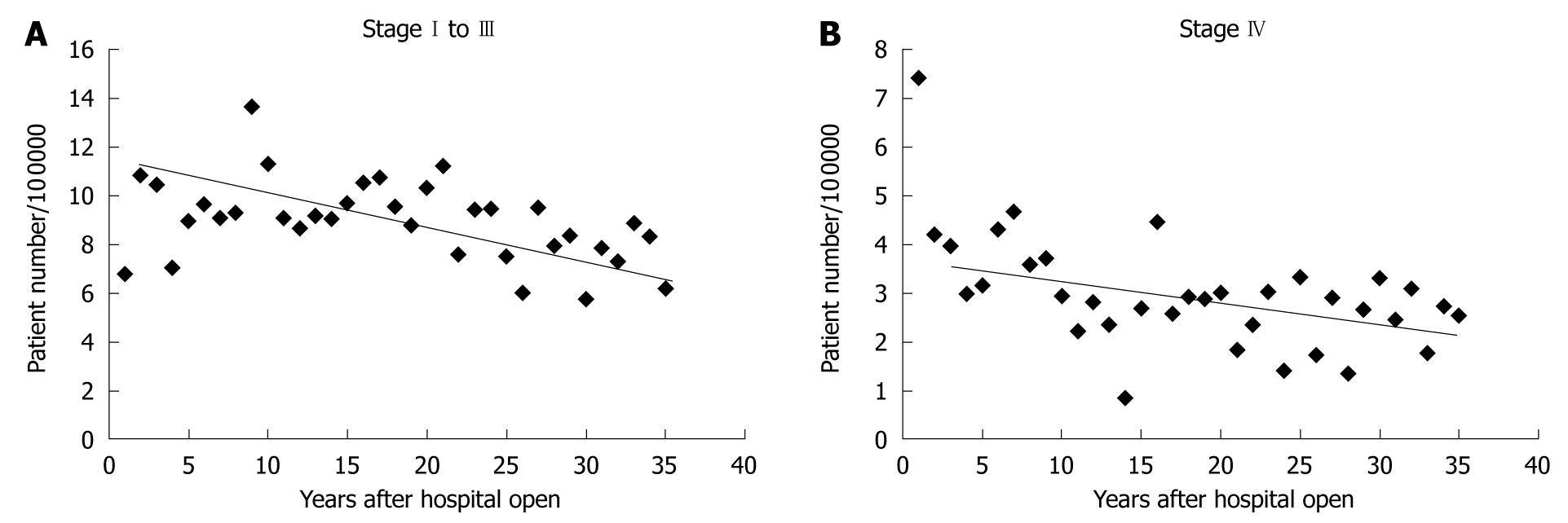
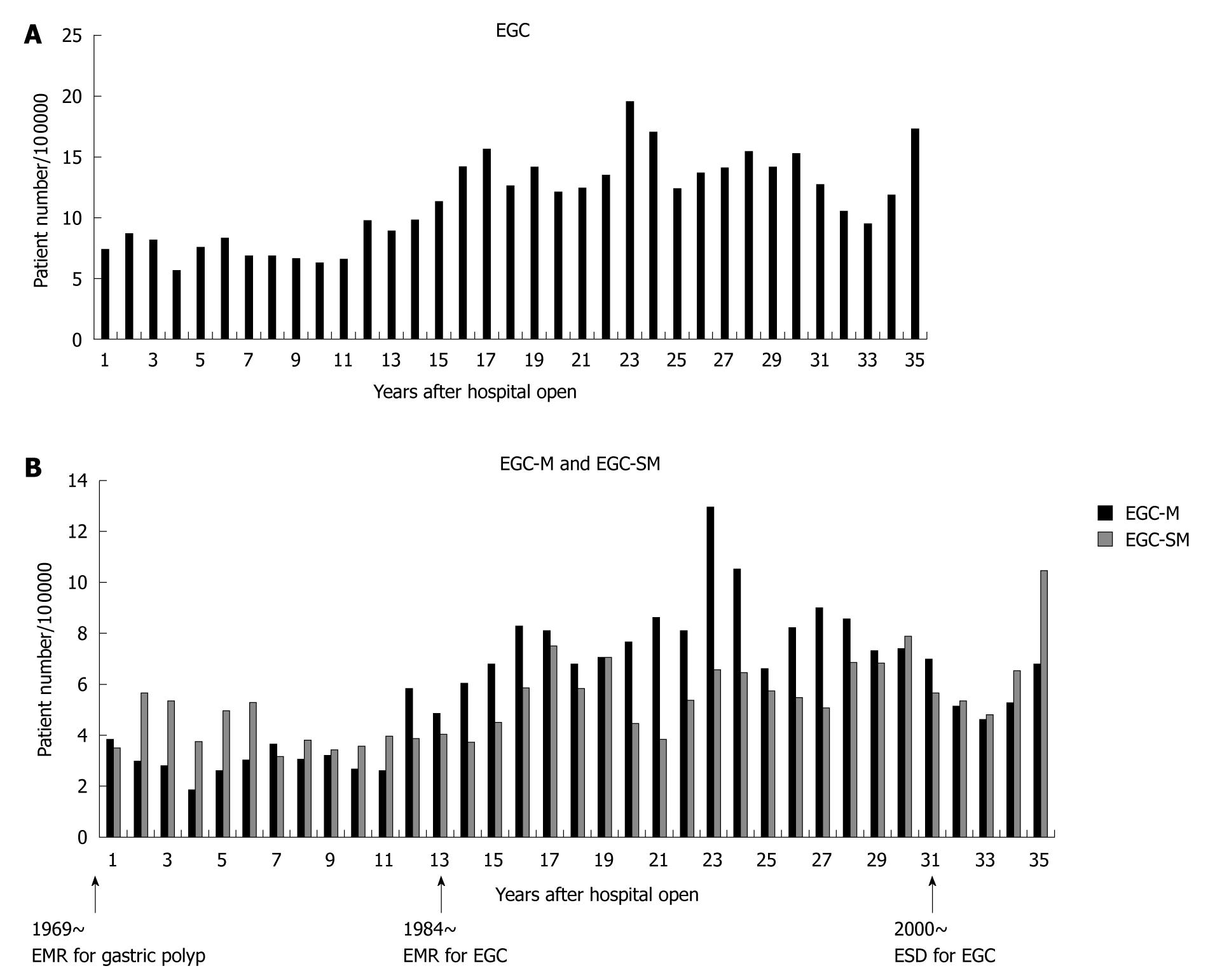
Despite a constant incidence of gastric cancer requiring surgery, the ratio of EGC to AGC persistently increased from low rates ( about 30%) to similar rates (about 50%) (Figure 3). The actual numbers of gastric cancer cases are shown in Figure 4. We subdivided gastric cancer requiring surgical resection into 4 categories, namely mucosal EGC (Early-M), submucosal EGC (Early-SM), AGC stage I to III, and AGC stage IV. Although very small in number, EGC stage IV cases were either Early-M or Early-SM according to our classification. The number of Early-M cases increased until 1994, and afterwards decreased probably due to the extended indication for endoscopic resection of mucosal EGC. On the other hand, the number of Early-SM cases persistently increased until recently, probably because it is inevitably an indication for surgical resection as it has potential for lymph node metastasis. As endoscopic screening has become more prevalent than ever, early detection of SM cancers have also increased. On the other hand, the number of AGC stage I to III (potentially curative) and stage IV was relatively constant, while the population increased in Sagamihara city. After calculating the incidence of each gastric cancer group according to the population in Sagamihara, the incidence of stage I to III and stage IV AGC showed a tendency to decrease (Figure 5). On the other hand, the incidence of EGC markedly increased as a whole. After the recent increased prevalence of endoscopic submucosal dissection and its potential extended application, Early-M and Early-SM exhibited a fluctuating ratio (Figure 6).
