Published online May 28, 2011. doi: 10.3748/wjg.v17.i20.2572
Revised: April 13, 2011
Accepted: April 20, 2011
Published online: May 28, 2011
AIM: To investigate the proteins involved in colonic adaptation and molecular mechanisms of colonic adaptation in rats with ultra-short bowel syndrome (USBS).
METHODS: Sprague Dawley rats were randomly assigned to three groups: USBS group (10 rats) undergoing an approximately 90%-95% small bowel resection; sham-operation group (10 rats) undergoing small bowel transaction and anastomosis; and control group (ten normal rats). Colon morphology and differential protein expression was analyzed after rats were given post-surgical enteral nutrition for 21 d. Protein expression in the colonic mucosa was analyzed by two-dimensional electrophoresis (2-DE) in all groups. Differential protein spots were detected by ImageMaster 2D Platinum software and were further analyzed with matrix-assisted laser desorption/ionization-time-of-flight/time-of-flight-mass spectrometric (MALDI-TOF/TOF-MS) analysis.
RESULTS: The colonic mucosal thickness significantly increased in the USBS group compared with the control group (302.1 ± 16.9 μm vs 273.7 ± 16.0 μm, P < 0.05). There was no statistically significant difference between the sham-operation group and control group (P > 0.05). The height of colon plica markedly improved in USBS group compared with the control group (998.4 ± 81.2 μm vs 883.4 ± 39.0 μm, P < 0.05). There was no statistically significant difference between the sham-operation and control groups (P > 0.05). A total of 141 differential protein spots were found in the USBS group. Forty-nine of these spots were down-regulated while 92 protein spots were up-regulated by over 2-folds. There were 133 differential protein spots in USBS group. Thirty of these spots were down-regulated and 103 were up-regulated. There were 47 common differential protein spots among the three groups, including 17 down-regulated protein spots and 30 up-regulated spots. Among 47 differential spots, eight up-regulated proteins were identified by MALDI-TOF/TOF-MS. These proteins were previously reported to be involved in sugar and fat metabolism, protein synthesis and oxidation reduction, which are associated with colonic adaption.
CONCLUSION: Eight proteins found in this study play important roles in colonic compensation and are associated with sugar and fat metabolism, protein synthesis, and molecular chaperoning
- Citation: Jiang HP, Chen T, Yan GR, Chen D. Differential protein expression during colonic adaptation in ultra-short bowel rats. World J Gastroenterol 2011; 17(20): 2572-2579
- URL: https://www.wjgnet.com/1007-9327/full/v17/i20/2572.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i20.2572
Short bowel syndrome (SBS) is a chronic malabsorptive syndrome resulting from extensive small bowel resections[1]. Residual small bowel less than 30 cm from proximal end or distal end is termed ultra-short bowel syndrome (USBS)[1]. It occurs when the bowl is less than 200 cm in situ[2]. The estimated population prevalence is approximately one per million[3]. In adults, SBS usually results from resection of unviable intestine secondary to vascular insufficiency, Crohn’s disease, malignancy or radiation[3]. In children with congenital intestinal anomalies, such as gastroschisis or atresia, necrotizing enterocolitis leads to insufficient intestinal length[4]. Malabsorption of macronutrients and micronutrients may predominate as a clinical manifestation, whereas other patients may struggle to maintain fluid and electrolytes homeostasis[5]. These patients may become dependent on parenteral nutrition (PN) support to maintain their energy balance. A study showed that the colon could undergo adaptation like the small intestine[6]. Epithelial hyperplasia occurred 24-48 h after small intestinal resection in experimental models[7-9]. Animal models showed that adaptation process is stimulated by enteral nutrition for enterocyte reproduction, resulting in the release of trophic factors[10]. Our previous studies have also found changes in morphology and ultrastructure of rat colon and adaptation of absorption functions[11]. However, the proteins and molecular mechanisms associated with the colonic adaptation are still unknown. In this study, we used a proteomics approach to identify the proteins associated with colonic adaptation in USBS rats, and discussed the molecular roles of the identified proteins in colonic adaptation.
Thirty healthy male Sprague Dawley rats (aged 3-4 mo, weighing 250-300 g) were randomly assigned to three groups (10 rats in each group): USBS group undergoing an approximately 90%-95% small bowel resection; sham-operation group undergoing small bowel transaction and anastomosis; and normal control group.
On the day of operation, animals were fasted from solid food, but with unrestricted access to water. Animals in the USBS group were anesthetized with chloral hydrate (0.3 mL/100 g) for surgical resection of the small bowel. The entire small intestine was resected from 1 cm distal to the Treitz ligament to 1 cm proximal to the ileocecal valve. End-to-end anastomosis of the jejunum to the ileum was performed.
After recovery from anesthesia, rats were fed Peptisorb (Holland Nutricia). The nutrient fluid had an osmotic pressure of 410 mosm/L and a concentration of 25.2%. The nutrient mix could provide 413.82 J of energy per 100 mL. The fluid contained carbohydrates and 85% protein made up the short-chain hydrolytic lactalbumin, including 50% medium-chain triglycerides and 50% long-chain triglycerides. Both the USBS group and sham-operation group were fed with 12.6% Peptisorb on days 1-3 after surgery. Intake was increased to 16.8% on days 4-6 and to 25.2% from day 7 after surgery to the end of the experiment on day 21. Rats were fed 80 mL nutrient mixture daily using a special bottle. Diphenoxylate (0.75 mg/kg daily) was added to the feeding solution to reduce diarrhea.
All rats were anesthetized using chloral hydrate (0.3 mL/100 g) prior to a terminal surgical procedure. A 0.5 cm colon was resected from near the ileocecal valve and HE stained in paraffin. The thickness of tunica mucosa coli and the height of plica were measured with an automatic image analysis. Five non-serial sections were measured from each specimen. Average value and standard deviation (SD) were calculated.
A 1-cm segment of colon was removed from near the ileocecal valve and incised longitudinally after enteral nutrition for 21 d. After being repeatedly washed with double-distilled water, the colon was quickly scraped with glass slides in an ice bath to collect intestinal mucosa. The intestinal mucosa were stored at -80°C or used for immediate schizolysis to extract protein.
Total mucosa was obtained by mixing colonic mucosa from rats in each group. The combined intestinal mucosa was then triturated in liquid nitrogen and lyzed in ice-cold lysis buffer (7 mol/L urea, 2 mol/L thiourea, 4% (w/v) CHAPS, 1 mmol/L DTT, 10 mmol/L PMSF and protease inhibitor cocktail). The cellular lysate was centrifuged at 13 200 r/mim for 30 min. The supernatant was collected and stored at -80°C. Protein concentration was determined using the Bradford assay, as previously described (REF).
Protein from intestinal mucosa (150 μg) was applied by 2-DE analysis. 2-DE was performed as previously described. Briefly, one-dimensional isoelectric focusing (IEF) was performed on 13-cm gel strips, pH 3-10 NL (Amersham Ettan IPGPhor IEF system). The following IEF protocol was used at 20°C: rehydration at 30 V for 16 h; 500 V for 1 h; 1000 V for 1 h; and 8000 V for 64000 Vh. After IEF, the strips were incubated for 15 min with 10 mL equilibration solution A [2% sodium dodecyl sulfate (SDS), 50 mmol/L Tris-HCl pH 8.8, 6 Murea, 30% v/v glycerol, 0.002% bromophenol blue, and 100 mg dithiothreitol (DTT)]. The strips were further equilibrated for 15 min with equilibration solution B, which was identical to solution A except that 250 mg iodoacetamide replaced the DTT. Samples were then transferred onto 12.5% SDS-PAGE for 2-D separation. Proteins in 2-DE gels were stained with silver. Images were scanned using Image-scanner. Each sample was analyzed three times.
The protein spots and their differential expressions were analyzed using ImageMaster 2D Platinum software. USBS group gel was compared with the other two groups. Two groups of differentially acquired protein spots were compared to find common differential protein spots. Protein spots achieving a > 2-fold increase in spot intensity and observed in three replicate gels from three independent experiments were scored and subjected to matrix-assisted laser desorption/ionization-time-of-flight/time-of-flight-mass spectrometric (MALDI-TOF/TOF-MS) analysis.
The differential protein spots were in-gel digested, with minor modifications. In brief, the differential protein spots were destained using 15 mmol/L K4Fe(CN)6 and 50 mmol/L sodium thiosulfate. Spots were digested with trypsin at 37°C for 16 h. Peptide mixtures were extracted from the gel spots. The extracted peptide mixtures were dried using the SpeedVac Centrifuge.
The peptide mixtures were analyzed on an ABI 4800 plus MALDI-TOF/TOF-MS (ABI, CA). Each spectrum was produced by accumulating the data from 500 consecutive laser shots in the mass range of 900-3500 Da. Seven maximum precursor ions with a signal-to-noise ratio > 50 were chosen for tandem mass spectrometry (MS-MS). The obtained MS and MS-MS data were processed by GPS Explorer software (V3.6). Proteins were identified by the MASCOT search engine (V2.1) in the IPI mouse database based on these MS and MS/MS spectra. Proteins were considered a match if the error for peptide mass was 100 ppm or lower and the mass accuracy was 0.2 Da or lower. Scores > 59 were considered statistically significant (P < 0.05).
Using information from the European Bioinformatics Institute (http://www.ebi.ac.uk), the differential proteins were classified based on function. The relationship between protein expression and biological function in the USBS group was further analyzed, as described below.
The protein-protein interaction network was analyzed by the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) system. The following sets of STRING programs were employed: organism, required confidence (score), interactions, and additional nodes shown as “homo sapiens,”“low confidence (0.150),”“no more than 10 interactions,” and “0.” For other parameters, default settings were used.
One-way analysis of variance and the Student-Newman-Keuls test were performed using SPSS 13.0 statistical software. Data were expressed as mean ± SD. P values less than 0.05 were considered statistically significant.
Among USBS rats, the survival rate was over 90%. Only one rat died of intestinal obstruction 6 d after operation. Rats were observed with dull coats and severe hair loss, especially within the first two weeks after the operation. Rats gradually regained a dense, shiny coat. Rats experienced severe diarrhea, rectal eversion, perineal eczema and infections, and perianal and tail skin ulcers forming ringtail disease.
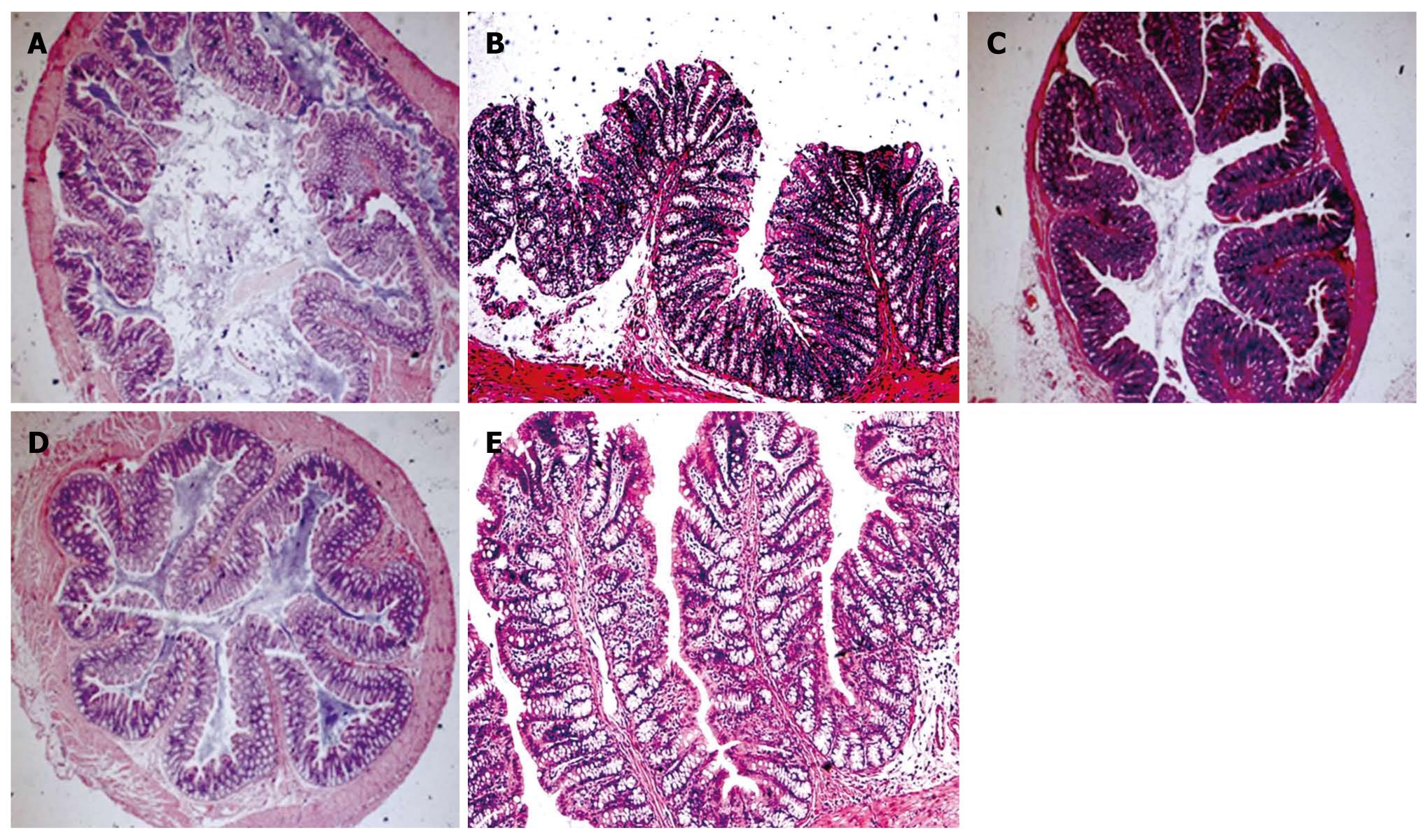
The colonic mucosal thickness was 302.1 ± 16.9, 276.6 ± 19.1 and 273.7 ± 16.0 μm in the USBS group, sham-operation group and control group, respectively. The colonic mucosal thickness was significantly higher in the USBS group than in other two groups (F = 7.46, P = 0.003). The colonic mucosal thickness was not significantly different between the sham-operation and control groups (P = 0.717). The height of colon plica of the three groups was 998.4 ± 81.2 μm, 893.7 ± 20.2 μm, and 883.4 ± 39.0 μm, respectively. The height of colon plica markedly improved in the USBS group compared with the control group (F = 13.96, P < 0.001). There were no significant differences in plica height between sham-operation and control groups (P = 0.665, Figure 1).
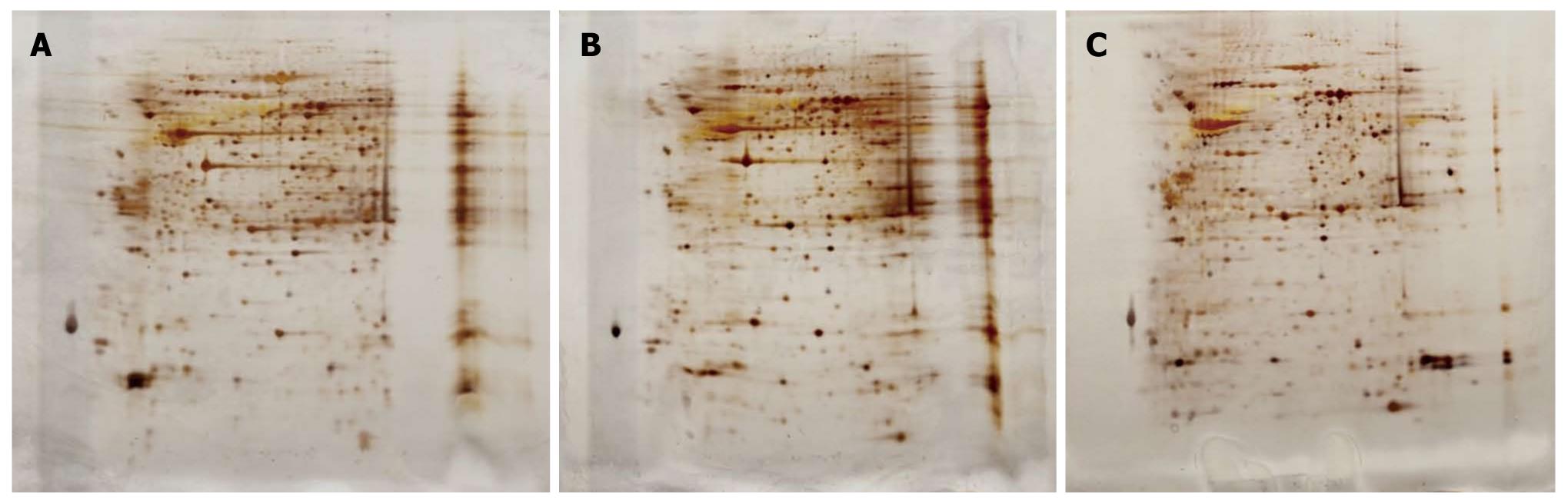
To identify the proteins involved in colonic adaptation, total proteins isolated from colonic mucosa of the three groups were separated on a 2-D gel (pH 3-10 NL), respectively. The gels were visualized by silver staining (Figure 2). In total, approximately 700 protein spots were detected in each of the silver-stained gels using ImageMaster software. Differential spots were scored when there was a > 2-fold change in spot intensity in three replicate gels from three independent experiments. The analysis revealed 141 differential protein spots, including 49 increased spots in USBS group and 92 decreased spots in the control group; and 133 differential protein spots, including 103 increased spots in USBS group and 30 decreased spots in the sham-operation groups. Among these differential protein spots, 47 differential protein spots were frequently observed in all three groups, including 30 increased spots and 17 decreased spots.
Protein spots were subjected to in-gel digestion with trypsin. Protein identities were determined with MS and MS-MS by searching the IPI mouse database using the MASCOT program. This allowed us to identify eight proteins from 43 differential spots, including protein disulfide-isomerase A3, phosphoglycerate kinase 1, pyruvate kinase isoforms M1/M2, alcohol dehydrogenase class-3, mitochondrial ribosomal proteins, pancreatic triacylglycerol lipase, Hnrph1, and Lambda-crystallin homolog. These proteins are listed in Table 1, along with their IPI accession number, molecular weight, isoelectric point, scores and fold changes among the groups.
| No. | Spot No. | Protein name | Accession No. | Protein MW/PI | Protein score | FD (24 h) |
| 1 | 528 | Phosphoglycerate kinase 1 | IPI00231426 | 44510 /8.02 | 243 | 1 000 000 |
| 2 | 725 | Pancreatic triacylglycerol lipase | IPI00198916 | 51407/36.31 | 60 | 1 000 000 |
| 3 | 946 | Hnrph1 protein | IPI00650124 | 20567/3 5.2 | 172 | 1 000 000 |
| 4 | 331 | Protein disulfide-isomerase A3 | IPI00324741 | 57043 /5.88 | 391 | 2.4 ± 0.2 |
| 5 | 599 | Pyruvate kinase isoforms M1/M2 | IPI00231929 | 57938.9/6.63 | 67 | 2.0 ± 0.5 |
| 6 | 603 | Alcohol dehydrogenase class-3 | IPI00568787 | 39550.2/7.45 | 70 | 2.2 ± 0.3 |
| 7 | 746 | Lambda-crystallin homolog | IPI00213610 | 35318/5.94 | 222 | 2.1 ± 0.4 |
| 8 | 1033 | Mitochondrial ribosomal protein L12 | IPI00203773 | 29422/99.7 | 122 | 2.3 ± 0.2 |
Eight differential proteins were preliminarily classified by functions using the European Bioinformatics Institute Bioinformatics Database (http://www.ebi.ac.uk). Protein disulfide-isomerase A3 is associated with molecular chaperoning. Phosphoglycerate kinase 1 and pyruvate kinase 3 isoforms M1/M2 are associated with glucose metabolism. Pancreatic triacylglycerol lipase is associated with fat metabolism. Lambda-crystallin homolog and alcohol dehydrogenase class-3 are associated with oxidation and reduction. Hnrph1 protein, and mitochondrial ribosomal protein L12 are associated with protein synthesis.
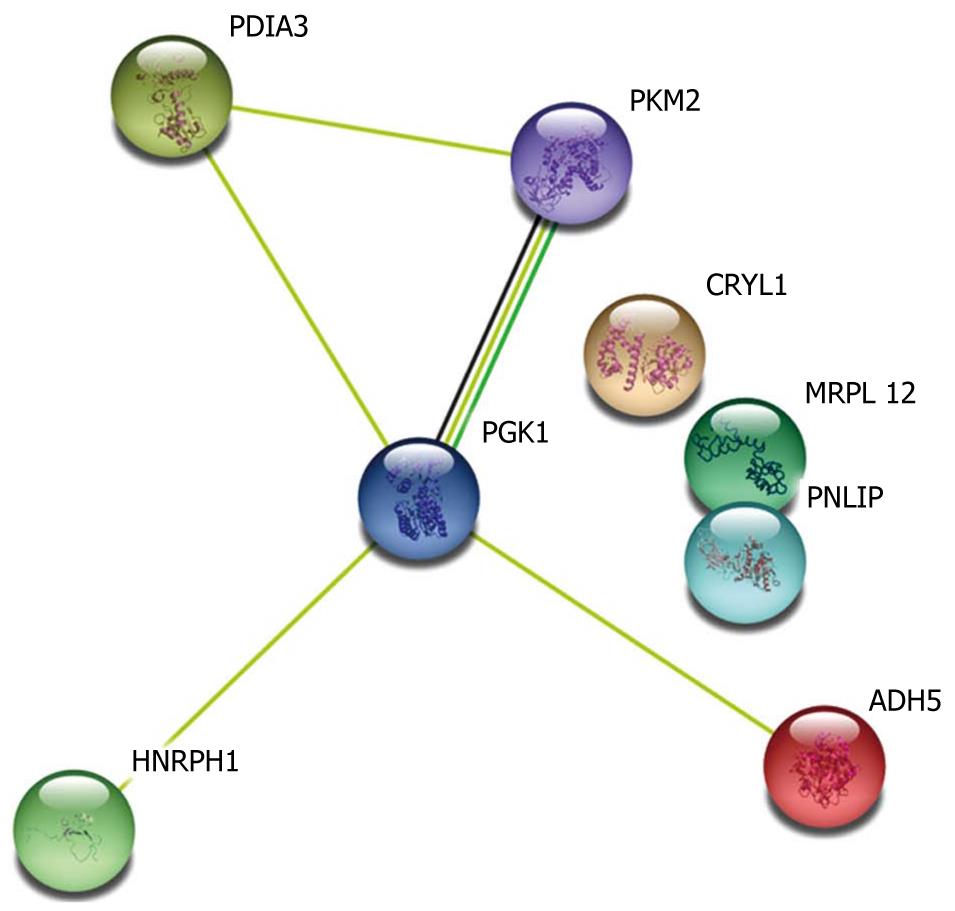
STRING is a system for mapping protein-protein interaction networks. We used the STRING system to construct the protein-protein interaction network of the differential proteins associated with colonic adaptation. Five (phosphoglycerate kinase 1, Hnrph1, protein disulfide-isomerase A3, pyruvate kinase 3 isoforms M1/M2, and alcohol dehyogenase 5) of the eight identified proteins were directly involved in the protein-protein interactions (Figure 3). Notably, phosphoglycerate kinase 1 was found to be a signal node in the network, suggesting that phosphoglycerate kinase 1 may be crucially involved in colonic adaptation. Previous studies have shown that the five proteins played an important role in energy metabolism in cells. It is well known that quickly-grown cells frequently exhibit increases in glycolytic metabolic pathway for adenosine triphosphate (ATP) generation to meet their energetic needs. Therefore, over-expression of these five proteins resulted in increased ATP production, rapid growth of colonic musocal cells and thickness of colonic musocal thickness.
Experiments have demonstrated that the colon can compensate after the extensive removal of the small intestine, with thickening of the colonic mucosa and heightening of the colon plica[1,5]. Epithelial hyperplasia was found 24-48 h after small intestinal resection[7-9]. Adaption process is stimulated by enteral nutrition by providing energy for enterocyte reproduction, stimulating the release of trophic factors[10]. Our previous experiments have shown that nutritional absorption of the colon can be increased by adaptation, and microvilli became more abundant, longer and thicker[11]. The colon may adapt to the absorption of a large volume of solutes[12-14]. Absorptive cell hyperplasia of colonic mucosa was found in the USBS rats assessed under electron microscope. These results showed that the colonic absorption area was greatly increased in the USBS rats. There were less apoptosis, less goblet cells and more absorptive epithelial cells in the USBS rats under transmission electron microscope. Adjacent cell membranes merged tightly and formed a rugged structure. There was an increase in cell connections and connection complexes in USBS rats. The junctions, bridge corpuscles, endoplasmic reticulum, Golgi apparatus, and mitochondrion were all increased. This resulted in improvement in the absorptive functions of water, xylose and 15N-glycine[15].
In this study, the entire small intestines of rats were removed to ensure maximum colonic adaptation. We tried to determine how the colon could compensate when the small intestine was lost. We found that USBS rats could survive using colonic compensation after all small intestines were lost, since rats were fed only with enteral nutrition and had a postoperative survival rate over 90%. The length, diameter and height of the mucosal fold in the mucosal thickness of the colon were increased in the USBS rats compared with the control group.
The colon can undergo adaptations in USBS patients and rats. Some materials have been shown to improve the adaptation. In recent years, the more studies of intestinal compensation have focused on compensatory mechanisms, adsorptive function, and cellular hyperplasia of short bowel syndrome (SBS) remnant intestines. Understanding of the mechanisms and ultimate treatment of the disease will eventually be reflected in protein expression levels and changes in post-translational modifications. We used a proteomics approach to observe differential protein expressions to fully understand the molecular mechanisms of colon adaptation in an attempt to help develop drugs that can promote colon compensation.
The term proteome was first proposed by Mac Wilkins and Keith Williams in 1994[16] to describe the complete set of proteins that are expressed and modified by an entire genome or cell. The intracellular and dynamic changes in protein composition, expression levels and modification states should be analyzed to observe protein-protein interactions and relationship with the disease, revealing the protein functions and activities important in the disease development.
In the diseases of the digestive system, proteomics have not been as widely used to study the USBS as gastric cancer, esophageal cancer and liver cancer[17-20]. A number of other growth and trophic factors have been implicated in animal models including enteroglucagon, epidermal growth factor, glutamine, growth hormone, chole-cystokinin, gastrin, neurotensin, leptin and insulin-like growth factors[21-23]. Fat-stimulated glucagon-like peptide-II may lead to hyperplasia[24,25]. In this study, 47 protein spots were found to be expressed nonrepetitively, including 30 upregulations and 17 downregulations. Their functions were reported to be associated with metabolism of sugar, protein and fat, oxidation-reduction and molecular chaperones. This implied that multiple proteins might affect the metabolism pathway involved in increasing absorptive functions and compensation.
Among these up-regulated proteins, protein disulfide-isomerase A3 is a special multifunctional protein rich in the endocytoplasmic reticulum. It has a strong non-specific peptide binding ability, and acts as both an enzyme and a chaperone[26]. As an enzyme, protein disulfide-isomerase A3 can assist in protein folding by catalyzing covalent bond changes that directly affect protein folding and functional conformations.
As a chaperone, protein disulfide-isomerase A3 may have acquired a new function during evolution to increase catalytic efficiency and assist in protein folding. It was up-regulated in carcinoma of the large intestine[27]. Ryu et al[28] found that protein disulfide-isomerase A3 was over-expressed when gastric cancer and paraneoplastic mucosal biological markers were studied with proteomics. This study indirectly demonstrated that protein disulfide-isomerase A3 is associated with hyperplasia of mucosa. Our previous experiments demonstrated that the endoplasmic reticulum in USBS rat colon was rich in protein disulfide-isomerase A3, suggesting that it was closely related to the adaptation of tunica mucosal coli cells and compensation of the colon.
Another up-regulated protein is pyruvate kinase, which participates in the last step of glycolysis, catalyzing phosphoenolpyruvate and adenosine diphosphate (ADP) into pyruvate and adenosine triphosphate (ATP). Pyruvate can generate ATP by zymolysis or by entering tricarboxylic acid (TCA) circulation. In animals, pyruvate kinase may serve as a gene, promoter, mRNA splicer, and signal transducer with polyadenylic acid. In this study, the pyruvate kinase was up-regulated in ultra-short intestinal mucosa. This implies that pentose phosphate shunts may be improved by pyruvate kinase. The excessive ribose-5-phosphate and one carbon unit produced by protein decomposition synthesize purine nucleosidase, improving the hyperplasia of tunica mucosa coli cells.
In this study, phosphoglycerate kinase 1 was richly expressed. Phosphoglycerate kinase 1 is the key enzyme of glycolysis and is also an essential enzyme for living. The main function of phosphoglycerate kinase 1 is to participate in the glycolysis procedure. It can catalyze 1,3-diphosphoglyceric acid into 3-phosphoglyceric acid. Some studies have shown that phosphoglycerate kinase 1 can affect DNA replication and repair the cell nucleus. It can also serve as an mRNA binding protein[29]. Other studies showed that phosphoglycerate kinase 1 up-regulation can cause carcinoma and vascularization. The oxidation status of larvaceous intra-cellular protein is a biological marker in human colon carcinoma[30]. These studies indicated that overexpressed phosphoglycerate kinase 1 can promote cellular proliferation, suggesting that phosphoglycerate kinase 1 is involved in colon compensation by inducing cell proliferation. The bioinformatics analysis in this study showed that phosphoglycerate kinase 1 acts as a hub in the protein-protein interaction networks involved in colon adaptation, suggesting that phosphoglycerate kinase 1 played a key role in colon adaptation and compensation.
Mitochondrial ribosomal proteins were also richly expressed in this study. Some studies have shown that mitochondrial ribosomal proteins can be regulated in translation as chondriogene mRNA. Up-regulation of mitochondrial ribosomal proteins is related to dell differentiation in tunica mucosal coli and colon carcinoma[31]. It has also been implied that mitochondrial ribosomal proteins participate in colon compensation since the number of chondriosomes is markedly increased in USBS rats.
Bioinformatics analysis and previous reports showed that eight proteins were closely related to sugar, fat metabolism, protein synthesis, redox, and molecular chaperones. These proteins likely play an important role in colonic compensation in USBS rats. Further studies to understand the characteristics of these differential proteins will help elucidate the molecular mechanisms of colonic compensation in USBS, and develop drugs to promote adaptation and alimentation.
People with short small bowel may have difficulties in nutritious absorption and may struggle to maintain nutritious balance. The increased morbility in intestinal tumors, traumas and mesenteric vascular thrombosis resulted in the increased incidence of short small bowel. It has been evidenced that rats can live without small intestine for a long period as their colon can undergo compensation.
More studies of intestinal compensation have focused on compensatory mechanisms, adsorptive function and cellular hyperplasia of remnant intestines. Mechanism and treatment of the disease will eventually be reflected in the protein expression levels and changes of post-translational modifications.
The protein expression changes in normal rats and short bowel rats have been studied based on mass spectrometric analysis and other advanced technologies. Eight proteins were found to be closely related with sugar, fat metabolism, protein synthesis, redox, and molecular chaperone. They might play important roles in nutritious absorption, and treatment of short bowel patients.
The authors offered the methodology for exploring the proteins in short bowel rats. Besides mass spectrometry, immunohistochemistry may be also a smart choice. Once the mechanism is clear and the therapeutic target is established, the malabsorption resulting from extensive small bowel loss will be hopefully solved.
Short bowel syndrome is a chronic malabsorptive syndrome resulting from extensive small bowel resections. Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles. It is used for determining masses of particles, the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and other chemical compounds.
This study combines proteomics with short bowel syndrome systematically. And it may form the theoretic foundation for treating short bowel syndrome.
Peer reviewer: Laura E Matarese, PhD, RD, LDN, FADA, CNSD, Assistant Professor of Surgery, University of Pittsburgh Medical Center, Director of Nutrition, Intestinal Rehabilitation and Transplantation Center, Thomas E. Starzl Transplantation Institute, UPMC Montefiore, 7 South, 3459 Fifth Avenue, Pittsburgh, PA 15213, United States
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Kocoshis SA, Beath SV, Booth IW, Garcia Oliva CA, Goulet O, Kaufman SS, Lai HS, Luque C, Ohtsuka Y. Intestinal failure and small bowel transplantation, including clinical nutrition: working group report of the second World congress of Pediatric Gastroenterology, Hepatolopy and Nutrition. Pediatr-Gastroenterol Nutr. 2004;39:655-661. |
| 2. | Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111-1134. |
| 3. | Bakker H, Bozzetti F, Staun M, Leon-Sanz M, Hebuterne X, Pertkiewicz M, Shaffer J, Thul P. Home parenteral nutrition in adults: a european multicentre survey in 1997. ESPEN-Home Artificial Nutrition Working Group. Clin Nutr. 1999;18:135-140. |
| 4. | Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16-S28. |
| 5. | Tilg H. Short bowel syndrome: searching for the proper diet. Eur J Gastroenterol Hepatol. 2008;20:1061-1063. |
| 6. | Lobo DN. Colonic adaptation: a therapeutic target for short-bowel syndrome? World J Surg. 2008;32:1840-1842. |
| 7. | Dowling RH, Booth CC. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967;32:139-149. |
| 8. | Nygaard K. Resection of the small intestine in rats. 3. Morphological changes in the intestinal tract. Acta Chir Scand. 1967;133:233-248. |
| 9. | Hanson WR, Osborne JW. Epithelial cell kinetics in the small intestine of the rat 60 days after resection of 70 per cent of the ileum and jejunum. Gastroenterology. 1971;60:1087-1097. |
| 10. | Feldman EJ, Dowling RH, McNaughton J, Peters TJ. Effects of oral versus intravenous nutrition on intestinal adaptation after small bowel resection in the dog. Gastroenterology. 1976;70:712-719. |
| 11. | Jiang HP, Guo QF, Zhang HW, Yuan L, Chen D. Observation of ultrastructure and absorption function of colon mucosa in rats with ultra-short bowel syndrom. Zhongguo Linchuang Yingyang Zazhi. 2010;18:360-365. |
| 12. | Nightingale JM, Lennard-Jones JE, Gertner DJ, Wood SR, Bartram CI. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33:1493-1497. |
| 13. | Pharaon I, Despres C, Aigrain Y, Grini A, Faure C, Matarazzo P, Navarro J, Cathelineau L, Cezard JP. Long-term parenteral nutrition in children who are potentially candidates for small bowel transplantation. Transplant Proc. 1994;26:1442. |
| 14. | Vargas JH, Ament ME, Berquist WE. Long-term home parenteral nutrition in pediatrics: ten years of experience in 102 patients. J Pediatr Gastroenterol Nutr. 1987;6:24-32. |
| 15. | DiBaise JK, Young RJ, Vanderhoof JA. Intestinal rehabilitation and the short bowel syndrome: part 1. Am J Gastroenterol. 2004;99:1386-1395. |
| 16. | Wasinger VC, Cordwell SJ, Cerpa-Poljak A, Yan JX, Gooley AA, Wilkins MR, Duncan MW, Harris R, Williams KL, Humphery-Smith I. Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis. 1995;16:1090-1094. |
| 17. | Vantini I, Benini L, Bonfante F, Talamini G, Sembenini C, Chiarioni G, Maragnolli O, Benini F, Capra F. Survival rate and prognostic factors in patients with intestinal failure. Dig Liver Dis. 2004;36:46-55. |
| 18. | Coran AG, Spivak D, Teitelbaum DH. An analysis of the morbidity and mortality of short-bowel syndrome in the pediatric age group. Eur J Pediatr Surg. 1999;9:228-230. |
| 19. | Keller J, Panter H, Layer P. Management of the short bowel syndrome after extensive small bowel resection. Best Pract Res Clin Gastroenterol. 2004;18:977-992. |
| 20. | Wilmore DW, Lacey JM, Soultanakis RP, Bosch RL, Byrne TA. Factors predicting a successful outcome after pharmacologic bowel compensation. Ann Surg. 1997;226:288-292; discussion 292-293. |
| 21. | Nightingale JM, Kamm MA, van der Sijp JR, Ghatei MA, Bloom SR, Lennard-Jones JE. Gastrointestinal hormones in short bowel syndrome. Peptide YY may be the ‘colonic brake’ to gastric emptying. Gut. 1996;39:267-272. |
| 22. | de Miguel E, Gómez de Segura IA, Bonet H, Rodríguez Montes JA, Mata A. Trophic effects of neurotensin in massive bowel resection in the rat. Dig Dis Sci. 1994;39:59-64. |
| 23. | Liu CD, Rongione AJ, Shin MS, Ashley SW, McFadden DW. Epidermal growth factor improves intestinal adaptation during somatostatin administration in vivo. J Surg Res. 1996;63:163-168. |
| 24. | Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806-815. |
| 25. | Sigalet DL, Bawazir O, Martin GR, Wallace LE, Zaharko G, Miller A, Zubaidi A. Glucagon-like peptide-2 induces a specific pattern of adaptation in remnant jejunum. Dig Dis Sci. 2006;51:1557-1566. |
| 26. | Wang CC, Tsou CL. Protein disulfide isomerase is both an enzyme and a chaperone. FASEB J. 1993;7:1515-1517. |
| 27. | Liu WJ, Qin HL, Ma YL. Proteomics study of intestinal mucosa in patients with colorectal cancer. Shandong Yiyao. 2008;48:1-3. |
| 28. | Ryu JW, Kim HJ, Lee YS, Myong NH, Hwang CH, Lee GS, Yom HC. The Proteomics Approach to Find Biomarkers in Gastric Cancer. Korean Med Sci. 2003;18:505-509. |
| 29. | Popanda O, Fox , G , Thielmann HW. Modulation of DNA polymerases alpha, delta and epsilon by lactate dehydrogenase and 3-phosphoglycerate kinase. Biochim Biophys Acta. 1998;139:102-117. |