INTRODUCTION
Intraductal papillary neoplasms of the bile duct (IPNB) are characterized by a grossly visible, exophytic proliferation of neoplastic cholangiocytes with delicate fibrovascular cores in large bile ducts[1-3]. IPNB not infrequently presents with mucinhypersecretion and prominent cysts in the affected bile ducts[4,5], and is regarded as a precursor of invasive cholangiocarcinoma[1-3]. IPNB has been reported in association with chronic biliary diseases, including hepatolithiasis, primary sclerosing cholangitis, and parasitic biliary diseases, as well as apparently normal bile ducts, though the exact histogenesis of IPNB remains unclear.
Here, we report a case of IPNB arising in a patient with hepatitis B virus (HBV)-related liver cirrhosis associated with hepatocellular carcinoma (HCC). In this case, the malignant transformation of hepatic progenitor cells (HPCs) may be responsible for the development of IPNB and also HCC.
CASE REPORT
A 76-year-old man was admitted to hospital with recurrent HCC in the right liver lobe for transcatheter arterial chemoembolization (TACE). He had been found to have hepatitis B surface antigen 17 years ago and had suffered from HBV-related cirrhosis for nearly 10 years. HCC was found in the right lobe, and a partial hepatectomy was performed, 4 years ago. On admission, the laboratory values of tumor markers were as follows: the serum α-fetoprotein (AFP) level was within the normal range, the carcinoembryonic antigen level was 114.6 ng/mL (normal range: < 22 ng/mL) and the carbohydrate antigen 19-9 level was 52 000 U/mL (normal range: < 37 U/mL). Contrast-enhanced computerized tomography revealed mild dilatation of the left intrahepatic bile duct and HCC after TACE in segment 6 of the liver. The patient died of progressive hepatic failure after hospitalization for 4 mo.
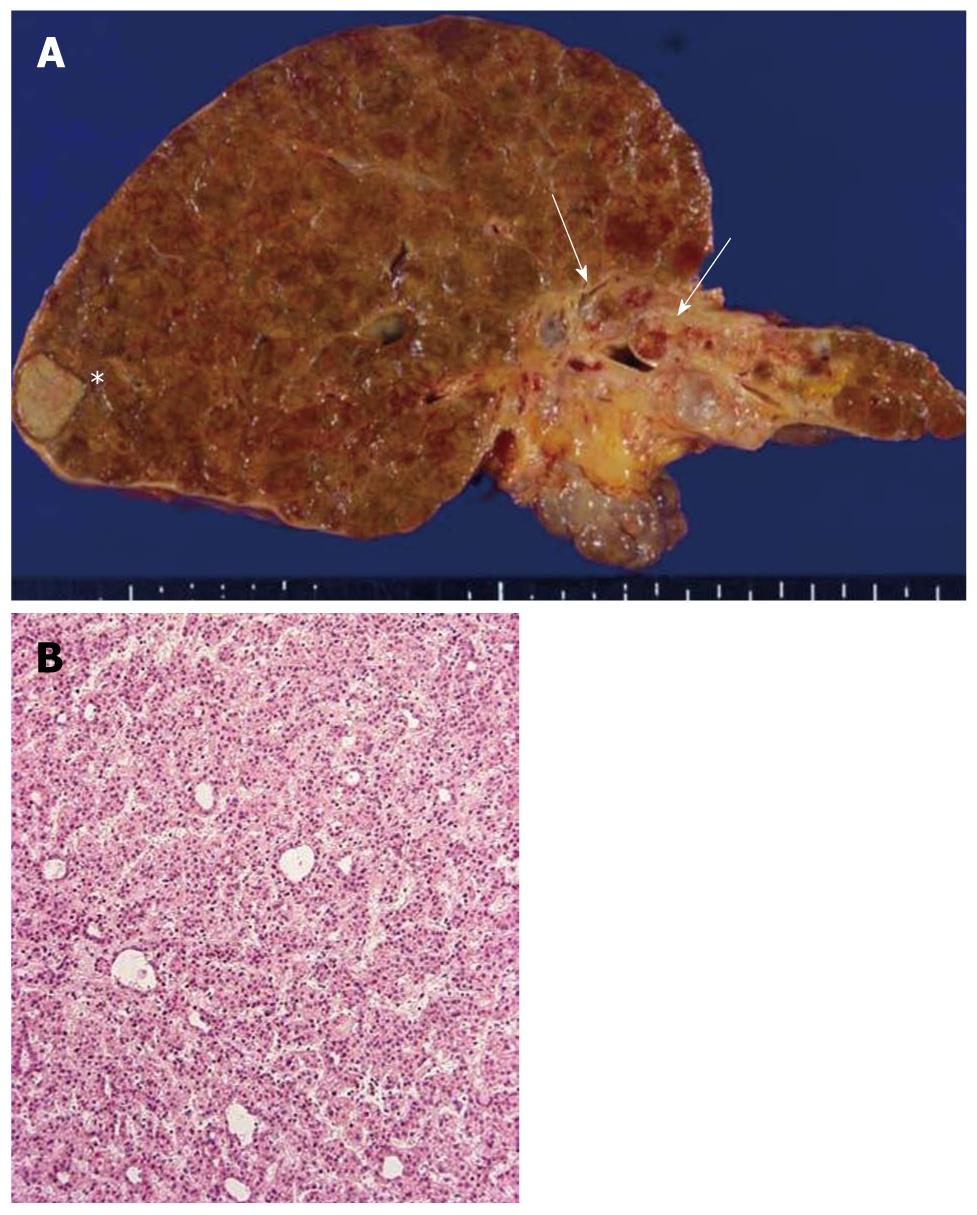
At autopsy, the liver weighed 615 g, and the majority of the liver showed cirrhotic nodules measuring less than 5 mm in diameter. Two distinct tumorous nodules (1.7 cm × 1.4 cm and 1.2 cm × 1.3 cm) were identified in the right lobe (Figure 1A), and these tumors showed largely coagulative necrosis compatible with HCC after TACE, while the remaining parts showed a trabecular growth pattern of HCC (Figure 1B). The rest of the liver was associated with mild to moderate necroinflammatory changes and ductular reactions.
Figure 1 Liver pathology.
A: The autopsied liver tissue shows cirrhosis with a solid whitish lesion (*) in segment 6 of the right lobe (hepatocellular carcinoma after transcatheter arterial chemoembolization) and cystic dilatation in the left intrahepatic bile ducts filling with yellowish-tan papillary masses and mucin production (arrows). The left liver appears atrophied; B: Well-differentiated hepatocellular carcinoma. Hematoxylin and eosin staining (H and E).
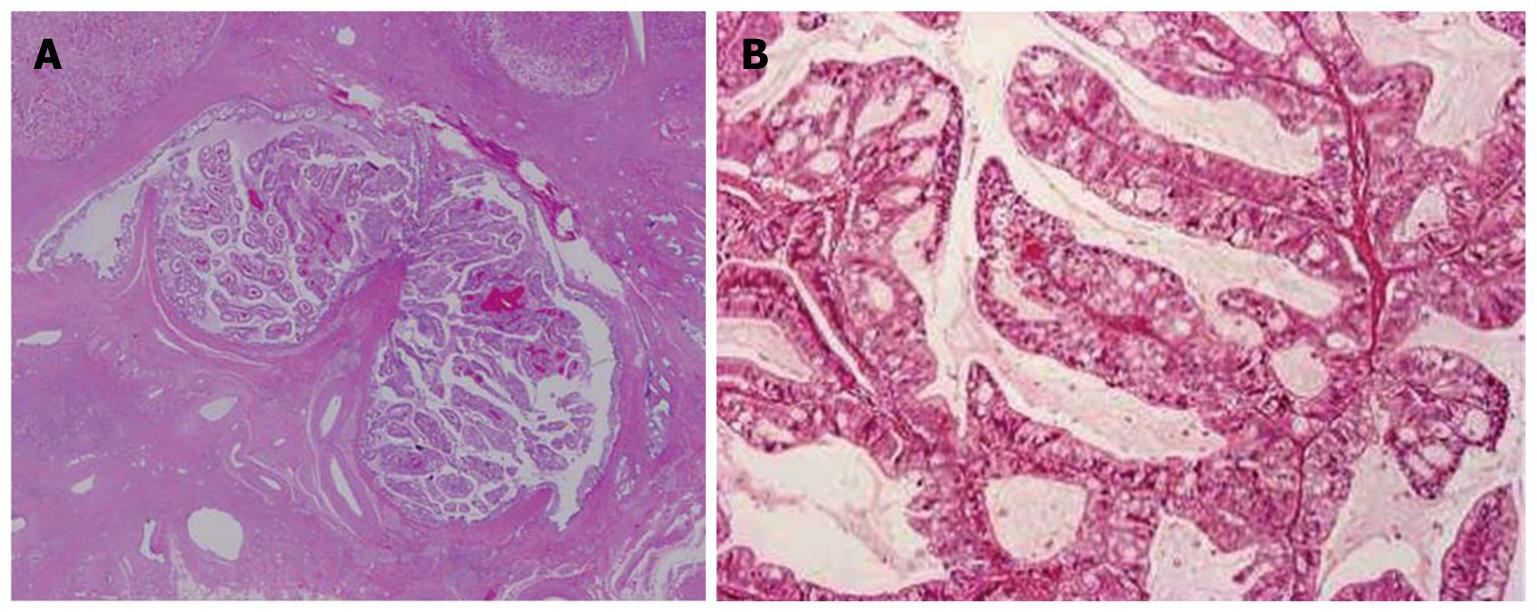
The intrahepatic large bile ducts in the left lobe showed cystic or fusiform dilatation, and were filled with yellowish-tan papillary masses and hypersecretedmucin (Figures 1 and 2A). The affected bile duct lumen was obstructed, and the left liver was atrophic due to the obstruction. Within the left dilated intrahepatic bile ducts, the neoplastic cholangiocytes showed exophytic and papillary proliferation with delicate fibrovascular stalks (Figure 2B). The neoplastic cholangiocytes showed multilayered nuclei, an increased nucleo-cytoplasmic ratio and nuclear hyperchromasia, and these papillary tumors were diagnosed as cholangiocarcinomas. Such cholangiocarcinoma cells spread along the luminal surface of the bile ducts, with a flat and micropapillary configuration. In the subepithelialstroma, no ovarian-like mesenchymalstroma was observed. Near the large-sized bile duct with IPNB, stromal invasion of cholangiocarcinoma accompanied by mucinous carcinoma in the left lobe was observed. Mucin was positive by Alcian blue (pH 2.5) and diastase-periodic acid Schiff stains. Taken together, these carcinomas were regarded as invasive IPNB. Immunohistochemical staining revealed that cytokeratin-7 (CK7) and CK19 were diffusely expressed, mucin (MUC) 5AC was moderately expressed, and CK20, CDX2, NCAM and MUC1 were focally expressed in IPNB. However, MUC2, MUC6, HepPar-1 and AFP were not observed.
Figure 2 Histological features of the papillary tumor of the left lobe.
A: Neoplastic biliary epithelia show intraductal papillary growth in the dilated bile duct lumen. Hematoxylin and eosin staining (H and E); B: The atypical biliary epithelium with a fine fibrovascular core is spreading. H and E.
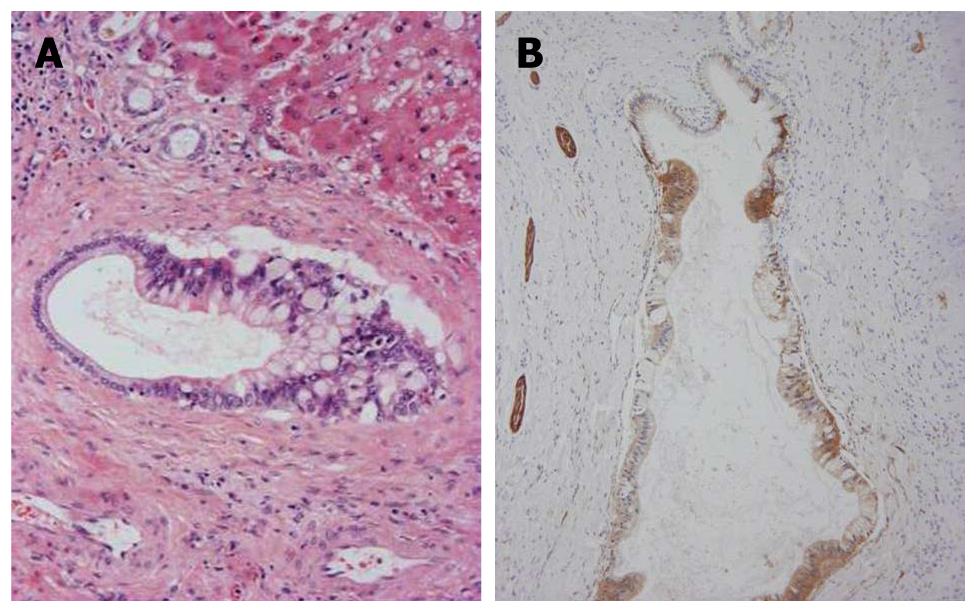
Interestingly, in the left lobe including the areas near or adjacent to the left hepatic bile duct, carcinomatous cholangiocytes partially replaced the normal biliary epithelia of some septal intrahepatic bile ducts and totally replaced the epithelia of the interlobular bile ducts (Figure 3A). They showed similar mucinous and immunohistochemical profiles as seen in IPNB, and NCAM was frequently expressed in spreading neoplastic cells (Figure 3B). Interestingly, some foci of the proliferated bile ductules located in the fibrous stroma surrounding regenerative nodules showed cellular and nuclear atypia (Figure 4A) and were positive for MUC1 and focal NCAM (Figure 4B). They were considered to represent an extensive intra-bile duct spread of cholangiocarcinoma cells from reactive bile ductules, interlobular bile ducts and septal bile ducts to the large bile ducts. In contrast, the hilar bile ducts and bile ducts in the right lobe were not affected.
Figure 3 Carcinomatouscholangiocytes.
A: Atypical biliary epithelial cells (cholangiocarcinoma) partially replace the epithelia of septal bile ducts. Hematoxylin and eosin staining (H and E); B: Neoplastic biliary epithelial cells spreading on the luminal surface of the septal bile ducts are positive for neural cell adhesion molecule (NCAM). Nerve fibers around the bile ducts are also positive. Immunostaining for NCAM and hematoxylin.
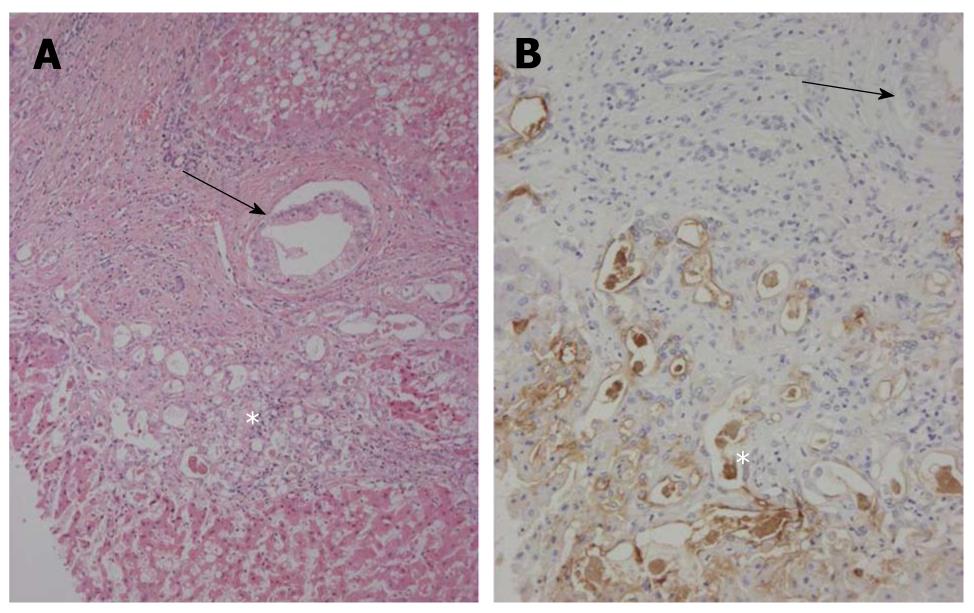
Figure 4 Bileductular cell proliferation.
A: Clusters of atypical bile ductular cells (*) are seen, and are regarded as malignant cells. An arrow shows the involvement of carcinoma cells of interlobular bile ducts. Hematoxylin and eosin staining (H and E); B: Atypical bile ductular cells (*) are positive for mucin (MUC)1. Neoplastic epithelial cells replacing interlobular bile ducts (arrow) are also focally positive. Immunostaining for MUC1 and hematoxylin.
DISCUSSION
HCC grows occasionally in the bile duct as a tumor cast and presents clinical symptoms related to biliary obstruction[6]. Here, we report a case of papillary cholangiocarcinoma secreting much mucin in the dilated intrahepatic large bile duct of the left lobe and showing invasion as a mucinous carcinoma. Immunohistochemically, the papillary carcinoma was strongly and diffusely positive for CK7 and CK19, and also expressed MUC5AC. CDX2, CK20, and MUC1 were also focally positive. This phenotypic profile suggests that the neoplastic cells retain a biliary phenotype (CK7 and CK19) and a gastric phenotype (MUC5AC), and intestinal metaplasia (CK20, CDX2) was also focally expressed. Carcinoma cells in the invasive area showed an apical membranous pattern for MUC1, while MUC2 (intestinal-type mucin) and MUC6 (pyloric gland-type mucin) were negative in the present case. These features are compatible with invasive IPNB. Compared with previous reports of IPNB[1-3], the present case is unique in 2 points: (1) atypical biliary cells regarded as cholangiocarcinoma cells spread not only in the large bile ducts but also in the small bile ducts and the reactive bile ductules; and (2) IPNB was found in HBV-related liver cirrhosis associated with HCC, which was separate from the cholangiocarcinomatous components.
Papillary cholangiocarcinoma arising from extrahepatic and/or intrahepatic bile ducts occasionally shows extensive intraductal luminal spread[7-12]. However, the extensive intraluminal spread of atypical biliary epithelial cells extending to the reactive bile ductules found in our case, has not been reported in the literature. Recently, Ashimaet al[13] demonstrated 2 cases of biliary intraepithelial neoplasia (BilIN), a flat biliary epithelial neoplastic lesion, with extensive intraductal spread associated with liver cirrhosis. Rougemontet al[14]also reported a case of extensive BilIN and multifocal intrahepatic cholangiocarcinoma associated with non-biliary cirrhosis. A review of the literature revealed 3 cases of biliary papillomatosis, corresponding to IPNB, associated with liver cirrhosis[3,15-18]. However, in these cases, the involvement of interlobular bile ducts and reactive bile ductules was not shown.
It has long been controversial whether hepatic malignancies arise from stem cells or HPCs undergoing a malignant transformation[19,20]. According to recent studies, some primary hepatic malignancies, including combined hepatocellular cholangiocarcinoma (HC-CC), originate from the transformation of HPCs[7]. Theiseet al[17] demonstrated one case of biliary papillomatosis (in the left liver lobe) associated with HC-CC (in the right liver lobe) on a background of liver cirrhosis secondary to hepatitis B, and speculated that the HC-CC was derived from neoplastic transformation of HPCs. However, they did not refer to the relationship of HC-CC with biliary papillomatosis.
It is possible that activated HPCs located in reactive bile ductules underwent neoplastic transformation leading to hepatocellular and biliary differentiation. The former may have been followed by the development of HCC at the first step, while the latter may have been followed by superficial spread from the neoplastic foci in reactive bile ductules to the interlobular bile duct, the septal intrahepatic bile ducts, and, eventually, the left hepatic bile ducts, where the cholangiocarcinoma developed as multiple papillary growths and then an invasive mucinous adenocarcinoma. Interestingly, NCAM, a HPC marker, was also focally expressed in invasive IPNB and in the small bile ducts replaced by neoplastic cholangiocytes (Figure 3), suggesting that the cholangiocarcinoma spreading into the intrahepatic bile ducts retained a HPC phenotype.
In conclusion, a rare case of IPNB arising in a patient with liver cirrhosis associated with HCC was reported. Neoplastic transformation of bile ductules containing HPCs may have been responsible for the HCC as well as the IPNB showing extensive bile duct spread.
Peer reviewer: KyuTaek Lee, MD, PhD, Professor, Division of Gastroenterology, Department of Medicine, Sungkyunkwan University, Samsung Medical center, 50 Ilwon-Dong, Kangnam-Ku, Seoul 135-710, South Korea
S- Editor Sun H L- Editor Cant MR E- Editor Ma WH