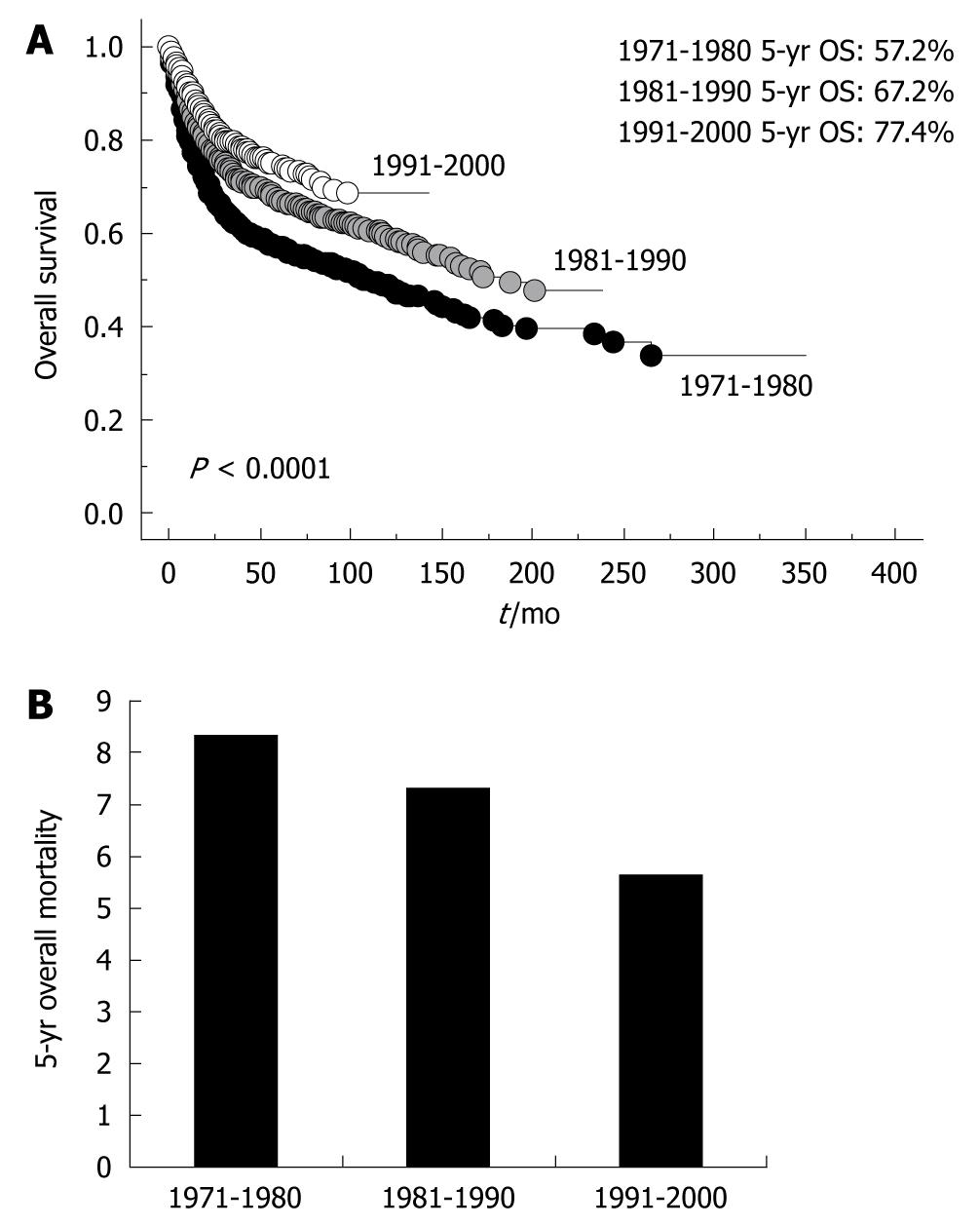
Overall survival of gastric cancer requiring surgical resection markedly improved until recently (Figure 7A). This may be due to the change in the distribution of stage of gastric cancer requiring surgical resection by planned intervention (early detection by endoscopy or double-contrast barium imaging and endoscopic resection). To confirm whether a long-term reduction in the mortality of gastric cancer was observed, we also examined 5-year mortality, and histological distribution of AGC requiring surgical resection. The numbers of deaths at 5 years were 307, 354, and 323 according to the time period (1971-1980, 1981-1990, 1991-2000), and mortality was calculated according to the population in Sagamihara. The mortality of gastric cancer requiring surgical resection at 5 years after surgery gradually decreased (Figure 7B). In order to determine the effect against reduced mortality by introduction of interventions, we examined the ratio of intestinal type AGC to diffuse type AGC stage I to III (Figure 1B). As a result, we could not confirm a changing distribution of intestinal type and diffuse type AGC stage I to III as in other developed countries, in spite of rigorous planned intervention, suggesting that early resection of intestinal type EGC did not necessarily result in a reduced incidence of intestinal type AGC.
