Published online Jul 7, 2011. doi: 10.3748/wjg.v17.i25.2992
Revised: February 21, 2011
Accepted: February 28, 2011
Published online: July 7, 2011
AIM: To evaluate the effect of RNA interference (RNAi) mediated silence of signal transduction and activation of transcription (STAT)3 on the growth of human pancreatic cancer cells both in vitro and in vivo.
METHODS: STAT3 specific shRNA was used to silence the expression of STAT3 in pancreatic cancer cell line SW1990. The anti-growth effects of RNAi against STAT3 were studied in vitro and in experimental cancer xenografts in nude mice. The potential pathways involved in STAT3 signaling were detected using reverse transcription polymerase chain reaction and western blotting.
RESULTS: The expression of the STAT3 was inhibited using RNAi in SW1990 cells. RNAi against STAT3 inhibited cell proliferation, induced cell apoptosis and significantly reduced the levels of CyclinD1 and Bcl-xL when compared with parental and control vector-transfected cells. In vivo experiments showed that RNAi against STAT3 inhibited the tumorigenicity of SW1990 cells and significantly suppressed tumor growth when it was directly injected into tumors.
CONCLUSION: STAT3 signaling pathway plays an important role in the progression of pancreatic cancer, and silence of STAT3 gene using RNAi technique may be a novel therapeutic option for treatment of pancreatic cancer.
- Citation: Huang C, Yang G, Jiang T, Cao J, Huang KJ, Qiu ZJ. Down-regulation of STAT3 expression by vector-based small interfering RNA inhibits pancreatic cancer growth. World J Gastroenterol 2011; 17(25): 2992-3001
- URL: https://www.wjgnet.com/1007-9327/full/v17/i25/2992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i25.2992
Pancreatic cancer is one of the most lethal solid malignancies and its overall 5-year survival is less than 5%. It represents one of the leading causes of cancer deaths in industrialized countries despite advances in medical and surgical modalities[1,2]. Up till now, surgical resection still remains the only treatment for pancreatic cancer[3,4]. However, because of the aggressiveness of this disease, most patients have had local or metastatic spread by the time of diagnosis, and surgical resection is possible in only a few patients. Even among patients undergoing a potentially curative resection, the long-term prognosis remains poor due to early recurrence and metastasis[5]. Unfortunately, effective systemic therapy capable of reversing its aggressiveness is unavailable and the specific molecular regulatory pathways involved in pancreatic cancer initiation and progression have not been fully identified[6,7]. Targeting the currently known signaling pathways, however, may lead to effective treatment for pancreatic cancer.
STAT3, a member of the signal transduction and activation of transcription (STAT) family, is a key cytoplasmic transcription factor activated by tyrosine kinase growth and cytokine receptors. Once tyrosine is phosphorylated, two STAT3 monomers form a dimer through reciprocal phosphotyrosine-SH2 interactions, and translocate to the nucleus where they bind to STAT3-specific DNA-response elements of target genes, and induce gene transcription[8,9]. Elevated activity of STAT3 has been found frequently in a wide variety of human tumors including pancreatic cancer[10-13] and STAT3 participates in the occurrence and development of cancers by promoting cell proliferation, inhibiting cell apoptosis, inducing immune escape, and promoting angiogenesis and metastasis[14,15].
STAT3 signaling pathway may represent a new molecular target for novel therapeutic approaches for human cancers. Several reports showed that blocking of STAT3 expression in human cancer cells suppresses proliferation in vitro and tumorigenicity in vivo. Antisense oligonucleotides and decoy oligonucleotides[16,17], tyrosine kinase inhibitors[18,19], dominant negative STAT3 protein[20], drug-like non-peptide small molecules[21], and RNA interference (RNAi)[22,23] can target STAT3 signaling pathways. Among them, RNAi is the most popular one.
RNAi is a phenomenon of gene silencing, resulting from specific degradation of homologous mRNA mediated by small interfering RNA (siRNA) produced through degradation of double-stranded RNA (dsRNA)[24,25]. Gene silencing involving RNAi requires the processing of long double-stranded RNA (dsRNA) into 19- to 21-nt RNAs, which is called small interfering RNA (siRNA). This process is mediated by Dicer, a type of endonuclease. Subsequently, the siRNA molecules are incorporated into the RNA-induced silencing complex (RISC). The active complexes recognize and cleave the homologous mRNAs, thus selectively inhibiting the expression of the target gene[26,27]. Currently, a prompt and highly-effective method has been developed in RNAi technique to inhibit the expression of specific genes, and has been widely applied in the research of viral diseases, genetic diseases and malignant tumors[28].
The present study was designed to evaluate the use of RNAi to knockdown STAT3 expression and activation, and their effects on human pancreatic cancer cell growth both in vitro and in vivo. The phenotypic growth changes resulting from the reduction of STAT3 expression were observed both in vitro and in vivo. We found that knockdown of STAT3 gene by RNAi significantly suppressed the expression of CyclinD1 and Bcl-xL, both of which were accompanied with marked inhibition of tumor cell growth in vitro and in vivo. Our results demonstrate that STAT3 signaling pathway plays an important role in the progression of pancreatic cancer and that knockdown of STAT3 gene using RNAi technique may be a novel therapeutic option for treatment of pancreatic cancer.
Human pancreatic cancer cell line SW1990 and PANC-1 were purchased from American Type Culture Collection (Manassas, USA). They were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 μg/mL streptomycin in a humidified incubator with an atmosphere of 5% CO2 and 95% air at 37°C.
In our previous studies, three coding regions corresponding to nucleotides 1819-1837, 1025-1043 and 237-255 of STAT3 sequence in the GenBank (NM003150) were selected to form siRNA target sequences. Three primer pairs were synthesized: one pair encoding nucleotides sites 1819-1837 (CTGCTAAGATTCAGTGAAA) followed by a 9 base “loop” (TTCAAGAGA) and the inverted repeat (STAT3-siRNA-1), the second one encoding nucleotides sites 1025-1043 (GCGTCCAGTTCACTACTAA) also followed by the loop and the inverted repeat (STAT3-siRNA-2), and the third one encoding nucleotides 237-255 (TCAGCACAATCTACGAAGA) again followed by the loop and the inverted repeat (STAT3-siRNA-3). We then constructed three STAT3 specific shRNA expression vectors (pRNAT-STAT3-siRNA-I, II, III) and found that pRNAT-STAT3 siRNA-II had the most obvious gene silencing effect. We also constructed scrambled siRNA expression vector as a negative control (pRNAT-Con). We established stable SW1990 pRNAT-Con transfectants (SW1990-Con) and SW1990 STAT3-RNAi transfectants (SW1990-RNAi) and found that stable transfection of pRNAT-STAT3-siRNA-II vector silenced STAT3 expression. The stably transfected cells were used for subsequent studies[29].
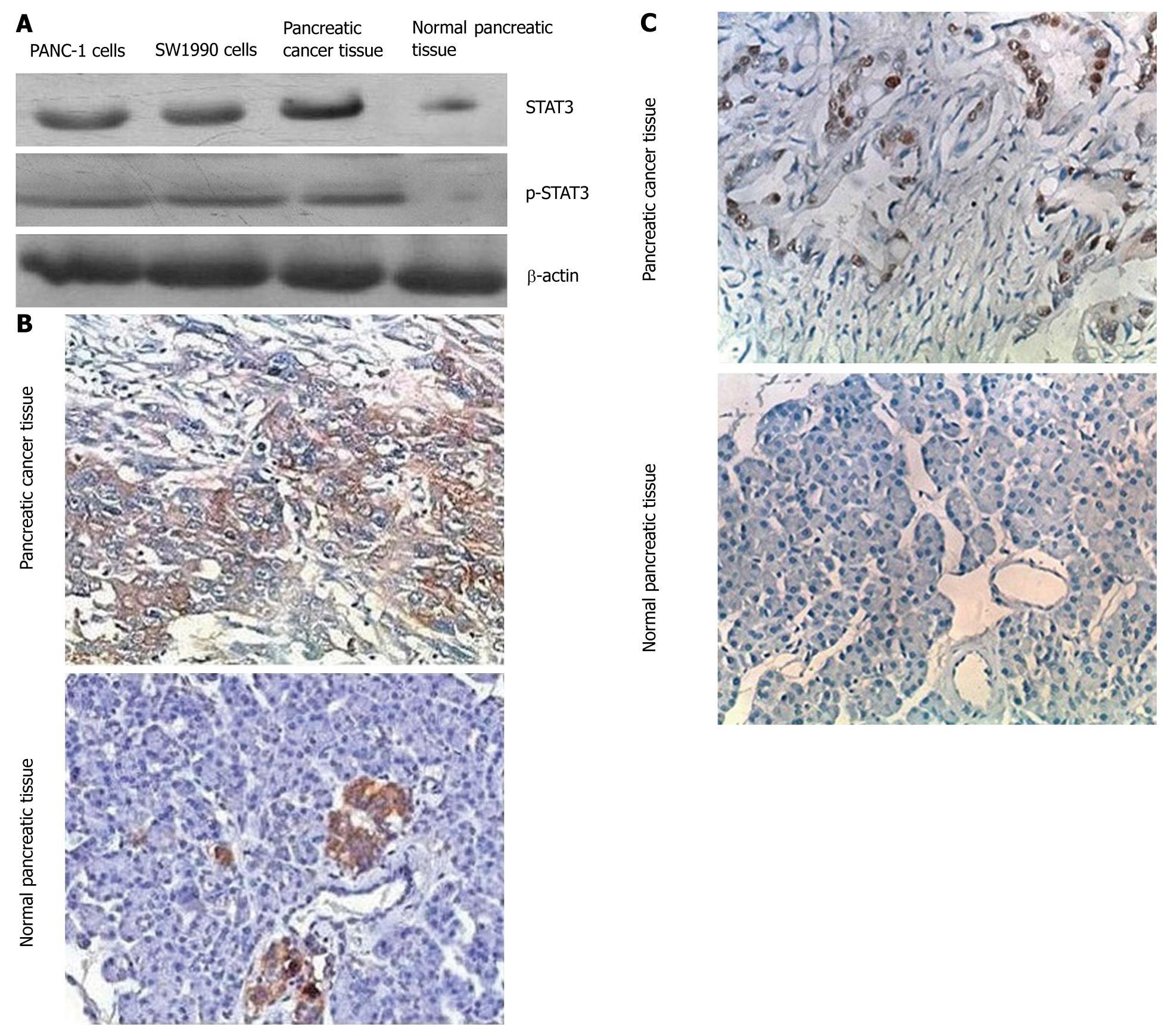
Primary pancreatic tumors were found in 71 patients suffering from pancreatic cancer. Informed consent was obtained for the use of tissues in this study from all the patients, who underwent surgical treatment at Affiliated First People’s Hospital of Shanghai Jiao Tong University. Ten normal pancreatic tissues were collected through regular multi-organ donor procedures. Paraffin wax samples from the 71 cases of primary pancreatic tumors and 10 with normal pancreatic tissues were cut into 4-μm-thick slices. These slices were dewaxed and the endogenous peroxidase activity was quenched after incubation in methanol containing 3% hydrogen peroxide for 10 min. The histologic sections were incubated with a rabbit anti-human STAT3 polyclonal antibody (Cell Signal, USA) or rabbit polyclonal IgG controls (Vector Laboratories, USA) in blocking buffer overnight at 4°C. The sections were then rinsed in PBS (containing 0.5% bovine serum albumin and 0.1% Tween-20) and incubated for 30 min with biotinylated goat anti-rabbit IgG (ABC staining kit, Santa Cruz, USA) diluted according to the manufacturer’s protocol. Next, a solution of avidin-conjugated horseradish peroxidase (ABC staining kit) was applied for 30 min, according to the manufacturer’s instructions. Peroxidase activity was developed in 0.5% (vol/vol) 3,3’-diaminobenzidine hydrochloride (DAB, Sigma, USA) in PBS containing 0.03% (vol/vol) hydrogen peroxide for 2 min. Sections were counterstained with Harris’ hematoxylin and mounted in gelatin (Sigma, USA). The criteria for immunohistochemical assay are as follows: positive cells contained brown particle staining in the nucleus or cytoplasm. Samples with < 5% positive cells were designated as negative (-); samples stained slightly (5%-25% positive) were designated as (+); samples stained moderately (25%-50% positive) as (++), and stained deeply ( > 50 % positive) as (+++).
To quantify cell proliferation, SW1990 cells and stably transfected cells (SW1990-Con, SW1990-RNAi) were seeded in a 96-well plate at a concentration of 5 × 103 /well (100 μL/well). Eight parallel wells were assigned to each group. Then 20 μL/well of 5 mg/mL MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added at 24 h, 48 h and 72 h after seeding and the cells were nurtured for another 4 h. The supernatant was removed and the product converted from MTT was dissolved by adding 150 μL/well dimethyl sulfoxide (DMSO) and shaking for 10 min. Optical density (OD) readings were obtained at 490 nm. The cell growth rate was represented by the relative ratio of OD490 at 24, 48 and 72 h, to OD490 at 0 h, respectively. The growth curve was drawn according to the cell growth rate. A soft agar colony formation assay was used to assess the anchorage-independent growth ability of cells. Specifically, SW1990, SW1990-Con and SW1990-RNAi cells were plated on a 0.6% agarose base in six-well plates (1.0 × 103 per well) in 1 mL DMEM medium containing 10% fetal bovine serum and 0.3% agarose. Colonies > 100 μm were counted 14 d after plating.
Cell apoptosis was assessed by apoptosis kit (Roche, USA), according to the manufacturer’s protocol. Briefly, SW1990 cells, and stably transfected cells SW1990-Con and SW1990-RNAi were collected to make single cell suspensions (5 × 106 cells). And 20 μL fluorescence-tagged Annexin-V and 20 μL pyridine iodinate (PI) were added into 1 mL incubation buffer to prepare the marking liquor. Cells were washed once by PBS and centrifuged at 500 ×g at 4°C for 5 min. The supernatant was discarded. The cell deposition was resuspended with 100 μL marking liquor and placed in dark at normal temperature for 10-15 min. Flow cytometric analysis showed that Annexin-V+/PI- cells were early apoptotic cells, while Annexin-V+/PI+ cells were late apoptotic and dead cells.
SW1990 cells and stably transfected cells, SW1990-Con and SW1990-RNAi , were collected and fixed. After incubation in RNase A for 30 min at 37°C, the cells were stained with PI. Flow cytometric analysis was done using a FACScan instrument (Becton Dickinson, Mountain view, CA) and CellQuest software
Male athymic BALB/c nude mice were obtained from the Animal Center of Chinese Academy of Sciences (Shanghai, China) and housed in laminar flow cabinets under specific pathogen-free conditions. The mice were used when they were 6-8 wk old. The use of animals in this study complies with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1985) and the current Chinese regulations and standards on the use of laboratory animals.
Male athymic BALB/c nude mice (6-8 wk old) were housed in laminar flow cabinets under specific pathogen-free conditions. SW1990, SW1990-Con and SW1990-RNAi cells were injected into the right flank of mice with a total volume of 100 μL (1.0 × 107 cells). The tumor-bearing mice were sacrificed 35 d after inoculation and the tumors were taken and weighed.
SW1990 cells were injected into the right flank of BALB/c nude mice with a total volume of 100 μL (1.0 × 107 cells). Tumors were allowed to grow in vivo for 2 weeks, reaching an average size of 5 mm in diameter. The animals were divided randomly into three groups (six mice per group): (1) PBS buffer alone (mock), (2) pRNAT-Con (20 μg/mouse), and (3) pRNAT-STAT3-siRNA-II (20 μg/mouse). The samples were diluted in 50 μL PBS buffer and injected percutaneously into the tumor using a syringe with a 27-gauge needle. Immediately after injection, tumors were pulsed with an electroporation generator. This process was repeated on day 21. Tumor sizes were measured every 5 d. Tumor masses (in cubic millimeter) were calculated as a × b2× 0.52 (a represents the length, b represents the width)[30]. The tumor-bearing mice were sacrificed on day 35, and the tumors treated with either pRNAT-Con or pRNAT-STAT3-siRNA-II were taken, weighed and sectioned for STAT3 immunostaining with rabbit anti-human STAT3 polyclonal antibody (Cell Signal, USA) as before.
Total RNA extraction from tumor cells was performed with Trizol Reagent (Life Technologies, USA). Two µg of total RNA was reverse-transcribed with the First Strand cDNA Synthesis Kit (Promega, USA) to synthesize cDNA samples. Subsequently, 2 μL cDNA product was subjected to PCR amplification with Taq DNA polymerase (Sangon, China) on a thermal cycler using the following primers. The oligo-nucleotide primers for STAT3 were constructed using a software “Primer Premier 5.0”. The oligo-nucleotide primers for Bcl-xL, Cyclin D1 and β-actin were constructed based on the published sequence. The PCR primers used to detect each factor were as follows: Bcl-xL, sense strand 5’-CCCAGAAAGGATACAGCTGG-3’, antisense strand 5’- GCGATCCGACTC ACCAATAC-3’, with a product length of 448 bp[31]; Cyclin D1, sense strand 5’- GAGACCATCCCCCTGACGGC-3’, antisense strand 5’-TCTTCCTCCTCCTCGGCGGC-3’, with a product length of 485 bp[31]; β-actin, sense strand 5’-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3’, antisense strand 5’-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3’, with a product length of 838 bp[32]. The PCR conditions were: one cycle of denaturing at 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 60°C for 1 min and 72°C for 1 min, before a final extension at 72°C for 10 min. The PCR products were loaded onto 2% agarose gels and visualized with ethidium bromide under UV light. This experiment was performed three times and a representative data was shown.
Whole-cell protein extracts and nuclear protein extracts from tumor cells were prepared with RIPA Lysis Buffer (Santa Cruz, USA) and Nuclear Extract Kit (Active Motif, USA), according to the manufacturer’s instructions, respectively. Protein concentrations were determined using a Bio-Rad assay kit (Bio-Rad, USA). Lysates containing 100 μg protein were mixed with loading-buffer with 5% β-mercaptoethanol, and heated for 5 min at 100°C. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes by semi-dry blotting. Membranes were incubated in blocking buffer (1 × TBS, 0.1% Tween 20, and 5% non-fat dry milk) for 1 h at room temperature, followed by hybridization with anti-p-STAT3 [tyr-705] antibody (Cell signal, USA, 1:1000 dilution), anti-STAT3 antibody (Cell signal, USA, 1:1000 dilution), anti-Bcl-xL antibody (Cell signal, USA, 1:1000 dilution), anti-CyclinD1 antibody (Cell signal, USA, 1:000 dilution) or anti-β-actin antibody (Labvision, USA, 1:100 dilution) at 4°C overnight. After three washes in TBS/0.1% Tween 20, the membranes were hybridizated with a horseradish peroxidase-conjugated secondary antibody rabbit IgG (Santa Cruz, USA, 1:5000 dilution) for 1 h at room temperature. After three washes in TBS/0.1% Tween 20, signals were detected by chemiluminescence using the Western blotting Luminol Reagent (Santa Cruz, USA). The same experiment was performed three times and a representative data was shown.
To determine whether STAT3 and p-STAT3 are overexpressed in pancreatic cancer tissues, we compared the level of STAT3 and p-STAT3 expression in normal pancreatic tissues with that in the pancreatic cancer tissue and pancreatic cancer cell lines (PANC-1 and SW1990) using immunohistochemical and Western blot analyses with an anti-STAT3 antibody and anti-p-STAT3 antibody. Both approaches showed that STAT3 and p-STAT3 were overexpressed in cancer tissues and pancreatic cancer cell lines (Figure 1). STAT3 and p-STAT3 protein levels were measured by Western blotting. Quantitative evaluation of the relative expression of STAT3 revealed that this protein was overexpressed by an average of 2.8-fold in the 71 primary pancreatic tumors compared with normal pancreatic tissues. As summarized in Table 1, the STAT3 levels were significantly different (P < 0.05) between the pancreatic tumor specimens and normal pancreatic specimens. Immunohistochemical analyses also showed that pancreatic cancer specimens had a high density staining for p-STAT3.
| Specimens | n | - | + | ++ | +++ | |
| STAT3 | Normal specimens | 10 | 9 | 1 | 0 | 0 |
| Cancer specimens | 71 | 19 | 14 | 23 | 15 | |
| p-STAT3 | Normal specimens | 10 | 10 | 0 | 0 | 0 |
| Cancer specimens | 71 | 21 | 11 | 26 | 13 |
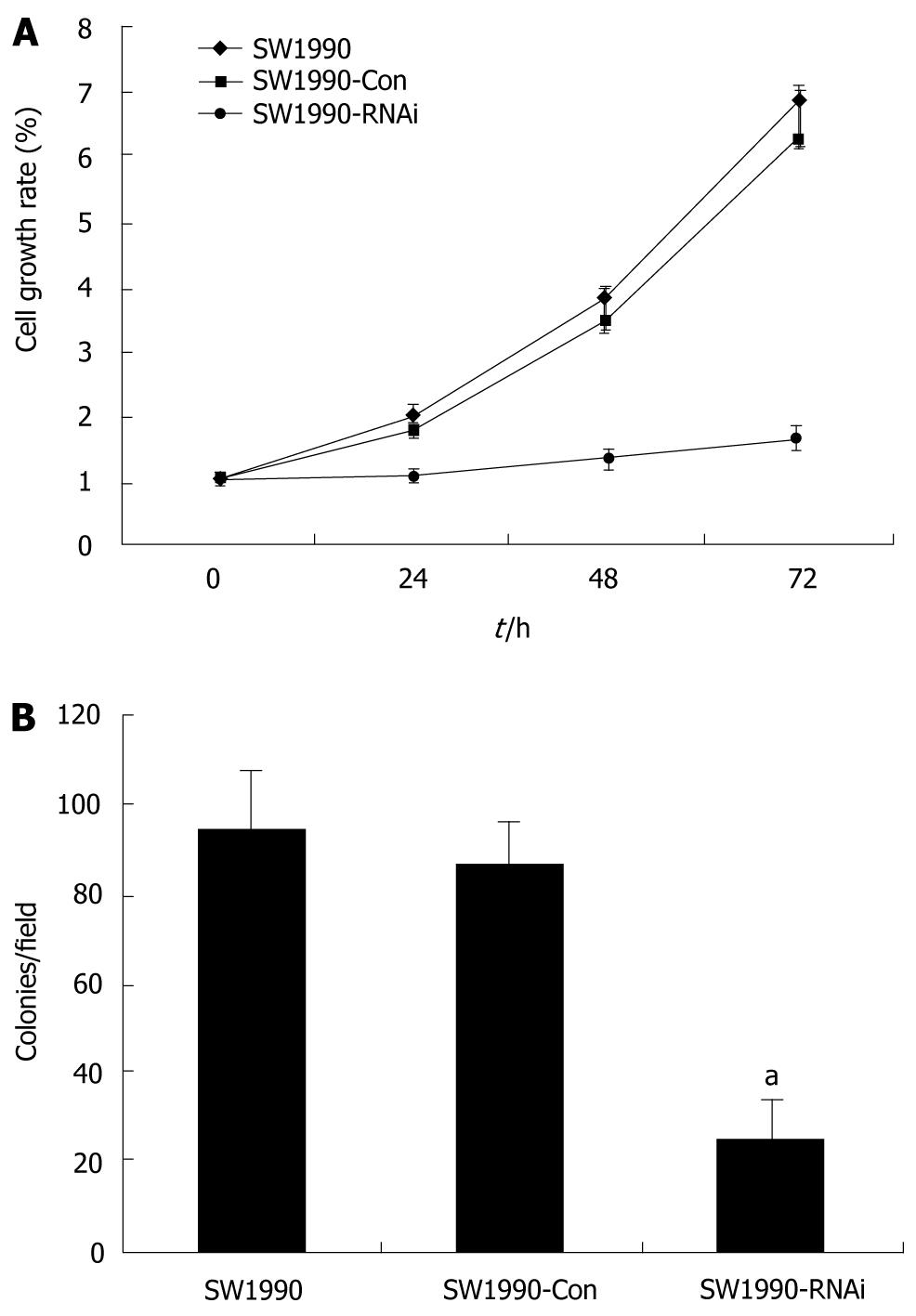
To determine whether inhibition of STAT3 affects cell proliferation and metabolic activity of parental SW1990 cells, SW1990-Con cells and SW1990-RNAi cells were determined at 24, 48 and 72 h by the MTT assay. The cell proliferation was reduced significantly after treatment with pRNAT-STAT3-siRNA-II (P < 0.05) as compared with that of parental SW1990 or SW1990-Con cells (Figure 2A). Furthermore, pRNAT-STAT3-siRNA-II reduced SW1990 cell colony formation by 72.6% (P < 0.05, Figure 2B).
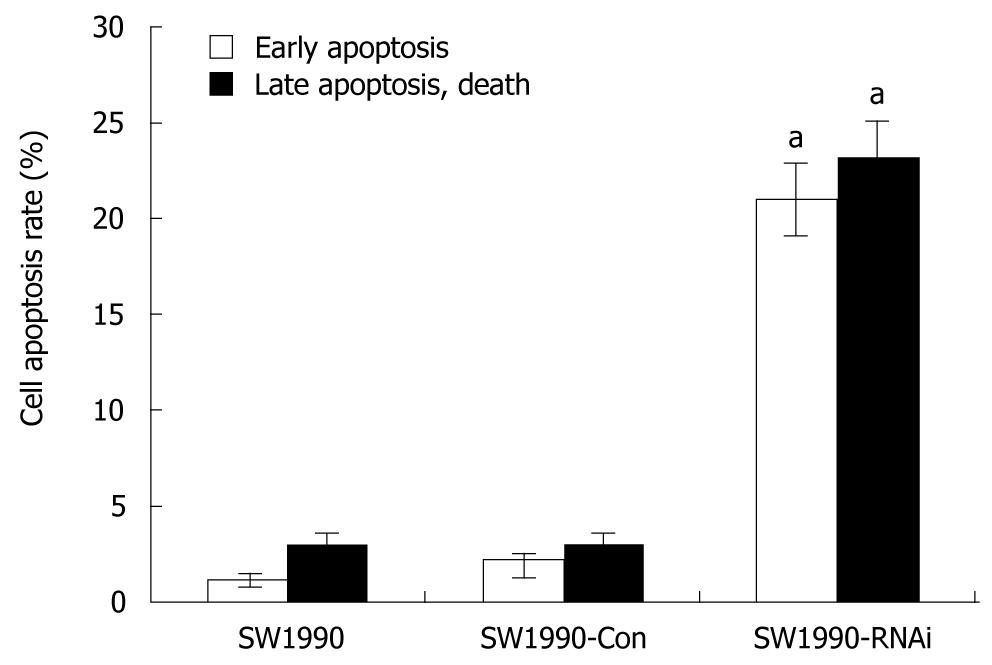
To analyze the mechanisms by which pRNAT-STAT3-siRNA-II inhibits cell proliferation, the cell cycle and cell apoptosis of SW1990 cells as well as stably transfected cells SW1990-Con and SW1990-RNAi, were analyzed by flow cytometry. As shown in Table 2, the percentage of cells at G0/G1 phase was increased from 38.76% (parental SW1990) to 65.39% (SW1990-RNAi) and the S-phase cells were decreased from 29.47% (parental SW1990) to 9.88% (SW1990-RNAi). Figure 3 also indicates that the difference was not statistically significant in the rates of the early apoptotic cells and the late apoptotic cells between SW1990 cells and SW1990-Con cells, while these rates in SW1990-RNAi cells increased significantly (P < 0.05) as compared with parental SW1990 or SW1990-Con cells. These data showed that silencing of STAT3 can arrest cells at G0/G1 phase and increase cell apoptosis.
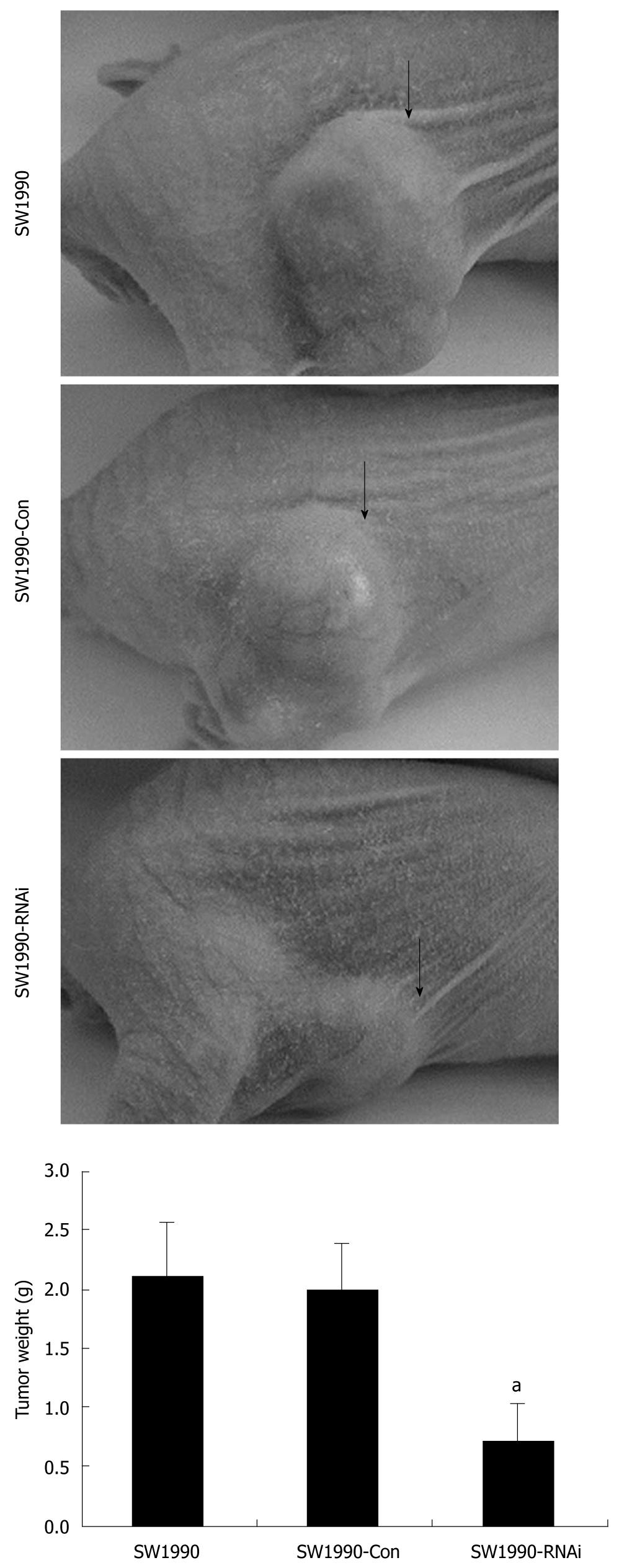
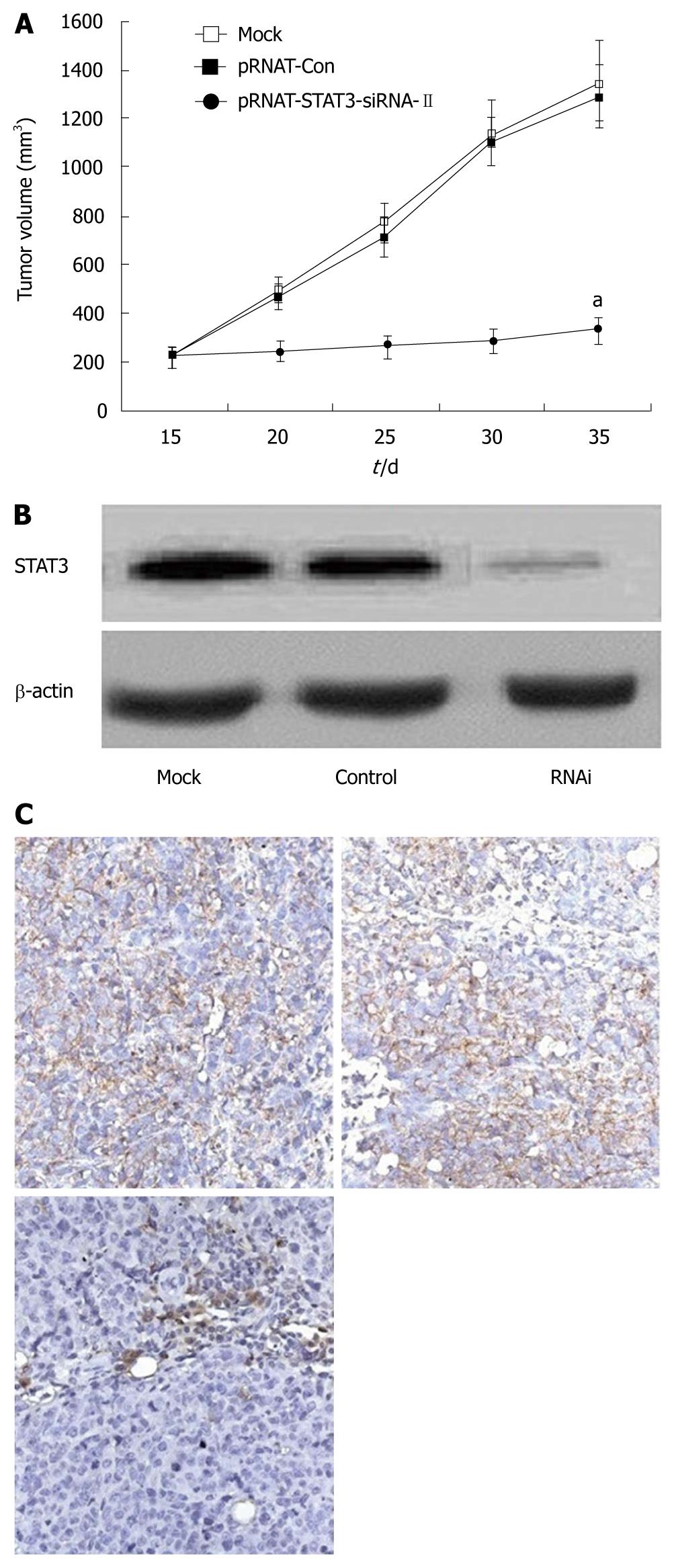
The tumorigenicity of SW1990 cells was examined after silencing of STAT3 in vivo. All mice developed tumors from parental SW1990 cells or pRNAT-Con-infected SW1990 cells (control) without significant difference in tumor weight. In contrast, only three of six mice developed tumors from pRNAT-STAT3-siRNA-II-infected SW1990 cells and the tumors were significantly smaller than those of the control mice (Figure 4). These results suggest that STAT3 plays an important role in tumorigenicity.
We further investigated the possibility of using STAT3 as a target gene for pancreatic cancer therapy in the nude mouse tumor xenograft model. Mice were transplanted s.c. with 1.0 × 107 SW1990 cells in the right flank. By day 14, palpable tumors had grown at the sites of injection. These mice were divided into three groups with six mice in each group and injected intratumorally with either PBS buffer alone (mock), pRNAT-Con, or pRNAT-STAT3-siRNA-II. This process was repeated on day 21. Animals were sacrificed on day 35. As shown in Figure 5A, the mean tumor size of mice treated with PBS buffer control (mock) was 1349.36 ± 164.41 mm3 on day 35; the mean tumor size in mice treated with pRNAT-Con was 1288.59 ± 129.26 mm3 and that of the group treated with pRNAT-STAT3-siRNA-II was 335.81 ± 55.74 mm3. The difference was not statistically significant in tumor size between the mock group and pRNAT-Con group (P > 0.05). The group treated with pRNAT-STAT3-siRNA-II showed marked tumor growth suppression compared with the pRNAT-Con (P < 0.05). STAT3 expression was significantly reduced after RNAT-STAT3-siRNA-II treatment (Figure 5B and C). These results suggested that silencing STAT3 has a therapeutic potential for pancreatic cancer.
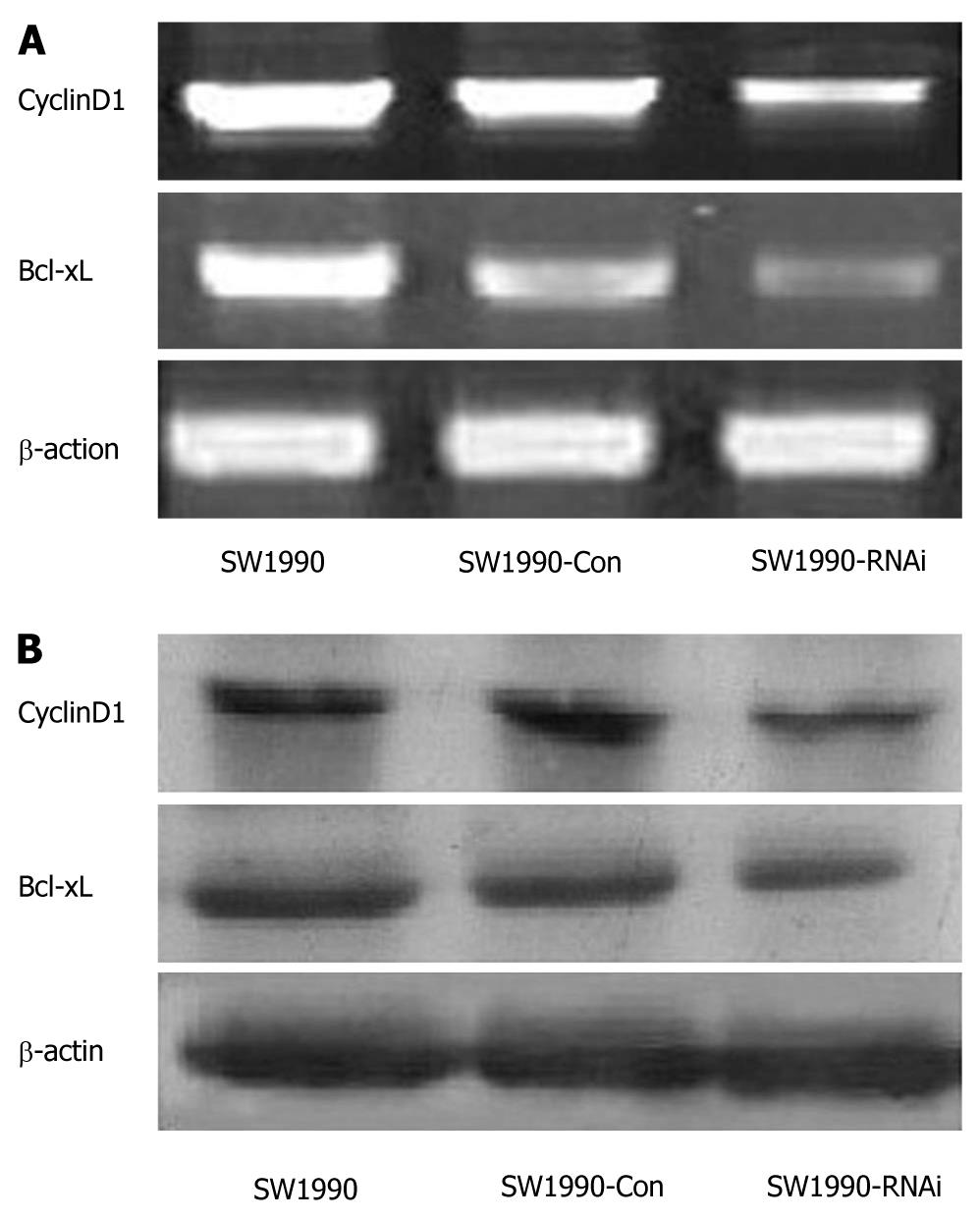
STAT3 activation contributes to oncogenesis through regulation of its target genes. To determine the effect of STAT3 downregulation on growth-related target gene expression, we assayed the expression of CyclinD1 and Bcl-xL by RT-PCR, both of which were directly involved in tumor cell proliferation and apoptosis. As shown in Figure 6A, the expression of CyclinD1 and Bcl-xL mRNAs in SW1990 cells was significantly inhibited after STAT3 silencing. The densitometric analyses revealed that the relative CyclinD1 expression in SW1990-RNAi cells was reduced to 52% compared with that of the parental SW1990 cells. And Bcl-xL relative expression in SW1990-RNAi cells was reduced to 39% of that of parental SW1990 cells. A similar inhibitory effect on protein levels is shown in Figure 6B, which demonstrated that the expression of CyclinD1 and Bcl-xL proteins in SW1990 cells was also significantly inhibited after STAT3 silencing. These results indicated that silencing of STAT3 gene suppressed CyclinD1 and Bcl-xL expression.
Pancreatic adenocarcinoma remains a widespread disease and difficult to be treated. Surgical resection can only cure a few cases, and most patients are not suitable for the surgical resection, and conventional chemotherapy and radiation remain largely ineffective. Thus, pancreatic adenocarcinoma represents one of the leading causes of cancer deaths in industrialized countries. With the expectation of increasing therapeutic efficacy, gene therapy is being investigated as a new treatment modality[33]. STAT3 has been considered a very promising target molecule for cancer therapy because it plays a pivotal role in tumorigenesis by cell cycle progression, apoptosis, angiogenesis, metastasis and tumor cell evasion of the immune system[14,15]. Strong evidence has proved that aberrant Stat3 signaling may play an important role in the development and progression of pancreatic adenocarcinoma. We also demonstrated that increasing STAT3 activation in pancreatic adenocarcinoma and blocking Stat3 activation by AG490 (a JAK-specific inhibitor) resulted in suppression of pancreatic cancer growth and invasion in vitro[34,35]. Collectively, these findings indicate that targeting STAT3 signaling may represent a novel approach to treat pancreatic adenocarcinoma.
RNAi represents a promising new experimental tool for the analysis of gene function and has become a key gene therapy technique in mammalian systems. Compared with traditional gene therapy, RNAi possesses the advantages of an exquisite precision and high efficacy in down-regulating gene expression[36,37].
In the present study, we used shRNAs targeting STAT3 to silence the expression of STAT3 in human pancreatic cancer cells SW1990. We successfully constructed the recombinant plasmid pRNAT-STAT3-RNAi-II and employed the recombinant plasmid to generate SW1990-RNAi cell line, which showed a significantly decreased STAT3 expression. Attenuation of STAT3 changed the growth behavior of human SW1990 cells both in vitro and in vivo. MTT assay and soft agar colony formation assay revealed that STAT3 silencing by RNAi inhibited SW1990 cell proliferation and anchorage-independent growth ability. Flow cytometry revealed that RNAi targeting STAT3 arrested SW1990 cells at G0/G1 phase and increased SW1990 cell apoptosis. Moreover, in vivo study showed that STAT3 silencing inhibited tumorigenecity and tumor growth of SW1990 cells in nude mouse tumor xenograft model.
The inhibitory mechanism in the tumor growth after STAT3 silencing with RNAi is considered as down-regulation of genes related with cell proliferation and apoptosis. CyclinD1 is believed to play a key role in the cell proliferation through promoting cell cycle[38] and overexpression of cyclin D1 was reported to correlate with poor prognosis in pancreatic cancer[39]. Recently, some studies have found that STAT3 signaling directly regulates CyclinD1 expression, tumor proliferation and growth and proved that CyclinD1 is a target gene of STAT3[40]. Our previous study found that inhibition of STAT3 signal with AG490 could inhibit growth of pancreatic cancer cells and decrease CyclinD1 expression[34]. This study also showed that the silencing of STAT3 markedly reduced the mRNA and protein expression of CyclinD1 in SW1990 cells.
Besides persistent proliferation, phenotypes of anti-apoptosis are also required for cancer cells to grow well in vivo. Bcl-xL, an anti-apoptotic gene of the BCL-2 family, is associated with poor survival and prognosis of pancreatic cancer patients[41]. Increased expression of Bcl-xL is dependent on the constitutively activated STAT3, and Bcl-xL has been proved to be a target gene of STAT3. Blocking STAT3 signal in human tumor cells has been shown to downregulate Bcl-xL expression and induce tumor-cell apoptosis[42]. As shown in our previous study, inhibition of STAT3 signal with AG490 could retard the growth of pancreatic cancer cells and decrease Bcl-xL expression[34]. This study also found that the silencing of STAT3 with RNAi significantly decreased the mRNA and protein expression of Bcl-xL in SW1990 cells.
In conclusion, the present study indicates that siRNA targeting STAT3 mRNA via a plasmid based system effectively sustains the silencing of STAT3 gene expression in SW1990 cells. The impaired STAT3 expression results in reduced SW1990 cell growth both in vitro and in vivo due to the the downregulation of the expression of CyclinD1 and Bcl-xL. Targeting STAT3 activation by RNAi may be a potential therapeutic strategy in the treatment of pancreatic adenocarcinoma.
signal transduction and activation of transcription (STAT)3 is a central cytoplasmic transcription factor. Uncontrolled activation of STAT3 plays a critical role in cell survival and proliferation during oncogenesis. The authors evaluated the effect of RNA interference (RNAi) mediated silence of STAT3 on the growth of human pancreatic cancer cells both in vitro and in vivo.
Activated STAT3 has been shown to promote tumor cell proliferation, metastasis, and angiogenesis by regulating associated genes. The authors determined whether the STAT3 signaling pathway regulates the growth of pancreatic cancer cells, and found that silencing of STAT3 with RNAi may offer a novel strategy for pancreatic cancer intervention.
The expression of the STAT3 was inhibited using RNAi in SW1990 cells. RNAi against STAT3 inhibited cell proliferation, induced cell apoptosis and significantly reduced the levels of CyclinD1 and Bcl-xL. In vivo experiments showed that RNAi against STAT3 inhibited the tumorigenicity of SW1990 cells and significantly suppressed tumor growth.
The present study indicates that siRNA targeting STAT3 mRNA via a plasmid based system effectively sustains the silence of STAT3 gene expression in SW1990 cells. The impaired STAT3 expression results in reduced SW1990 cell growth both in vitro and in vivo. Therefore, targeting STAT3 activation by RNAi may be a more effective approach in the treatment of pancreatic cancer.
STAT3 is a key cytoplasmic transcription factor activated by tyrosine kinase growth factor and cytokine receptors. Once tyrosine is phosphorylated, two STAT3 monomers form a dimer through reciprocal phosphotyrosine-SH2 interactions, and translocate to the nucleus where they bind to STAT3-specific DNA-response elements of target genes, and induce gene transcription. It has been demonstrated that STAT3 participates in the occurrence and development of cancers.
In this study, the authors evaluate the effect of RNAi mediated silence of STAT3 on the growth of human pancreatic cancer cells. They showed that RNAi for STAT3 not only inhibited cell proliferation and induced cell apoptosis in vitro but also suppressed pancreatic tumor growth in vivo. As the authors stated, the present study suggested the possibility that silence of STAT3 gene using RNAi may be a novel therapeutic option for treatment of pancreatic cancer. Overall, the manuscript is well written.
Peer reviewer: Masahiro Iizuka, MD, PhD, Director, Akita Health Care Center, Akita Red Cross Hospital, 3-4-23, Nakadori, Akita, 010-0001, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
| 1. | Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897-909. |
| 3. | Loos M, Kleeff J, Friess H, Büchler MW. Surgical treatment of pancreatic cancer. Ann N Y Acad Sci. 2008;1138:169-180. |
| 4. | Adams RB, Allen PJ. Surgical treatment of resectable and borderline resectable pancreatic cancer: expert consensus statement by Evans et al. Ann Surg Oncol. 2009;16:1745-1750. |
| 5. | Yokoyama Y, Nimura Y, Nagino M. Advances in the treatment of pancreatic cancer: limitations of surgery and evaluation of new therapeutic strategies. Surg Today. 2009;39:466-475. |
| 6. | Pliarchopoulou K, Pectasides D. Pancreatic cancer: current and future treatment strategies. Cancer Treat Rev. 2009;35:431-436. |
| 7. | Schneider G, Hamacher R, Eser S, Friess H, Schmid RM, Saur D. Molecular biology of pancreatic cancer--new aspects and targets. Anticancer Res. 2008;28:1541-1550. |
| 10. | Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, Cascone T, Mills GB, Heymach JV, Johnson FM. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res. 2009;15:6852-6861. |
| 11. | He M, Young CY. New approaches to target the androgen receptor and STAT3 for prostate cancer treatments. Mini Rev Med Chem. 2009;9:395-400. |
| 12. | Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, Hwang SG, Park PW, Rim KS, Hong SP. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009;24:646-651. |
| 13. | Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, Schimer M, Wiedenmann B and Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891-905. |
| 14. | Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315-324. |
| 15. | Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798-809. |
| 16. | Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA. 2003;100:4138-4143. |
| 17. | Lewis HD, Winter A, Murphy TF, Tripathi S, Pandey VN, Barton BE. STAT3 inhibition in prostate and pancreatic cancer lines by STAT3 binding sequence oligonucleotides: differential activity between 5’ and 3’ ends. Mol Cancer Ther. 2008;7:1543-1550. |
| 18. | Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645-648. |
| 19. | Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C, Nissen MH, Röpke C, Wasik MA, Odum N. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia. 2001;15:787-793. |
| 20. | Ni Z, Lou W, Leman ES, Gao AC. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res. 2000;60:1225-1228. |
| 21. | Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci USA. 2005;102:4700-4705. |
| 22. | Klosek SK, Nakashiro K, Hara S, Goda H, Hamakawa H. Stat3 as a molecular target in RNA interference-based treatment of oral squamous cell carcinoma. Oncol Rep. 2008;20:873-878. |
| 23. | Jiang K, Krous LC, Knowlton N, Chen Y, Frank MB, Cadwell C, Centola M, Jarvis JN. Ablation of Stat3 by siRNA alters gene expression profiles in JEG-3 cells: a systems biology approach. Placenta. 2009;30:806-815. |
| 25. | Lee SH, Sinko PJ. siRNA--getting the message out. Eur J Pharm Sci. 2006;27:401-410. |
| 26. | Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Genet. 2001;2:110-119. |
| 27. | Elbashir SM, Lendeckel W and Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188-200. |
| 28. | Elbashir SM, Harborth J, Weber K and Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199-213. |
| 29. | Qiu Z, Huang C, Sun J, Qiu W, Zhang J, Li H, Jiang T, Huang K, Cao J. RNA interference-mediated signal transducers and activators of transcription 3 gene silencing inhibits invasion and metastasis of human pancreatic cancer cells. Cancer Sci. 2007;98:1099-1106. |
| 30. | Fahmy RG, Dass CR, Sun LQ, Chesterman CN, Khachigian LM. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med. 2003;9:1026-1032. |
| 31. | Toyonaga T, Nakano K, Nagano M, Zhao G, Yamaguchi K, Kuroki S, Eguchi T, Chijiiwa K, Tsuneyoshi M and Tanaka M. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201:107-116. |
| 32. | Zhu Z, Yao J, Wang F and Xu Q. TNF-alpha and the phenotypic transformation of human peritoneal mesothelial cell. Chin Med J (Engl). 2002;115:513-517. |
| 34. | Huang C, Qiu ZJ, Liu C, Sun HC. Effect of blocking STAT3 signaling pathway on growth of human pancreatic cancer cells. Zhong Liu. 2006;26:414-417. |
| 35. | Huang C, Cao J, Huang KJ, Zhang F, Jiang T, Zhu L, Qiu ZJ. Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 2006;97:1417-1423. |
| 36. | Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107:222-239. |
| 38. | Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812-821. |
| 39. | Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG. Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res. 1997;57:1634-1637. |
| 40. | Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;19:5419-5427. |