Published online Jun 21, 2011. doi: 10.3748/wjg.v17.i23.2821
Revised: March 17, 2011
Accepted: March 24, 2011
Published online: June 21, 2011
AIM: To investigate apparent diffusion coefficient (ADC) values as an indication of reconditioning of acute hepatic injury (AHI) after allogeneic mononuclear bone marrow cell (MBMC) transplantation.
METHODS: Three groups were used in our study: a cell transplantation group (n = 21), transplantation control group (n = 21) and normal control group (n = 10). AHI model rabbits in the cell transplantation group were injected with 5 mL of MBMC suspension at multiple sites in the liver and the transplantation controls were injected with 5 mL D-Hanks solution. At the end of the 1st, 2nd and 4th wk, 7 rabbits were randomly selected from the cell transplantation group and transplantation control group for magnetic resonance diffusion-weighted imaging (MR-DWI) and measurement of the mean ADC values of injured livers. After MR-DWI examination, the rabbits were sacrificed and the livers subjected to pathological examination. Ten healthy rabbits from the normal control group were used for MR-DWI examination and measurement of the mean ADC value of normal liver.
RESULTS: At all time points, the liver pathological scores from the cell transplantation group were significantly lower than those in the transplantation control group (27.14 ± 1.46 vs 69.29 ± 6.16, 22.29 ± 2.29 vs 57.00 ± 1.53, 19.00 ± 2.31 vs 51.86 ± 6.04, P = 0.000). The mean ADC values of the cell transplantation group were significantly higher than the transplantation control group ((1.07 ± 0.07) × 10-3 mm2/s vs (0.69 ± 0.05) × 10-3 mm2/s, (1.41 ± 0.04) × 10-3 mm2/s vs (0.84 ± 0.06) × 10-3 mm2/s, (1.68 ± 0.04) × 10-3 mm2/s vs (0.86 ± 0.04) × 10-3 mm2/s, P = 0.000). The pathological scores of the cell transplantation group and transplantation control group gradually decreased. However, their mean ADC values gradually increased to near that of the normal control. At the end of the 1st wk, the mean ADC values of the cell transplantation group and transplantation control group were significantly lower than those of the normal control group [(1.07 ± 0.07) × 10-3 mm2/s vs (1.76 ± 0.03) × 10-3 mm2/s, (0.69 ± 0.05) × 10-3 mm2/s vs (1.76 ± 0.03) × 10-3 mm2/s, P = 0.000]. At any 2 time points, the pathological scores and the mean ADC values of the cell transplantation group were significantly different (P = 0.000). At the end of the 1st wk, the pathological scores and the mean ADC values of the transplantation control group were significantly different from those at the end of the 2nd and 4th wk (P = 0.000). However, there was no significant difference between the 2nd and 4th wk (P = 0.073 and 0.473, respectively). The coefficient of correlation between the pathological score and the mean ADC value in the cell transplantation group was -0.883 (P = 0.000) and -0.762 (P = 0.000) in the transplantation control group.
CONCLUSION: Tracking the longitudinally dynamic change in the mean ADC value of the AHI liver may reflect hepatic injury reconditioning after allogeneic MBMC transplantation.
- Citation: Shang QL, Xiao EH, Zhou QC, Luo JG, Wu HJ. Pathological and MR-DWI study of the acute hepatic injury model after stem cell transplantation. World J Gastroenterol 2011; 17(23): 2821-2828
- URL: https://www.wjgnet.com/1007-9327/full/v17/i23/2821.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i23.2821
Mononuclear bone marrow cells (MBMCs) are adult stem cells that have multi-potential differentiation capabilities and low immunogenicity. Direct allogeneic MBMC transplantation can repair various organ injuries such as hepatic injury without obvious immune rejection[1]. There are many studies evaluating the therapeutic effect of MBMC transplantation therapy for hepatic injury. However, few studies have been undertaken to determine the feasibility of magnetic resonance diffusion-weighted imaging (MR-DWI) to evaluate the therapeutic effect of MBMC transplantation therapy on models of acute hepatic injury (AHI).
MR-DWI is an atraumatic and functional imaging technique that is very sensitive to molecular diffusion from the random and microscopic translational motion of molecules known as Brownian motion[2]. MR-DWI can image the difference in microscopic diffusion movements of water molecules in various tissues. The most noticeable merit of MR-DWI is providing an apparent diffusion coefficient (ADC) value that can distinguish the microscopic diffusion movement of water molecules in different tissues in vivo by assigning numerical values[3-7]. When a tissue has a pathological change, the microscopic diffusion movement of water molecules changes and the mean ADC value should also change. It has been generally accepted that MR-DWI is valuable in qualitatively and quantitatively diagnosing cerebral ischemia in the hyper-inchoate period[8]. During recent years, many studies of hepatic pathological changes using MR-DWI have been reported[3-7]. These showed that MR-DWI of the liver seems promising for the characterization of many diseases (especially focal liver lesions) by calculating ADC values.
Similarly, after MBMC transplantation therapy, there should be a dynamic change in the microscopic diffusion movement of water molecules in hepatic tissue during the repair process of AHI. Thus, the aim of our study was to evaluate the contribution of the mean ADC value in reflecting the repair process of AHI after MBMC transplantation therapy by comparison with the pathological change. The pathological mechanisms behind the dynamic change of the mean ADC value from injured hepatic tissue will be discussed in further detail.
Experiments were performed using 57 healthy, male New Zealand White rabbits weighing -2.5 kg with an average age of -2 mo. All animal work was conducted in accordance with the guidelines provided by the Institutional Animal Control and Utilization Committee. Five rabbits were randomly selected and used to isolate MBMCs. Dulbecco’s modified Eagle’s medium and fetal bovine serum were purchased from Gibco (New York, USA). Mononuclear cell separation medium was purchased from Tianjin Haoyang Company (Tianjin, China). D-Hanks solution, an electronic balance, 3% pentobarbital sodium, sterile surgical instruments, an optical microscope, cell separation tools and 2% trypan blue were supplied by the Second Xiangya Hospital. D-galactosamine (D-GaIN) was purchased from Jiangsu Nantong Tonglu Co. Ltd. (Nantong, China). Imaging was performed using a 1.5-Tesla Signa Twinspeed MR scanner (General Electron Medical Systems, USA) with a small diameter cylindrical brain radiofrequency coil.
Acute hepatic injury was induced by D-galactosamine (D-GalN). D-GalN was dissolved in sterile 0.9% NaCl at a concentration of 10 g/100 mL (w/v). Forty-two rabbits were randomly selected to establish the AHI models. According to the weight of each rabbit, D-GalN solution was injected into the upper abdomen at a dosage of 1.0 g/kg. This amount was determined by preliminary experiments. The rabbits’ weight, drug dosage and detailed administration times were recorded. The 42AHI rabbits were randomly and equally divided into 2 groups: a cell transplantation group and a transplantation control group. Liver function assays were performed 24 h after drug administration and pathological examinations of liver sections taken during cell transplantation were performed to verify the establishment of the AHI model. The remaining 10 healthy rabbits were assigned to the normal control group, and only MR-DWI examination was performed for measurement of the normal liver mean ADC value.
After being sacrificed by air injection into the ear vein, the bodies of 5 healthy male rabbits were sterilized by incubation in 75% ethanol. Their limb bones were then isolated and bone marrow was repetitively flushed using D-Hanks solution containing heparin. Soft tissue clumps were removed with 100 pore filters. Recovered cell suspensions were aliquoted into multiple centrifuge tubes. MBMCs were then obtained through density gradient centrifugation. Cell number was counted and the viability examined using trypan blue. The percentage of live cells had to be > 95%. The MBMCs were resuspended in D-Hanks solution at a density of 4 × 106/mL. Five milliliters of cell suspension was aliquoted into 10 mL glass syringes and kept in an incubator at 37°C with 5% CO2 for a short time before transplantation.
MBMC transplantations were performed between 24 and 48 h after establishing the AHI models. Each AHI rabbit from the cell transplantation group was properly anesthetized and immobilized on an operating table. The skin of the upper abdomen was prepared and sterilized, then the liver was exposed with a 2 cm cut beneath the xiphoid process. After slowly injecting 5 mL of the MBMC suspension at multiple sites in the liver, the needle was withdrawn and the wound sutured to stop bleeding. The wound was treated with penicillin and covered with a sterile dressing. After transplantation, each model rabbit was given intramuscular injections of penicillin in the buttocks over a 3-d period with normal feeding. In addition to 5 mL of D-Hanks solution substituted for the MBMC suspension, manipulations of the rabbits in the transplantation control group were the same as those in the cell transplantation group.
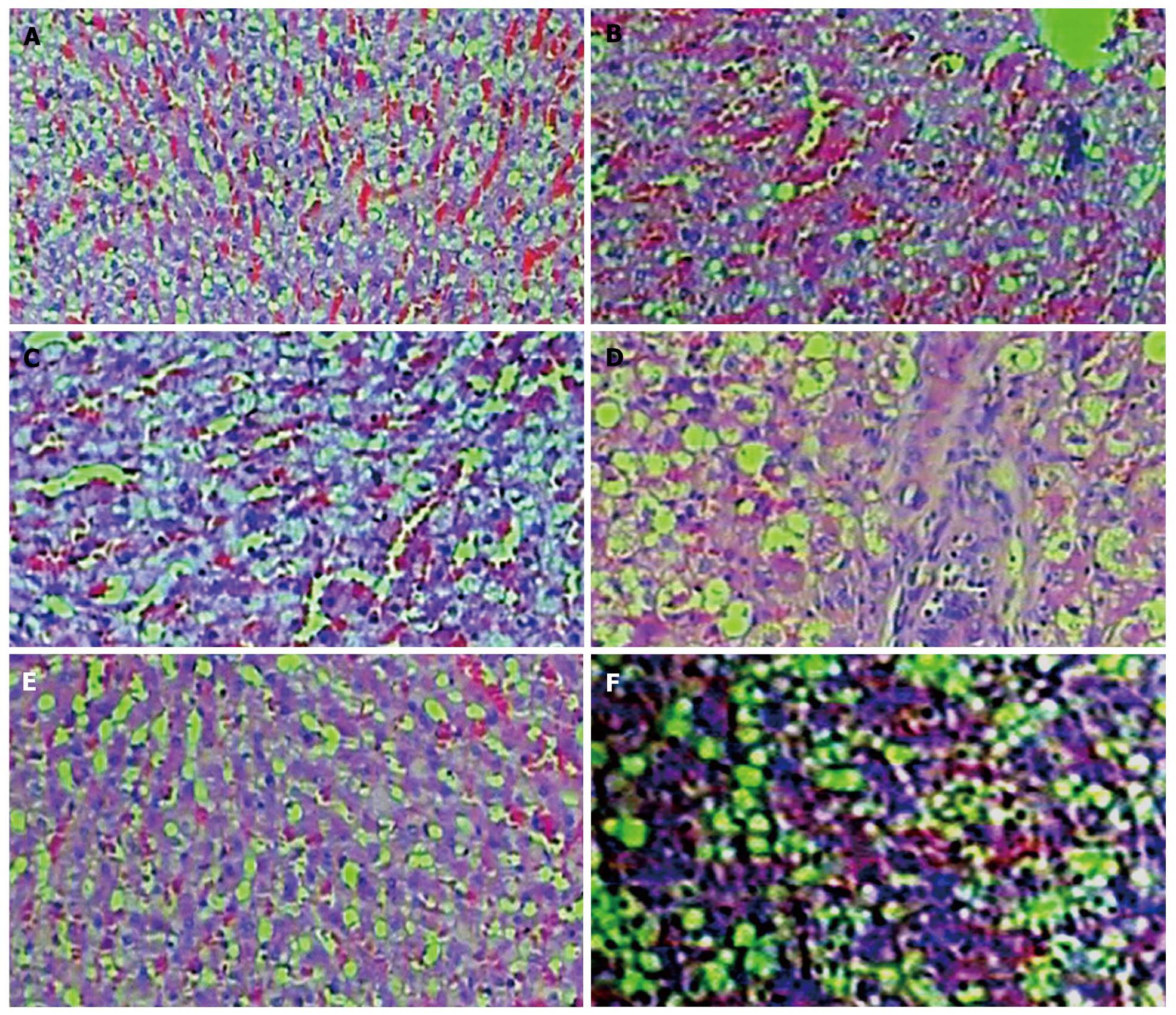
At the end of the 1st, 2nd and 4th wk after transplantation, 7 rabbits were randomly selected for MR-DWI examination of the liver at each time point in the cell transplantation group and transplantation control group. Then they were sacrificed for histological examination. Rabbit liver tissue blocks were fixed in 4% paraformaldehyde and were processed for paraffin embedding. Microsections were prepared and stained with hematoxylin and eosin, then examined under an optical microscope. The criteria for the liver pathological scores were established according to the characteristic of this study, histology activity index and previous studies[9] (Table 1). Six pathological microsections from each model rabbit were randomly selected to count the pathological scores by 2 experienced pathologists using a double-blind method. The sum of the pathological scores from 10 random high power fields (400 ×) from each microsection was regarded as the pathological score of that microsection.
| Score | Cellular necrosis and liver hyperplasia | Inflammatory cell infiltration in the area of the header and lobule | Injury of vascular endothelium and thrombus |
| 0 | Without | Without | Without |
| 1 | Spotty liver cell degeneration, necrosis without change of hepatic sinusoid and lobule shape | Infiltration area < 1/3 lobule or low inflammatory cell infiltration in the area of the header | Hyperemia of hepatic sinusoid or thrombus |
| 2 | Scattering severe liver cell degeneration, necrosis of whole lobule or unobvious liver cell hyperplasia | Infiltration area: 1/3-2/3 of lobule or comparatively wide-bound inflammatory cell infiltration | Injury of vascular endothelium or inflammatory cell infiltration under vascular endothelium |
| 3 | Large sheet liver cell degeneration and necrosis involving multiple lobules | Infiltration area: > 2/3 of lobule or inflammatory cells surround header | Extensive injury of vascular endothelium and thrombus |
Rabbits in the normal control group were subjected only to liver MR-DWI examination. At the end of the 1st, 2nd and 4th wk after transplantation, 7 rabbits from the cell transplantation group and from the transplantation control group were randomly selected at each time point for liver MR-DWI examination.
Rabbits were anesthetized and immobilized, then MR-DWI (axial) was carried out with a 1.5-Tesla Signa Twinspeed MR scanner equipped with a small diameter cylindrical brain radiofrequency coil. The scanning parameters of the MR-DWI included a spin echo echoplanar imaging series, b value 0 and 400 s/mm2, repetition time 6000 ms, echo time 45 ms, all diffusion directions, frequency coding direction R/L, field of view 20 cm × 15 cm, number of excitations 8, thickness layer 3 mm, 0.5 mm space, and matrix 128 × 128.
ADC values were obtained using Function Software on a GE workstation. Three different regions of interest (ROIs) (-50 mm2 each) were chosen in the liver parenchyma in every clear axial slice of each liver and their ADC values were measured. The mean value of the above was considered to be the ADC value of each liver.
Based on the mean ADC values from ROIs and the pathological scores, the differences between the various groups and time points, and the correlation between the mean ADC value and pathological score were assessed. The statistical significance was calculated by an independent sample t-test, analysis of variance and linear correlation using SPSS 11.0 software. P values < 0.05 were considered to indicate statistical significance.
The mean ADC value of the normal control group was (1.76 ± 0.03) × 10-3 mm2/s. At all time points after transplantation, the mean liver ADC values from the cell transplantation group were significantly higher than those of the transplantation control group (P = 0.000) (Table 2, Figure 1).
| Group | 1st wk | 2nd wk | 4th wk |
| Cell transplantation group | 1.07 ± 0.07 | 1.41 ± 0.04 | 1.68 ± 0.04 |
| Transplantation control | 0.69 ± 0.05 | 0.84 ± 0.06 | 0.86 ± 0.04 |
| t | 11.452 | 21.735 | 37.876 |
| P | 0.000 | 0.000 | 0.000 |
The mean liver ADC values from the cell transplantation group and transplantation control group gradually increased to near those of the normal control group over time. At the end of the 1st wk after transplantation, they were both significantly lower than those of the normal control group (t = 23.612, P = 0.000; t = 52.416, P = 0.000).
Between any 2 time points, the differences in the mean liver ADC values from the cell transplantation group were statistically significant (P = 0.000). At the end of the 1st wk after transplantation, the mean ADC values from the transplantation control group were significantly lower than those at the end of the 2nd and 4th wk (P = 0.000). However, there was no significant difference between the end of the 2nd and 4th wk (P = 0.473).
The livers’ pathological scores from the cell transplantation group and transplantation control group gradually decreased over time (Table 3, Figure 2). At all time points after transplantation, the pathological scores of the livers from the cell transplantation group were significantly lower than those from the transplantation control group (P = 0.000) (Table 3, Figure 2).
| Group | 1st wk | 2nd wk | 4th wk |
| Cell transplantation group | 27.14 ± 1.46 | 22.29 ± 2.29 | 19.00 ± 2.31 |
| Transplantation control | 69.29 ± 6.16 | 57.00 ± 1.53 | 51.86 ± 6.04 |
| t | -17.619 | -33.379 | -13.444 |
| P | 0.000 | 0.000 | 0.000 |
Between any 2 time points, the differences in pathological scores from the cell transplantation group were statistically significant (P = 0.000). At the end of the 1st wk after transplantation, the pathological scores from the transplantation control group were significantly higher than those at the end of the 2nd and 4th wk after transplantation (P = 0.000). However, there was no significant difference between the end of 2nd and 4th wk (P = 0.073).
When the b value was 400 s/mm2, there was a significant negative correlation between the pathological score and mean ADC value in the cell transplantation group and transplantation control group (r = -0.883, P = 0.000; r = -0.762, P = 0.000).
The efficacy of allogeneic MBMC transplantation therapy for AHI has already been proven[10,11]. Our results showed that the pathological changes such as cellular edema and inflammatory cell infiltration in the hepatic tissue of the transplantation control group were more obvious than those in the cell transplantation group at any similar time point. The pathological scores from the cell transplantation group were significantly lower than those of the transplantation control group at all time points. These results imply that allogeneic MBMC transplantation into an acute injury of the liver could improve hepatic injury reconditioning. This trend is similar to that found in other studies[10,11].
Because of the high resolution and sensitivity, recent studies have focused on in vivo real-time tracking and detecting the fate of transplanted stem cells with MRI[11,12]. However, there are few studies using MR-DWI to evaluate the therapeutic efficacy of MBMC transplantation for acute hepatic injuries.
When the b value is more than 300 s/mm2, physiological factors such as perfusion have little influence on the mean ADC value of hepatic tissue[13-15]. Therefore, in our study we performed the MR-DWI examination of rabbit liver with 400 s/mm2 for the b value, as determined by preliminary experiments.
Our study showed that at the end of the 1st wk after transplantation, the mean liver ADC values from the cell transplantation group and transplantation control group were much lower than the normal control group and both gradually increased to near the normal control over time. The correlation between the pathological scores and mean ADC values in the cell transplantation group or transplantation control group was significantly negative. This suggests the possibility of determining the reconditioning of an injured liver after allogeneic MBMC transplantation by tracking the longitudinally dynamic change of the mean ADC value from the AHI liver tissue.
By observing the pathological sections from the cell transplantation group and transplantation control group, we found the main pathological change was varying degrees of cytotoxic edema of the hepatocytes in all pathological sections. Therefore, we hypothesized that the pathological mechanism behind the change of the mean ADC value in hepatic tissue was mainly connected with cytotoxic edema of the hepatocytes in our study. Because the D-GalN injected into the peritoneal cavity was absorbed to injure the hepatic tissue causing Na-K pumps to be dysfunctional, the concentration of intracellular electrolytes increases. This causes water molecules inside cells to increase significantly, while extracellular water molecules decrease significantly. The cytotoxic edema of hepatocytes causes a decrease in gaps between hepatocytes, so that the space where extracellular water molecules can randomly move decreases. Therefore, the mean ADC value of hepatic tissue in the AHI model started to decrease[13-18] and was lower than that of the normal hepatic tissue.
In our study, the mean ADC values from the cell transplantation group and transplantation control group gradually increased over time and their pathological scores gradually decreased. The pathological sections showed their hepatic tissue injury gradually healed. At any similar time point, the mean ADC values from the cell transplantation group were significantly higher than those from the transplantation control group, and the pathological scores of the cell transplantation group were significantly better than those of the transplantation control group. According to previous studies[19-22] and our pathological sections, we hypothesized that there were 2 main hepatic reconditioning mechanisms causing these results. Firstly, the transplanted MBMCs promoted hepatic tissue reconditioning. Secondly, the hepatic injury caused the autologous hepatic reconditioning mechanism to activate. Because of the hepatic reconditioning, the hepatic cells’ cytotoxic edema gradually decreased, and the number of extracellular water molecules and the space where extracellular water molecules could move randomly both gradually recovered. Thus, the macroscopic mean ADC values from the cell transplantation group and transplantation control group gradually increased[23,24]. Because of the 2 mentioned hepatic reconditioning mechanisms, the reconditioning in the cell transplantation group was faster than the transplantation control group without transplanted MBMCs. Therefore, at any time point, the mean ADC values from the cell transplantation group were significantly higher than those from the transplantation control group.
Of course, if there were extensive hepatolysis and necrosis, the number of extracellular water molecules and the space where extracellular water molecules randomly move in the AHI model liver would greatly increase. This would cause the mean ADC value of the hepatic tissue in the AHI model to exceed that of the normal hepatic tissue[25,26]. However, we did not observe this phenomenon in our study. Through observing pathological sections from all time points, we found sporadic scattering but no extensive hepatolysis and necrosis. The dominant pathological change in the hepatic tissue was varying degrees of hepatocellular cytotoxic edema. Perhaps the reason was that the dosage of D-GalN used was not high enough to cause extensive hepatolysis in our study. Considering the influence of hepatolysis on the ADC value of hepatic tissue, we chose many ROIs in all clear images from each AHI model’s liver to measure ADC values. The mean ADC value was calculated from all ROIs and ADC values from each AHI liver. Thus, we could increase the accuracy of the ADC value of each AHI model’s liver to the utmost extent.
In conclusion, when the b value is equal to 400 s/mm2, tracking the longitudinally dynamic change of the mean ADC value of the AHI liver could determine injured hepatic tissue reconditioning after allogeneic MBMC transplantation.
Many studies have proven that allogeneic mononuclear bone marrow cell (MBMC) transplantation therapy is an effective way to repair liver injury. The most notable merit of magnetic resonance diffusion-weighted imaging (MR-DWI) is the provision of an apparent diffusion coefficient (ADC) value that can distinguish the microscopic diffusion movement of water molecules in various tissues in vivo by assigning a numerical value. After MBMC transplantation therapy, there should be a dynamic change in the microscopic diffusion movement of water molecules in the hepatic tissue during the repair process of acute hepatic injury. Thus, the authors attempted to use MR-DWI to evaluate the contribution of the acute injured liver’s mean ADC value in reflecting the repair process after MBMC transplantation therapy.
Allogeneic MBMC transplantation therapy can accelerate the repair of liver injury. Many studies are focusing on the mechanisms behind the transplanted MBMCs repair of the liver injury. Recently, some researchers have been attempting to track and detect the fate of transplanted MBMCs with magnetic resonance imaging.
It has been proven that allogeneic MBMC transplantation can repair liver injury. There are many studies on evaluating the therapeutic effect of MBMC transplantation therapy for hepatic injury, but few studies have determined the feasibility of MR-DWI to evaluate the therapeutic effect of MBMC transplantation therapy on models of acute hepatic injury.
The results of this study suggest that the dynamic change of the mean ADC value of the acute hepatic injury model’s liver can determine the injured liver’s reconditioning after allogeneic MBMCs transplantation.
MR-DWI is a functional imaging technique that can image the difference in microscopic diffusion movements of water molecules in various tissues. This technique only requires the examinee to lie still on the examining table while the examination is carried out. It is atraumatic and very safe.
This article investigates the role of MR diffusion-weighted images for detection of acute liver injury following allogeneic bone marrow cell transplantation in a rabbit model. The conclusion reached confirmed the value and usefulness of this modality. The method is non-traumatic and easily performed. The methodology and design of the study was sound. This study is of significance in the field of bone marrow transplantation.
Peer reviewer: Christopher Christophi, Professor and Head of The University of Melbourne Department of Surgery, Austin Hospital, Melbourne, 145 Studley Road, Victoria 3084, Australia
S- Editor Sun H L- Editor Cant MR E- Editor Ma WH
| 1. | Akihama S, Sato K, Satoh S, Tsuchiya N, Kato T, Komatsuda A, Hirokawa M, Sawada K, Nanjo H, Habuchi T. Bone marrow-derived cells mobilized by granulocyte-colony stimulating factor facilitate vascular regeneration in mouse kidney after ischemia/reperfusion injury. Tohoku J Exp Med. 2007;213:341-349. |
| 2. | Koike N, Cho A, Nasu K, Seto K, Nagaya S, Ohshima Y, Ohkohchi N. Role of diffusion-weighted magnetic resonance imaging in the differential diagnosis of focal hepatic lesions. World J Gastroenterol. 2009;15:5805-5812. |
| 3. | Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250:466-473. |
| 4. | Schaudt A, Kriener S, Schwarz W, Wullstein C, Zangos S, Vogl T, Mehrabi A, Fonouni H, Bechstein WO, Golling M. Role of transarterial chemoembolization for hepatocellular carcinoma before liver transplantation with special consideration of tumor necrosis. Clin Transplant. 2009;23 Suppl 21:61-67. |
| 5. | Kamel IR, Bluemke DA, Eng J, Liapi E, Messersmith W, Reyes DK, Geschwind JF. The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2006;17:505-512. |
| 6. | Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao JC, Hsu JS, Liu GC. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants--initial experience. Radiology. 2006;239:448-456. |
| 7. | Muhi A, Ichikawa T, Motosugi U, Sano K, Matsuda M, Kitamura T, Nakazawa T, Araki T. High-b-value diffusion-weighted MR imaging of hepatocellular lesions: estimation of grade of malignancy of hepatocellular carcinoma. J Magn Reson Imaging. 2009;30:1005-1011. |
| 8. | Kloska SP, Wintermark M, Engelhorn T, Fiebach JB. Acute stroke magnetic resonance imaging: current status and future perspective. Neuroradiology. 2010;52:189-201. |
| 9. | Scheuer PJ, Standish RA, Dhillon AP. Scoring of chronic hepatitis. Clin Liver Dis. 2002;6:335-347, v-vi. |
| 10. | Jin SZ, Meng XW, Han MZ, Sun X, Sun LY, Liu BR. Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failure. World J Gastroenterol. 2009;15:2657-2664. |
| 11. | Ju S, Teng GJ, Lu H, Zhang Y, Zhang A, Chen F, Ni Y. In vivo MR tracking of mesenchymal stem cells in rat liver after intrasplenic transplantation. Radiology. 2007;245:206-215. |
| 12. | Modo M, Cash D, Mellodew K, Williams SC, Fraser SE, Meade TJ, Price J, Hodges H. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage. 2002;17:803-811. |
| 13. | Erdem G, Erdem T, Muammer H, Mutlu DY, Firat AK, Sahin I, Alkan A. Diffusion-weighted images differentiate benign from malignant thyroid nodules. J Magn Reson Imaging. 2010;31:94-100. |
| 14. | Dale BM, Braithwaite AC, Boll DT, Merkle EM. Field strength and diffusion encoding technique affect the apparent diffusion coefficient measurements in diffusion-weighted imaging of the abdomen. Invest Radiol. 2010;45:104-108. |
| 15. | Eiber M, Beer AJ, Holzapfel K, Tauber R, Ganter C, Weirich G, Krause BJ, Rummeny EJ, Gaa J. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest Radiol. 2010;45:15-23. |
| 16. | Choi JS, Kim MJ, Choi JY, Park MS, Lim JS, Kim KW. Diffusion-weighted MR imaging of liver on 3.0-Tesla system: effect of intravenous administration of gadoxetic acid disodium. Eur Radiol. 2010;20:1052-1060. |
| 17. | Eccles CL, Haider EA, Haider MA, Fung S, Lockwood G, Dawson LA. Change in diffusion weighted MRI during liver cancer radiotherapy: preliminary observations. Acta Oncol. 2009;48:1034-1043. |
| 18. | Sun X, Wang H, Chen F, De Keyzer F, Yu J, Jiang Y, Feng Y, Li J, Marchal G, Ni Y. Diffusion-weighted MRI of hepatic tumor in rats: comparison between in vivo and postmortem imaging acquisitions. J Magn Reson Imaging. 2009;29:621-628. |
| 19. | Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213-222. |
| 20. | Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The efficacy of diffusion-weighted imaging for the detection of colorectal cancer. Hepatogastroenterology. 2009;56:128-132. |
| 21. | Kandpal H, Sharma R, Madhusudhan KS, Kapoor KS. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol. 2009;192:915-922. |
| 22. | Yu JS, Kim JH, Chung JJ, Kim KW. Added value of diffusion-weighted imaging in the MRI assessment of perilesional tumor recurrence after chemoembolization of hepatocellular carcinomas. J Magn Reson Imaging. 2009;30:153-160. |
| 23. | Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Saxena R, Aisen AM. Value of diffusion-weighted MRI for assessing liver fibrosis and cirrhosis. AJR Am J Roentgenol. 2009;193:1556-1560. |
| 24. | Maniam S, Szklaruk J. Magnetic resonance imaging: Review of imaging techniques and overview of liver imaging. World J Radiol. 2010;2:309-322. |
| 25. | Wu X, Wang H, Chen F, Jin L, Li J, Feng Y, DeKeyzer F, Yu J, Marchal G, Ni Y. Rat model of reperfused partial liver infarction: characterization with multiparametric magnetic resonance imaging, microangiography, and histomorphology. Acta Radiol. 2009;50:276-287. |
| 26. | Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Aisen AM. The value of diffusion-weighted imaging in characterizing focal liver masses. Acad Radiol. 2009;16:1208-1214. |