In the current study, we did not observe a declining trend of intestinal type gastric cancer in contrast to other developed countries[2]. The incidence of gastric cancer requiring surgical resection was unchanged in our hospital over the observed time period (35 years), which is consistent with the nationwide statistics that show a constant incidence of gastric cancer in Japanese people in recent years[5]. Our data therefore suggested that intestinal type gastric cancer has not changed in our country in spite of planned interventions such as rigorous screening for EGC, in which annual screening with a double-contrast barium technique and endoscopy is recommended for persons over the age of 40 years with subsequent endoscopic resection as necessary[6]. Recent introduction of endoscopic resection for a proportion of intestinal type mucosal EGC may have been expected to result in a reduced incidence of AGC or even EGC requiring surgery, but a reduced rate of intestinal type was not observed. Intestinal type gastric cancer is associated with H. pylori infection, which may result from a poor hygienic environment in early childhood[7]. Elderly Japanese had been living through World War II, when there was likely to be a poor hygienic environment in their childhood, which may be reflected in the high incidence of H. pylori infection in the elderly in Japan[8]. From such considerations, a decreasing trend in intestinal type gastric cancer as in other advanced countries may be expected 10 to 20 years later in Japan. Careful observation is needed to determine the future trend in gastric cancer in Japan.
On the other hand, our current data obviously demonstrated that the clinical outcome of gastric cancer requiring surgery has greatly improved recently (Figure 7A), and that mortality after surgery has declined (Figure 7B). Recent statistics in Japan also showed that mortality of gastric cancer exhibited a decreasing trend[5]. Gastric cancer consists of either EGC or AGC, but mortality largely reflects the prognostic outcome of AGC, and a reduced incidence of AGC must be associated with reduced mortality (Figures 3 and 5). Although AGC stage IV showed a similar tendency as other AGC, we could not conclude whether stage IV disease had actually declined, because far advanced AGC (stage IV) has usually been treated in the Gastrointestinal Department of Internal Medicine. Internal Medicine in our university rigorously screens for EGC and treats with endoscopic resection[9,10] as well as chemotherapy for far AGC[11,12]. Such active interventions for EGC may result in a reduction in mortality of gastric cancer requiring surgery. This also reflects previous investigations that indicate the critical significance of large-scale screening for gastric cancer in reducing mortality[13,14].
We were disappointed at the lack of change in intestinal type gastric cancer rates in this study. A recent report about the effect of eradication of H. pylori infection on metachronous gastric cancer after endoscopic resection of EGC suggested that the eradication itself may reduce the incidence of intestinal type gastric cancer[15]. In that randomized trial of 544 patients with positive H. pylori infection, metachronous gastric cancer after endoscopic resection for EGC largely included intestinal type gastric cancer (32/33), which may suggest that further planned intervention including eradication of H. pylori infection can reduce the incidence of intestinal type gastric cancer requiring surgery. Moreover, our current analysis included remnant gastric cancer, and intestinal type predominates in remnant cancer (about 70%)[16], indicating that eradication of H. pylori would have great preventive potential not only for primary gastric cancer, but also the secondary remnant gastric cancer in terms of intestinal type gastric cancer. Thus we should investigate the clinical significance of eradication of H. pylori infection to reduce the incidence of intestinal type gastric cancer in a population-based study. In Japan, a potent toxic strain of H. pylori with cagA positivity is more prevalent (almost 100%) than in other countries (about 60%)[17-19]. Thus further more general intervention may be needed to further reduce the incidence and mortality of gastric cancer in Japan.
On the other hand, a recent increase in cardia cancer is an emerging threat in gastric cancer worldwide[20,21]. There has also been a rising trend in esophageal adenocarcinoma, in which obesity, gastroesphageal reflux disease (GERD), and Barrett’s esophagus are major etiologic factors, and cardia cancers share certain epidemiologic features with adenocarcinomas of the distal esophagus and gastroesophageal junction, suggesting that they represent a similar disease. In the past 30 years, the incidence of gastric cardia adenocarcinoma rose by 5- to 6-fold in developed countries[22], but not in the developing countries or East Asia[2]. Gastric cardia tumors now account for nearly half of all stomach cancers among men from the Western world[21]. In Japan, foods have become westernized with an increase in obesity in the population, resulting in a rapidly increasing incidence of GERD[23], and cardia cancer is also increasing as we elucidated in this current study (Figure 2B). In Japan, the proportion of gastric cardia tumors remains relatively small among the total gastric cancer as compared to that in Western countries, but as it is supposed to be unrelated to H. pylori infection[24], identification of methods other than eradication of H. pylori infection are necessary to reduce the incidence of cardia cancer.
In conclusion, we revealed both the recent trend of gastric cancer requiring surgery in Japan and the difference from that in other developed countries. We found that intestinal type gastric cancer has not declined in Japan, and thus additional strategies beyond the present interventions such as mass eradication of H. pylori infection in the population may be needed to further reduce the incidence. On the other hand, gastric cardia cancer remains a small proportion of the gastric cancers in Japan, but its incidence is increasing as in Western countries. This finding warrants urgent development of novel strategies to reduce the risk of cardia cancer.
