Published online Jun 21, 2011. doi: 10.3748/wjg.v17.i23.2855
Revised: April 19, 2011
Accepted: April 26, 2011
Published online: June 21, 2011
AIM: To investigate the sonographic features and diagnostic value of endoscopic ultrasonography (EUS) for duodenal lipomas (DLs).
METHODS: A total of eight consecutive patients with DL diagnosed pathologically were included in the study. One EUS expert reviewed the ultrasonic images for all lesions, including the original layer of the duodenal wall, the echo intensity and the echo homogeneity. The size of the lesions and the perifocal structures were also investigated. The diagnosis by EUS was compared with the histological results.
RESULTS: Using routine endoscopy, only one case was correctly diagnosed as DL. Four cases were classified as submucosal tumors, and three cases were mistaken for stromal tumors. All tumors appeared as round or oval intensive hyperechoic lesions with distinct anterior borders that originated from the submucosal layer on EUS. Tumors ranged from 8 to 36 mm in size, with an average size of 16 mm. Homogeneous echogenicity was seen in all cases except one that had a tubular structure inside the tumor. Echo attenuation was observed only in the area behind the tumors in five cases, and it was observed both inside and behind the tumors in three cases in which the posterior border was obscure or invisible. Seven (87.5%) cases were correctly diagnosed as DL, and one (12.5%) was mistaken as Brunner’s gland adenoma by EUS. Pathologically, all tumors originated from the submucosal layer and consisted of mature fat cells without heteromorphism. Among the fat cells, there was a small amount of thick-wall vessels infiltrating the lymphocytes, and abundant fibrous connective tissues.
CONCLUSION: On EUS, DL is featured as an intensive homogeneous hyperechoic submucosal lesion with marked echo attenuation and without involvement of the mucosa.
- Citation: Chen HT, Xu GQ, Wang LJ, Chen YP, Li YM. Sonographic features of duodenal lipomas in eight clinicopathologically diagnosed patients. World J Gastroenterol 2011; 17(23): 2855-2859
- URL: https://www.wjgnet.com/1007-9327/full/v17/i23/2855.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i23.2855
Gastrointestinal lipomas are uncommon benign tumors that occur anywhere along the gut. The most common location for these lesions is the colon, followed by the ileum and the jejunum[1]. Lipomas found in the duodenum are rare and the literature regarding duodenal lipomas (DLs) is only limited to case reports[2-8], and no systematic study of diagnostic means for DLs has been reported. Since preoperative diagnosis of DLs is difficult, and large DLs can mimic malignant tumors on endoscopy, patients could be subjected to extensive surgical procedures that are sometimes destructive. Endoscopic ultrasonography (EUS) is an optimal method for detecting gastrointestinal submucosal tumors (SMTs) such as stromal tumors and leiomyomas; however, the features and diagnostic value of EUS for examining DLs have not been well established because of the rareness of this disease. In this study, we studied the sonographic features and diagnostic value of EUS for identifying DLs.
A total of eight consecutive patients with DL were included. The diagnosis of DL for all patients was pathologically established after surgical excision in five patients and endoscopic resection in three patients during the period from June 2000 to December 2010 in the First Affiliated Hospital, School of Medicine, Zhejiang University, China. The patient group was composed of five males and three females, and aged from 42 to 78 years, with a mean of 60 years. Except for one patient who was asymptomatic, DLs presented as bleeding in four patients, dyspepsia in two patients, and epigastric pain in one patient (Table 1). Laboratory examinations, including liver function tests, serum lipids, and tumor markers (carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 125, and alpha-fetoprotein), showed no obvious abnormalities except for anemia in five patients.
| Case | Sex | Age | Symptoms | Location | Treatment | EUS features | |||||
| Layer | Size (mm) | Echogenicity | Homogeneity | Border | Echo attenuation | ||||||
| 1 | M | 50 | None | Bulb | Endoscopic resection | 3rd | 12 | Hyperecho | Homogenous | Distinct | Behind |
| 2 | F | 64 | Melena | 2nd portion | Surgery | 3rd | 10 | Hyperecho | Homogenous | Distinct | Behind |
| 3 | M | 63 | Dyspepsia | 2nd portion | Surgery | 3rd | 25 | Hyperecho | Homogenous | Indistinct | Behind and inside |
| 4 | F | 54 | Melena | 2nd portion | Surgery | 3rd | 12 | Hyperecho | Homogenous | Distinct | Behind |
| 5 | M | 67 | Dyspepsia | 2nd portion | Endoscopic resection | 3rd | 8 | Hyperecho | Homogenous | Distinct | Behind |
| 6 | M | 78 | Melena | 2nd portion | Endoscopic resection | 3rd | 11 | Hyperecho | Homogenous | Distinct | Behind |
| 7 | F | 62 | Epigastric pain | 2nd portion | Surgery | 3rd | 15 | Hyperecho | Homogenous | Indistinct | Behind and inside |
| 8 | M | 42 | Melena | 2nd portion | Surgery | 3rd | 36 | Hyperecho | Heterogeneous | Indistinct | Behind and inside |
The EUS system included Olympus EU-M2000 sonogram processing equipment, an Olympus GIF-2T-240 double-cavity electronic gastroscope, Olympus MAJ drive systems with a high-frequency echo probe, UM-DP12-25R miniature ultrasonic probes with a frequency spectrum of 12-15 MHz, and a Daker WP-800 water pump (Olympus Medical System Corp., Tokyo Japan).
One EUS expert reviewed the ultrasonic image of all lesions, including the original layer of the duodenal wall, the echo intensity, and the echoic homogeneity. The size of the lesions and the perifocal structures were also investigated. The EUS diagnosis was compared with the histological results.
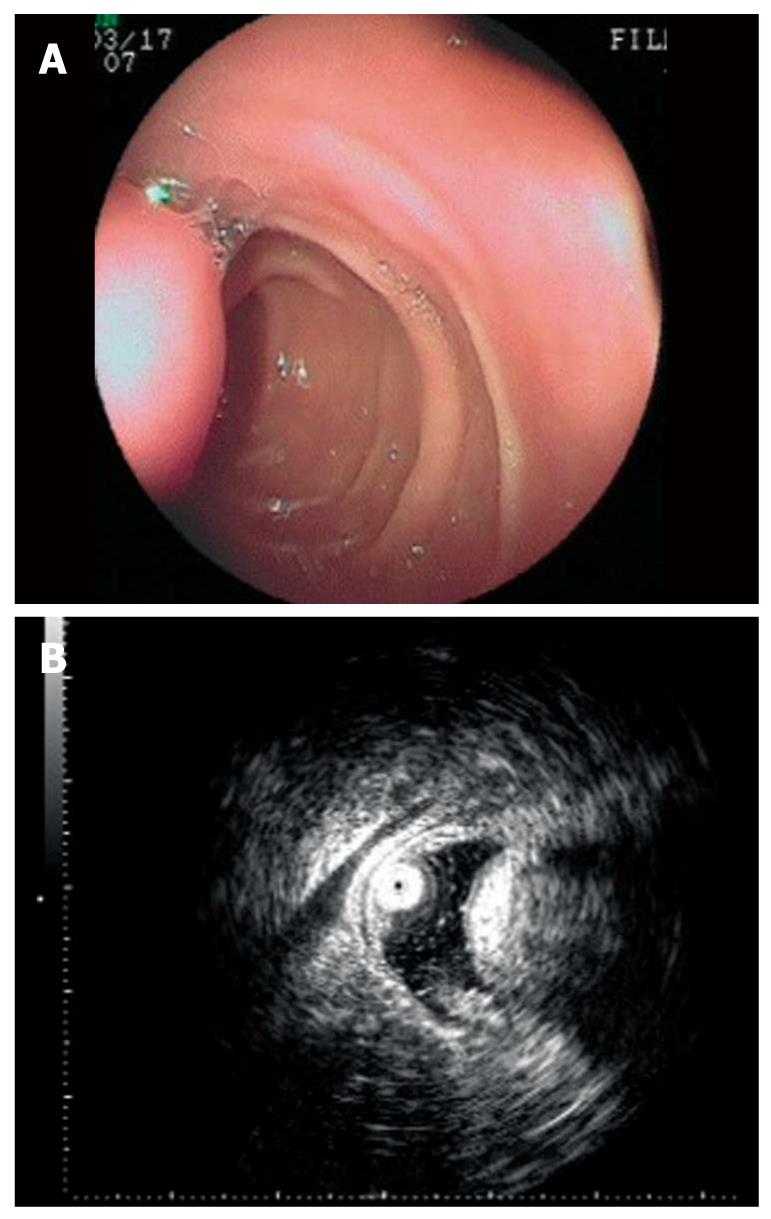
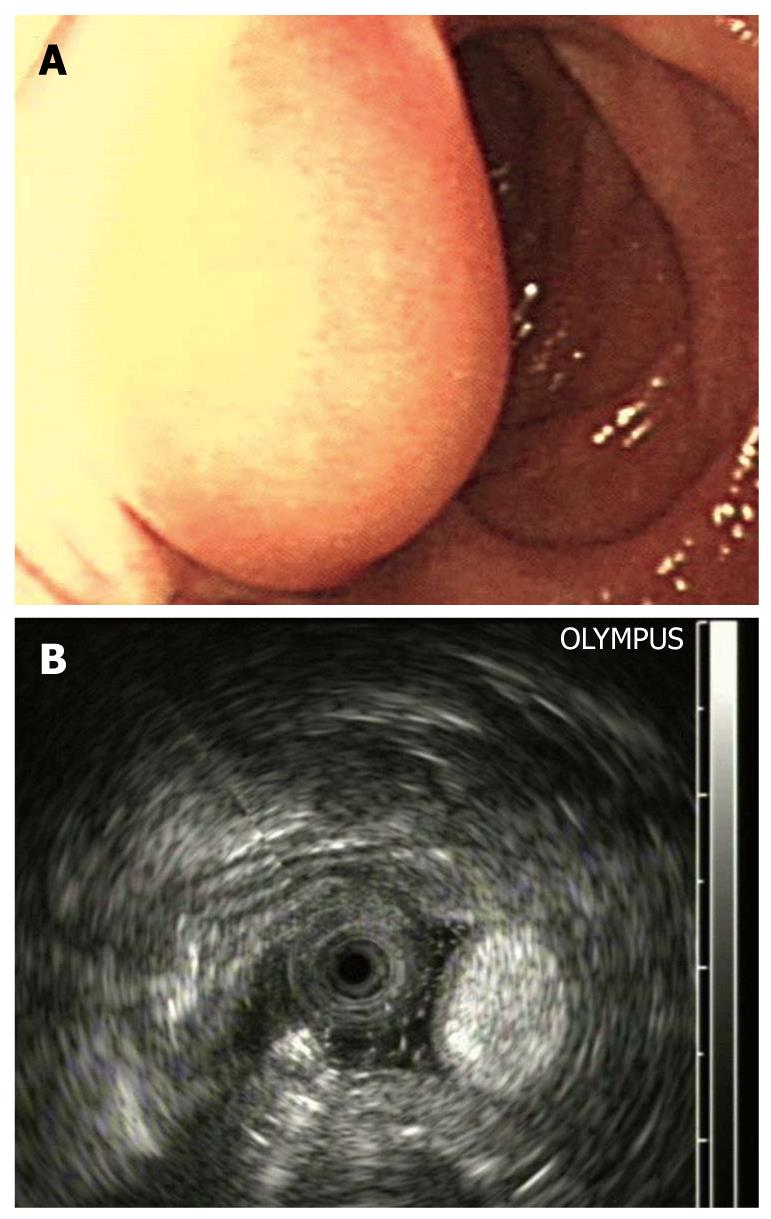
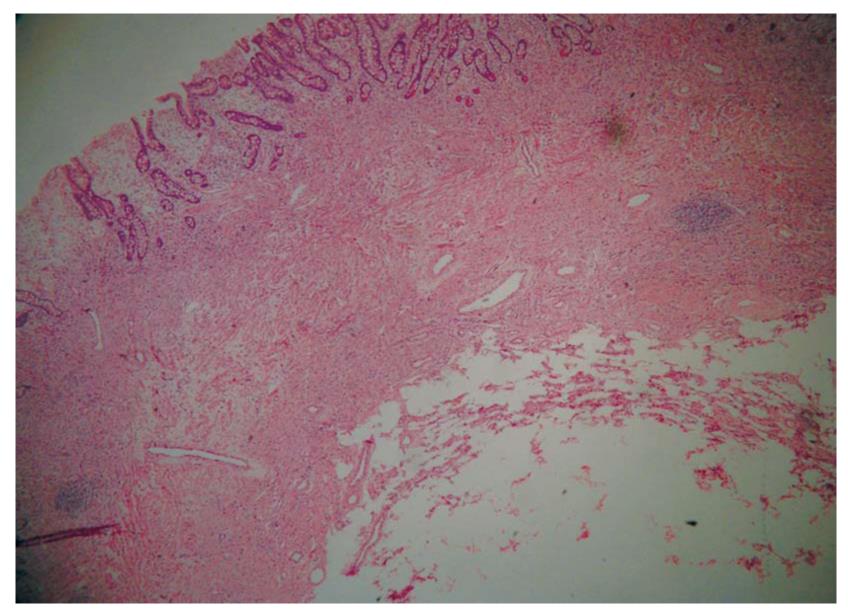
The tumors were located at the bulb in one case and in the descending part of the duodenum in seven cases. The lesions, with a hemispherical or oval shape, were sessile in six cases and had a stalk in two. The surface of the lesions was intact in three cases (Figure 1A) and had an ulcer (Figure 2A) or an erosion in five cases. Prior to EUS, only one case was correctly diagnosed as DL by routine endoscopy because of the yellowish and soft appearance (Figure 3A). Four cases were classified as SMTs, and three cases were mistaken for stromal tumors.
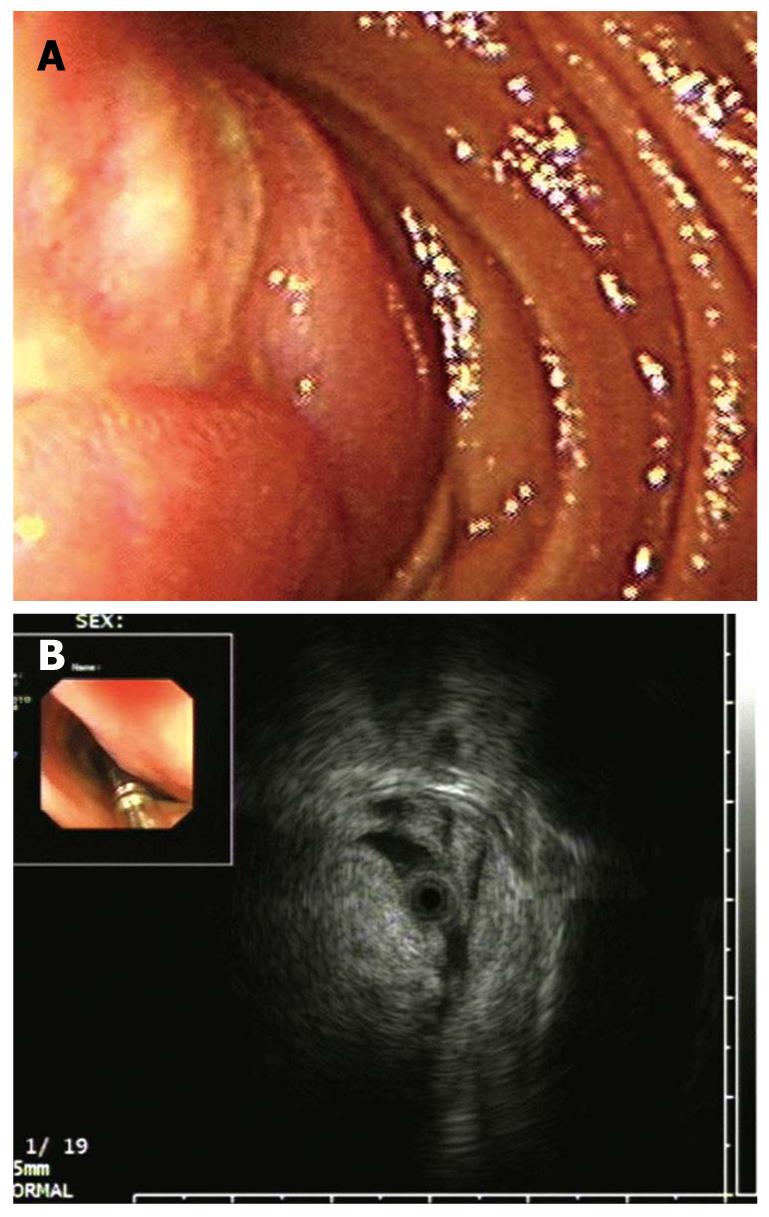
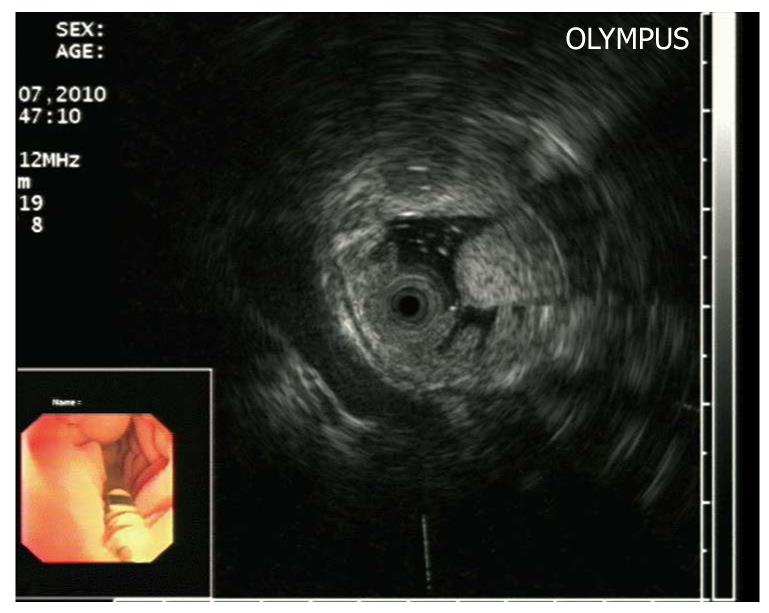
The endoscopic ultrasonography (EUS) findings in all cases are shown in Table 1. All tumors appeared as intensive hyperechoic lesions with a distinct anterior border that originated from the third EUS layer without involvement of the overlying first and second layers (Figures 1B, 2B, 3B and 4). Tumors ranged from 8 to 36 mm in size, with an average size of 16 mm. The margins of the tumors were clear in five cases (Figures 3B and 4), and the posterior borders of three lesions were obscure or invisible because of marked echo attenuation (Figures 1B and 2B). Homogeneous echogenicity was seen in all cases except for one that had a tubular structure inside the tumor (Figure 2B). Echo attenuation was observed only in the area behind the tumors in five cases (Figures 3B and 4) and both inside and behind the tumors in three cases (Figures 1B and 2B). With EUS, seven patients were correctly diagnosed as having DLs, but one patient with DL was mistaken as having Brunner’s gland adenoma (BGA) due to the appearance of a tubular structure.
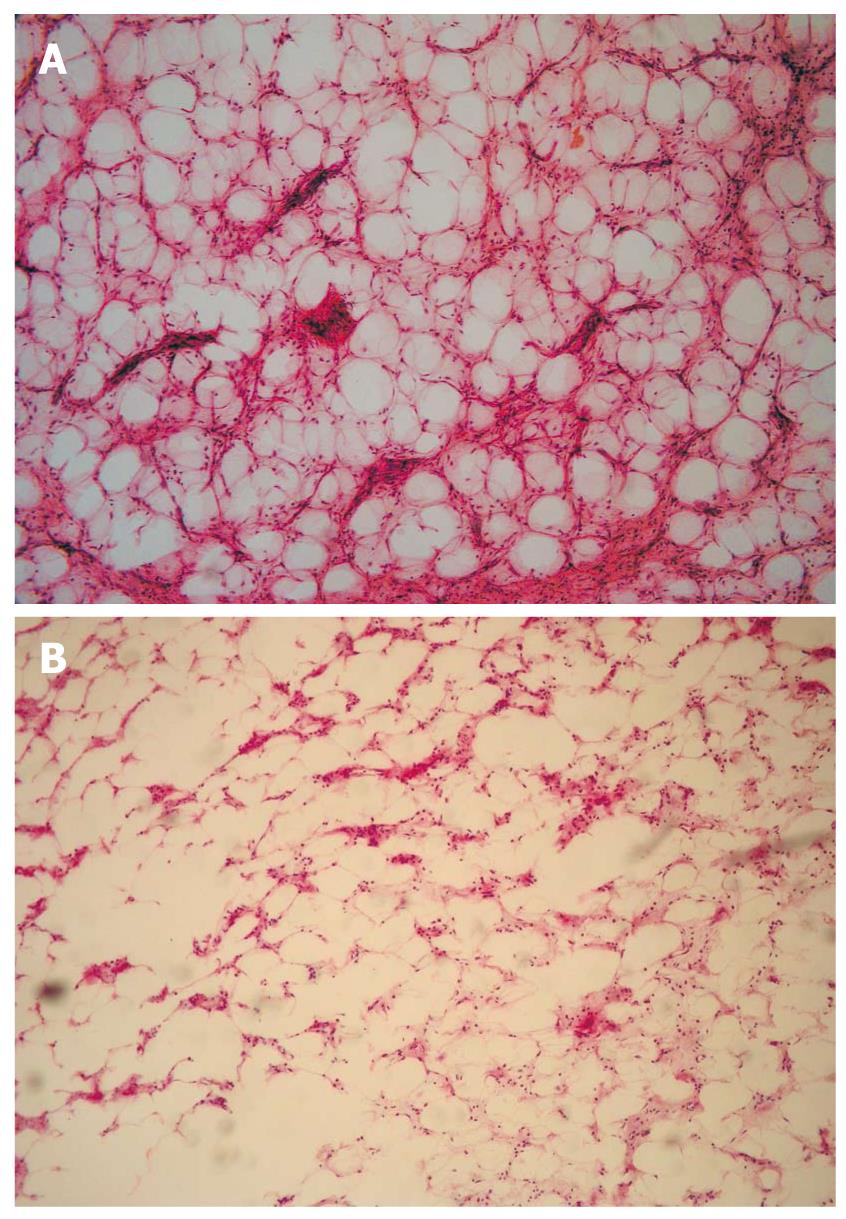
The pathological diagnosis after routine endoscopic biopsy was chronic inflammation of the mucosa, whereas the postoperative diagnosis after surgical excision or endoscopic resection was DL. The tumors presented by gross appearance as a node or finger in six cases and as a sub-lobe or cauliflower in two cases. All tumors originated from the submucosal layer, sometimes involving the muscularis propria, with a fully or partially coated fiber peplos. Microscopically, the tumors were composed of mature fat cells without heteromorphism. Among the fat cells, there were small amounts of thick-wall vessels, infiltrating lymphocytes, and abundant fibrous connective tissues (Figures 5, 6, 7).
Lipomas of the duodenum are rare, with fewer than 230 cases reported in the literature, and most of the described cases are from autopsy records rather than clinical experience[9]. DLs were mostly detected after bleeding or obstruction occurred. Among the patients in this study, four (50%) experienced bleeding, two (25%) experienced dyspepsia, one (12.5%) had epigastric pain, and one (12.5%) was asymptomatic. The symptoms of DL are nonspecific, however, and they are not useful for differential diagnosis.
Endoscopy is the preferred means for detecting upper gastrointestinal diseases. However, it can only suggest the presence of submucosal protruding lesions, but cannot provide additional details even though lesions have been detected[10,11]. In this study, four patients were diagnosed as having SMTs by endoscopy, and three patients were mistaken as having stromal tumors. Only one patient was correctly diagnosed with lipoma by routine endoscopy due to the yellowish and soft appearance of the lesion (Figure 2B).
EUS has been reported to be an effective modality for assessing gastrointestinal tumors and evaluating the original layer of the submucosal tumor, the homogeneity of the internal parenchymal echo, and the echogenicity of the lesions[10]. The typical EUS observations of DLs are intensive homogeneous hyperechoic lesions originating from the submucosa, with echo attenuation behind and/or inside the rear area. Thus, the signs of DLs are similar to the ultrasonic features of a fatty liver.
Intensive hyperecho is the most noticeable EUS feature of DLs. A newly-devised echogenicity classification system for SMTs by Okanobu[10] demonstrated the highest echo level of lipomas (levels 5-6). The marked echo-attenuation is another EUS feature of DLs. In our series, there was a more apparent echo decline in the area behind the focus than anywhere else in all cases regardless of the size of the lesions, suggesting a greater attenuation coefficient for DLs than for normal intestinal wall tissues. Furthermore, visible echo decline was seen inside the lesion when the size of the focus was larger than 15 mm. The anterior border was as distinct as the overlying first and second layers of the DLs; however, the appearance of the posterior border depended on the related echo decline. The latter portion and the posterior border of DLs larger than 25 mm are often invisible with the 12-MHz probe. The internal parenchymal echo of DLs is generally homogenous[11]. Occasionally, blood vessels may present as tubular structures inside the focus.
According to two ultrasonic characteristics, we could distinguish DLs from the majority of other SMTs such as leiomyomas, stromal tumors, or cysts. It is noteworthy that Brunner’s gland adenomas (BGAs) also show hyperechoic lesions originating from the submucosa, and therefore, DLs may be mistaken for BGAs[11,12]. In our study, one patient was misdiagnosed with duodenal BGA. BGAs appear primarily in the bulb portion or at the junction of the bulb and the descending duodenum, whereas DLs are located primarily in the descending portion of the duodenum. In addition, the echogenicity of BGAs is not as intensive or homogeneous as that of lipomas. Although the second EUS layer often becomes blurry or invisible in BGAs because of involvement of the lamina propria, it is always readable in DLs.
The reason why all gastrointestinal lipomas appeared as hyperechoic is not clear. Interestingly, normal subcutaneous fat tissues appeared as a hypoechoic zone with a small amount of hyperechoic fiber ropes. An earlier report showed that 29% of superficial soft tissue lipomas were hypoechoic, 22% were isoechoic, 29% were hyperechoic, and 20% were of a mixed pattern[13]. Thus, the echo types of lipomas mostly depend on the quantity of the boundary in relation to the mixture of fat and other connective tissues.We found abundant fibrous connective tissues among the fat cells of DLs. The heterogeneous mixture generated innumerable acoustic boundaries that appeared hyperechoic because of the marked acoustic impedance difference between the fat and fibrous tissues.
In summary, EUS has significant value for the differential diagnosis of DLs. The appearance of a round or oval lesion originating from the submucosal layer with intensive homogenous hyperecho and marked echo-attenuation, without involvement of the mucosal layers, suggests a diagnosis of DL. Abundant fibrous connective tissues among the fat cells may be the acoustic basis of this appearance.
Lipomas located in the duodenum are rare. Because the preoperative diagnosis for duodenal lipomas is difficult to establish and some large lipomas can mimic malignant tumors on endoscopy, patients could be subjected to extensive surgical procedures that sometimes were destructive. Endoscopic ultrasonography (EUS) is the optimal method for the diagnosis of gastrointestinal stromal tumors, but its diagnostic value for duodenal lipomas has not been well established because of its rareness.
Up to date, less than 230 cases of lipomas have been reported in the literature, but most of them are from autopsy records rather than clinical experience. Clinically, duodenal lipomas are mostly revealed by bleeding or obstruction. Endoscopy can only detect the submucosal lesion but fail to judge its nature. EUS is an effective modality in the assessment of gastrointestinal submucosal tumors and evaluation of the original layer, homogeneity of internal parenchymal echo and echogenicity of the lesions.
EUS is of significant value in the diagnosis and differential diagnosis of duodenal lipomas. The sonographic features were round or oval lesions originated from submucosal layer with intensive homogenous hyperecho and marked echo-attenuation, without involvement of the mucosal layers.
According to this study, duodenal lipomas can be defined by EUS, thus needless surgery can be avoided. By EUS, they can differentiate duodenal lipomas from other submucosal tumors. The sonographic features of duodenal lipomas can be used for the diagnosis of lipomas in esophagus, stomach and colon.
This is an original report on the correlations of the sonographic findings and clinicopathological features of duodenal lipomas. The material is characterized by small case series but the conclusive suggestions are appropriate and interesting. Duodenal lipoma is a rare clinicopathological entity. Therefore, it is interesting to get an eight consecutive duodenal lipoma cases. The EUS is probably the best option to diagnose this pathology.
Peer reviewer: Alexander Becker, MD, Department of Surgery, Haemek Medical Center, Afula 18000, Israel
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Taylor AJ, Stewart ET, Dodds WJ. Gastrointestinal lipomas: a radiologic and pathologic review. AJR Am J Roentgenol. 1990;155:1205-1210. |
| 2. | Sou S, Nomura H, Takaki Y, Nagahama T, Matsubara F, Matsui T, Yao T. Hemorrhagic duodenal lipoma managed by endoscopic resection. J Gastroenterol Hepatol. 2006;21:479-481. |
| 3. | Blanchet MC, Arnal E, Paparel P, Grima F, Voiglio EJ, Caillot JL. Obstructive duodenal lipoma successfully treated by endoscopic polypectomy. Gastrointest Endosc. 2003;58:938-939. |
| 4. | Tung CF, Chow WK, Peng YC, Chen GH, Yang DY, Kwan PC. Bleeding duodenal lipoma successfully treated with endoscopic polypectomy. Gastrointest Endosc. 2001;54:116-117. |
| 5. | Hizawa K, Kawasaki M, Kouzuki T, Aoyagi K, Fujishima M. Unroofing technique for the endoscopic resection of a large duodenal lipoma. Gastrointest Endosc. 1999;49:391-392. |
| 6. | Krachman MS, Dave PB, Gumaste VV. Bleeding duodenal lipoma. J Clin Gastroenterol. 1992;15:180-181. |
| 7. | Kang JY, Chan-Wilde C, Wee A, Chew R, Ti TK. Role of computed tomography and endoscopy in the management of alimentary tract lipomas. Gut. 1990;31:550-553. |
| 9. | Abu Daff SN, Abu Daff NS. Laparoscopic enucleation of a duodenal lipoma, with review of the literature. Saudi Med J. 2008;29:455-457. |
| 10. | Okanobu H, Hata J, Haruma K, Mitsuoka Y, Kunihiro K, Manabe N, Tanaka S, Chayama K. A classification system of echogenicity for gastrointestinal neoplasms. Digestion. 2005;72:8-12. |
| 11. | Xu GQ, Wu YQ, Wang LJ, Chen HT. Values of endoscopic ultrasonography for diagnosis and treatment of duodenal protruding lesions. J Zhejiang Univ Sci B. 2008;9:329-334. |
| 12. | Xu GQ, Zhang H, Li YM, Chen HT, Ji F, Chen CX, Ren GP, Ni XY. Analysis on clinical features of duodenal Brunner’s gland adenoma. Zhonghua Xiaohua Zazhi. 2006;26:511-514. |
| 13. | Fornage BD, Tassin GB. Sonographic appearances of superficial soft tissue lipomas. J Clin Ultrasound. 1991;19:215-220. |