The reduction in gastric cancer observed over many years in the Western world without a planned intervention is unique in the history of malignant disease, but the trend of gastric cancer in Japan remains largely unknown in the rapid course of therapeutic advancement.
The trend in an urban single high-volume institute would reveal the most recent trend in gastric cancer requiring surgery, and elucidate critical clinical concerns.
The study revealed that the ratio of diffuse type/intestinal type gastric cancer was unexpectedly unchanged despite planned and rigorous interventions. However, the actual incidence of potentially curative advanced gastric cancer showed a tendency to decrease. The incidence of early gastric cancer requiring surgery tended to increase as a whole, consistent with the increased prevalence of endoscopic surveillance. As a result, overall survival and mortality of gastric cancer requiring gastric resection drastically improved recently as in other areas of Japan.
In Japan, planned interventions by early detection of gastric cancer requiring surgery may improve mortality of the disease, but an unexpected trend of persistent existence of intestinal type gastric cancer suggested that a more robust planned medical intervention (removal of Helicobacter pylori for high risk patients) or intensive causative research (identification of disease etiology) in addition to the present interventions may be needed to reduce the incidence as in Western countries.
The work is an important retrospective analysis of 4163 gastric cancer patients with proven histology of adenocarcinoma of the stomach, who underwent gastrectomy during a long period and at one institution. The authors analyzed the trends of gastric cancer, reporting interesting data. The work is a well designed and well written study. I think that this study is of interest for the readers of the journal.
Peer reviewer: Francisco Jose Vizoso, MD, PhD, Unidad de Investigación, Hospital de Jove, Avda/Eduardo Castro s/n, 33290 Gijon, Spain
S- Editor Sun H L- Editor Cant MR E- Editor Zheng XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13539] [Article Influence: 677.0] [Reference Citation Analysis (1)] |
| 2. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [PubMed] |
| 3. | Kaneko S, Yoshimura T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975-1989. Br J Cancer. 2001;84:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Parkin DM. International variation. Oncogene. 2004;23:6329-6340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 499] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 5. | National Cancer Center CISC: Graph database. 2010. Available from: http://www.ncc.go.jp/jp/statistics/. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 499] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 6. | Kakizoe T. Cancer Statistics in Japan. Tokyo: Foundation for Promotion of Cancer Research 1999; . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 499] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 7. | Feldman RA. Epidemiologic observations and open questions about disease and infection caused by Helicobacter pylori. Helicobacter pylori: molecular and cellular biology. Wymondham: Horizon Scientific Press 2001; 29-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 499] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 8. | Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, Miki K, Graham DY. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760-766. [PubMed] |
| 9. | Tanabe S, Koizumi W, Kokutou M, Imaizumi H, Ishii K, Kida M, Yokoyama Y, Ohida M, Saigenji K, Shimao H. Usefulness of endoscopic aspiration mucosectomy as compared with strip biopsy for the treatment of gastric mucosal cancer. Gastrointest Endosc. 1999;50:819-822. [PubMed] |
| 10. | Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, Kida M, Oida M, Saigenji K. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708-713. [PubMed] |
| 11. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1413] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 12. | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 471] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 13. | IARC Unit of Descriptive Epidemiology: WHO cancer mortality databank. Cancer Mondial. 2001; Available from: http://www-dep.iarc.fr/ataava/globocan/who.htm. |
| 14. | Guo HQ, Guan P, Shi HL, Zhang X, Zhou BS, Yuan Y. Prospective cohort study of comprehensive prevention to gastric cancer. World J Gastroenterol. 2003;9:432-436. [PubMed] |
| 15. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 924] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 16. | Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007;94:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Vicari JJ, Peek RM, Falk GW, Goldblum JR, Easley KA, Schnell J, Perez-Perez GI, Halter SA, Rice TW, Blaser MJ. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50-57. [PubMed] |
| 18. | Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710-1714. [PubMed] |
| 19. | Azuma T, Yamakawa A, Yamazaki S, Fukuta K, Ohtani M, Ito Y, Dojo M, Yamazaki Y, Kuriyama M. Correlation between variation of the 3’ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J Infect Dis. 2002;186:1621-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [PubMed] |
| 21. | Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235-256. [PubMed] |
| 22. | Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510-513. [PubMed] |
| 23. | Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 24. | Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |