Published online Apr 14, 2011. doi: 10.3748/wjg.v17.i14.1915
Revised: December 10, 2010
Accepted: December 17, 2010
Published online: April 14, 2011
AIM: To investigate the relation of reactive oxygen species (ROS) to hypoxia induced factor 1α (HIF-1α) in gastric ischemia.
METHODS: The animal model of gastric ischemia reperfusion was established by placing an elastic rubber band on the proximal part of the bilateral lower limb for ligature for 3 h and reperfusion for 0, 1, 3, 6, 12 or 24 h. Ischemic post-conditioning, three cycles of 30-s reperfusion and 30-s femoral aortic reocclusion were conducted before reperfusion. Histological and immunohistochemical methods were used to assess the gastric oxidative damageand the expression of HIF1-α in gastric ischemia. The malondialdehyde (MDA) content and superoxide dismutase (SOD), xanthine oxidase (XOD) and myeloperoxidase (MPO) activities were determined by colorimetric assays.
RESULTS: Ischemic post-conditioning can reduce post-ischemic oxidativestressand the expression of HIF-1α of gastric tissue resulting from limb ischemia reperfusion injury. MDA, SOD, XOD and MPO were regarded as indexes for mucosal injuries from ROS, and ROS was found to affect the expression of HIF-1α under gastric ischemic conditions.
CONCLUSION: ROS affects HIF-1α expression under gastric ischemic conditions induced by limb ischemia reperfusion injury. Therefore, ROS can regulate HIF-1α expression in gastric ischemia.
- Citation: Wang T, Leng YF, Zhang Y, Xue X, Kang YQ, Zhang Y. Oxidative stress and hypoxia-induced factor 1α expression in gastric ischemia. World J Gastroenterol 2011; 17(14): 1915-1922
- URL: https://www.wjgnet.com/1007-9327/full/v17/i14/1915.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i14.1915
Recent studies have suggested that limb ischemia can induce ischemia reperfusion (IR) of remote organs (lung, liver, kidney and gastrointestinal tract), and limb ischemia reperfusion-induced gastric mucosal injury can cause the occurrence of stress ulcer. Although the incidence of acute postoperative gastric ulcers decreased after the introduction of H2 receptor-blockers and HK-pump inhibitors, there are still some problems to be solved. Oxidative stress plays a very important role through the free radicals and or reactive oxygen species (ROS)[1] in gastric ischemia reperfusion injury, and ROS production during reperfusion may play a role in the pathogenesis of gastric mucosal injury induced by IR. Many studies demonstrated that besides the lesions induced by ischemia, the reperfusion causes additional cellular damage not only in the primary sites but also in remote structures[2,3], such as gastric tissues.
The main factor involved in tissue lesions in the course of ischemia is hypoxia. It initiates intracellular signaling pathways, hence leading to the activation of the hypoxia-induced factor 1 (HIF-1)[4]. HIF-1 is a critical regulator of the transcriptional response to low-oxygen (O2) conditions (hypoxia/anoxia) in mammalian cells under both physiological and pathophysiological circumstances[5]. Heterodimeric protein is composed of a constitutively expressed HIF-1β subunit, and an O2-regulated HIF-1α subunit. Since the clinical data first indicated that HIF-1α may play an important role in human cancer progression in the 1999[6], significant knowledge has been accumulated and it has played a major role in gastric tumor and ischemia through activation of various genes that are linked to regulation of angiogenesis, cell survival, energy metabolism, and apoptotic and proliferative responses[7-9].
The mechanism of ROS in HIF-1α expression in ischemia could be solely related to H2O2 initial concentrations and production. Some studies have shown increased ROS expression in hypoxia, and increased HIF-1α expression has been found to contribute to mitochondrial activity, and especially ROS formation during hypoxia[10,11]. However, little is known about oxidative stress and the role of HIF-1α in rat gastric injury after limb ischemia reperfusion injury (LI-RI). We aimed to investigate the oxidative stress and HIF-1α protein expression in gastric ischemia induced by limb ischemia reperfusion, and to test if HIF-1α is regulated by reactive oxygen species. We used rat hind limbs ischemia as the primary lesion and the gastric mucosa as the remote site in order to find out a potential etiologic factor of acute gastric ulcers .
Male Wistar rats weighing 220-250 g were purchased from the Animal Experimental Center, Gansu College of Traditional Chinese Medicine (Lanzhou, China). All procedures were performed in accordance with the Declaration of Helsinki of the World Medical Association. The kits used to determine superoxide dismutase (SOD), malondialdehyde (MDA), xanthine oxidase (XOD) and myeloperoxidase (MPO) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and rat HIF-1α assay kits were purchased from Wuhan Boster Bioengineering Institute (Wuhan, China).
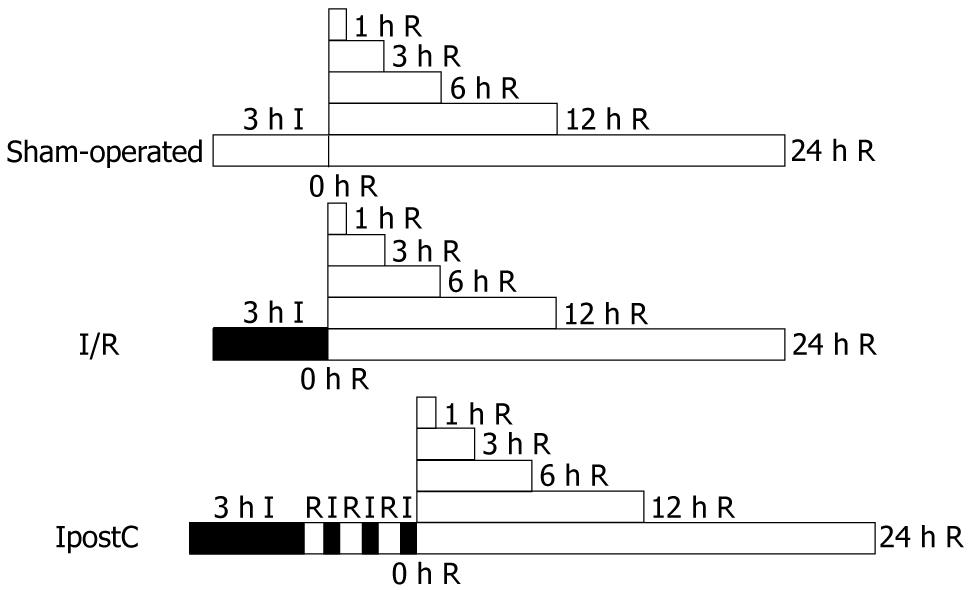
Animals were divided into three groups randomly, and each group contained 36 rats. Group 1, (sham-operated, control), a rubber band was used without any constriction; group 2, (ischemia/reperfusion, I/R), gastric ischemia/reperfusion injury (GI-RI) was induced with bilateral lower limb ligated for 3 h by placing an elastic rubber band under a pressure of 290-310 mmHg on the proximal part of the lower limb[12,13] and then released to allow reperfusion; and group 3, (ischemic post-conditioning, IpostC), at the start of reperfusion, three cycles of 30-s reperfusion and 30-s femoral aortic reocclusion[14] were conducted before reperfusion as shown in Figure 1. Global ischemia in hind limb was verified by the absence of blood flow in femoral aorta and vein. Each group was housed in wire mesh cages at room temperature and in a 12/12 h day/night cycle. Prior to the experiment, all rats were fasted for 24 h and allowed access to tap water ad libitum. The animals were anesthetized by inhalation 2%-3% isoflurane[15] and the anesthesia was only maintained in the course of the experimental operation. The three groups underwent 0, 1, 3, 6, 12 or 24 h reperfusion. Following reperfusion, blood samples from the inferior vena cava were collected, and six rats were humanely killed by venous bloodletting and the stomachs were immediately removed to collect tissue samples at each time point, respectively.
The stomach was homogenized in 0.9% saline solution using a homogenizer. The homogenate was then centrifuged at 2000-3000 rpm for 10 min at 4°C. The supernatant obtained was used to determine the MDA content and SOD, XOD and MPO activities according to the manufacturer’s instructions. MDA content was determined spectrophotometrically at 532 nm by the thiobarbituric acid method, and was expressed in nmol/mg of protein. The protein concentrations were determined by Coomassie brilliant blue protein assay. SOD activity was evaluated spectrophotometrically at 550 nm by the the xanthine oxidase method, and SOD activity was expressed in U/mg of protein. XOD was determined spectrophotometrically at 530 nm using a commercial XOD kit, and XOD activity was expressed in U/g of protein. MPO activity was determined spectrophotommetrically at 460 nm by the O-dianisidine method, and MPO activity was expressed as U/g of wet tissue. Each measurement was performed in triplicate.
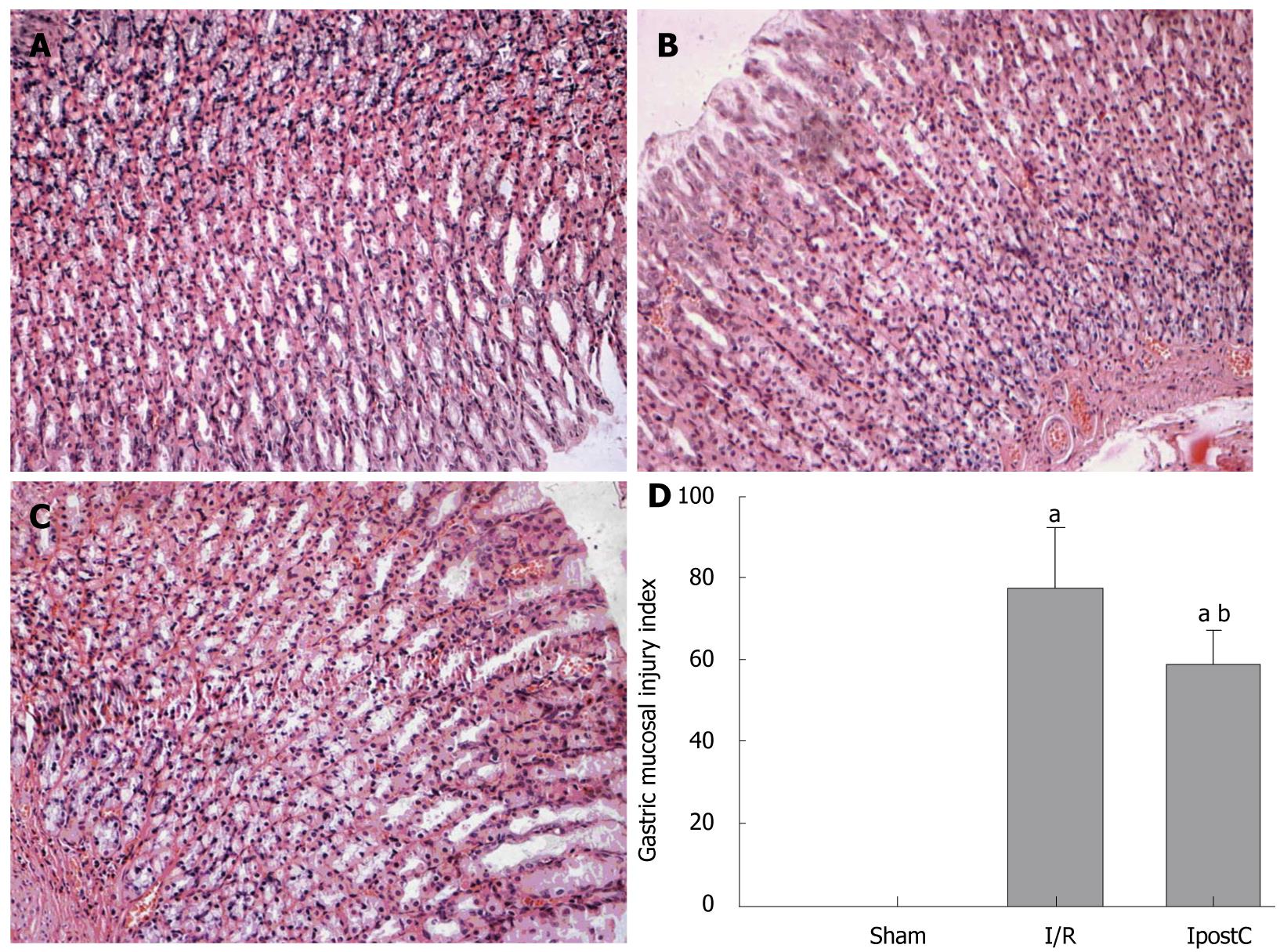
The murine stomach was incised along the lesser gastric curvature and fixed in 10% phosphate-buffered formalin, paraffin-embedded and sectioned at 4 μm in thickness. After deparaffinization and gradual hydration, they were examined using hematoxylineosin staining. Based on a cumulative-length scale where an individual lesion was limited to the mucosal epithelium (including pinpoint erosions, ulcers, and hemorrhagic spots), the index was scored according to its length: 1, ≤ 1 mm; 2, > 1 mm and ≤ 2 mm; and 3, > 2mm and ≤ 3 mm. For lesions > 1 mm in width, the score was doubled. The sum total of the scores of all lesions represented the gastric mucosal injury index as outlined by Zhang et al[16]. To avoid bias, the index was determined by a researcher who was blind to the treatment.
The stomach fixed in 10% phosphate-buffered formalin was paraffin-embedded and sectioned 4 μm thick. After deparaffinization and gradual hydration, it was examined using hematoxylin-eosin staining. Morphologic assessment was performed by an experienced pathologist who was unaware of the treatment under a light microscope.
The best tissue section for immunohistochemistry was selected and the corresponding formalin-fixed, paraffin-embedded resection specimens were obtained. Immunohistochemical detection of HIF-1α was performed using the image pro-plus 6.0 analysis system (Media Cybernetics Co., America) based on a StreptAvidin-Biotin Complex formation. Sections 4 mm in thickness were deparaffinised and the antigen was retrieved by microwaving in 10 mmol/L citrate buffer (pH 6.0) for 20 min followed by blocking steps according to the manufacturer’s protocol. Mouse monoclonal antibody (Wuhan Boster Co., China), diluted at 150-200, was applied and the slides were incubated overnight at 41°C. The biotinylated goat anti-rat secondary antibody (Wuhan Boster Co., China), was applied using additional blocking precautions to minimize the amplification of nonspecific background. The antibody was visualized using diaminobenzidine and the sections were counterstained with haematoxylin, dehydrated and mounted. Substitution of the primary immunoadsorption with immunizing peptide served as negative control. Batch-to-batch variation was assessed by choosing two sections showing high and low HIF-1α expressions and running additional sections from these biopsies in each batch.
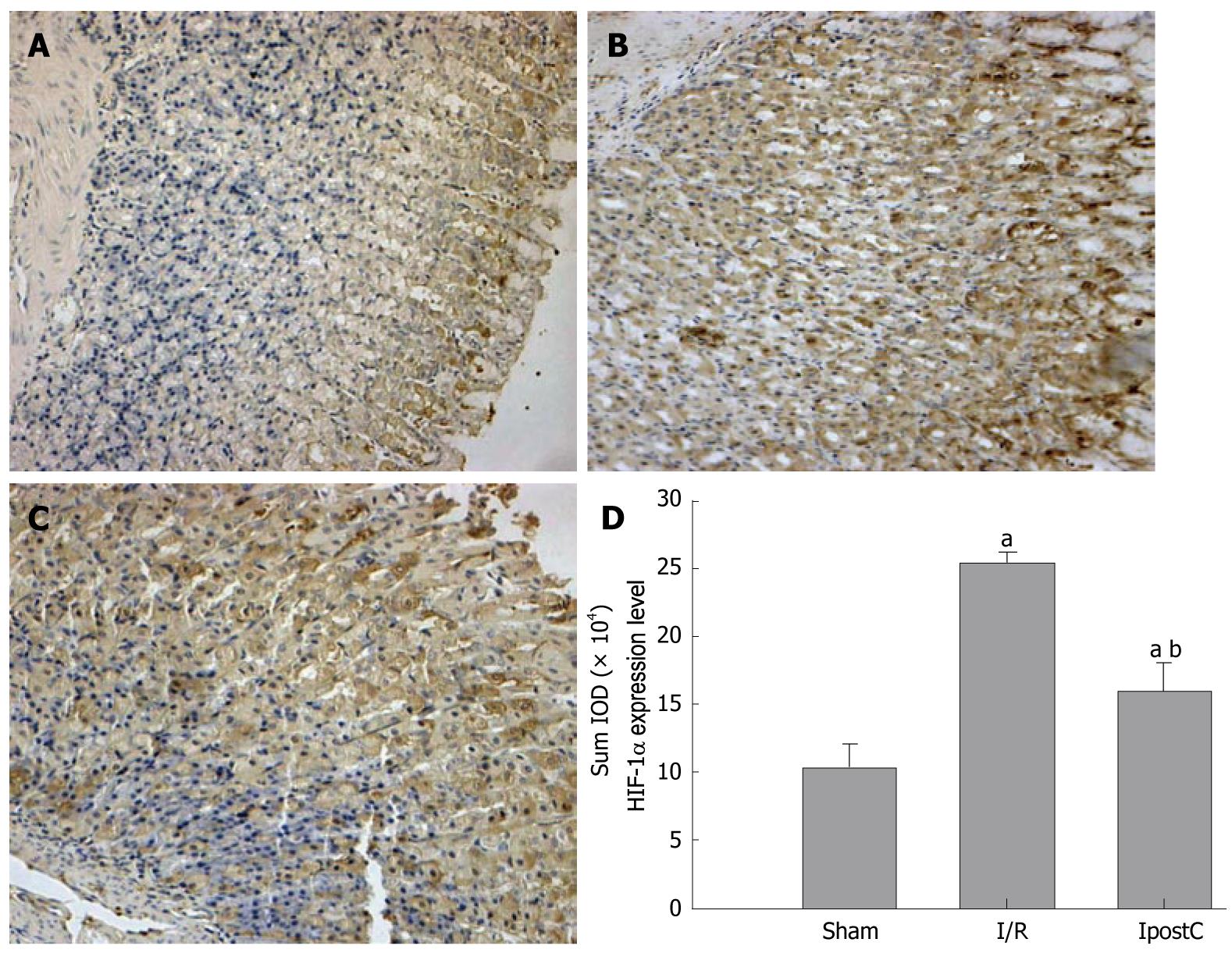
The extent of hypoxia gastric tissue staining was quantified on 24-h specimens. Digital images of the gastric tissue overlying 3 regions of the lesser gastric curvature (mucosae, muscularis mucosae and glands) were obtained using a microscope at × 20 magnification. The total thickness of the gastric tissue and positive staining cells were measured using Image Pro Software. In each field, 5 measurements were obtained and averaged. The HIF-1α protein level was expressed as the sum of the integrated optical density (SUMIOD) value in different groups. HIF-1α was also assessed by an experienced pathologist who was unaware of the treatment.
Data were entered into a database and analyzed using SPSS software (SPSS, Chiacgo, IL, USA) and were expressed as mean ± SD. Data were analyzed by Repeated Measures Analysis, and the means of all groups were compared using the least significant difference (LSD) test for multiple comparisons. P < 0.05 was considered as significant difference.
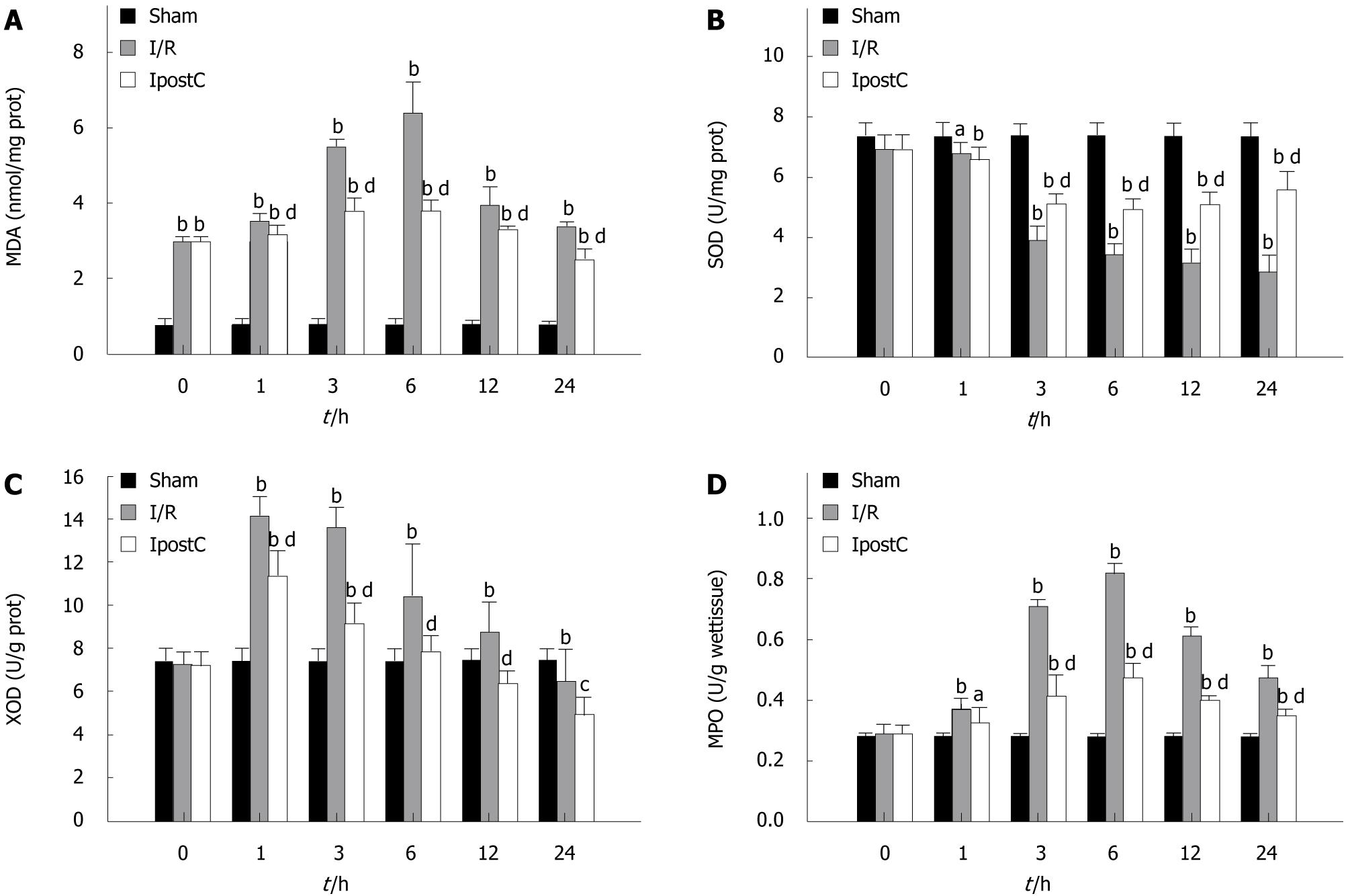
Significant elevation in MDA content and decrease in SOD activity were observed in the gastric tissue of I/R group when compared with the sham-operated group. Treatment with IpostC prevented marked elevation in MDA content and decrease in SOD activities (Figure 2A and B). XOD and MPO activities were also much higher than in the sham-operated group, whereas administration of IpostC reversed this change (Figure 2C and D).
Gastric IR resulted in significant injury as evidenced by gastric mucosal edema, gastric epithelial hemorrhage, hyperemia and erosion, and was infiltrated with inflammatory cells between the muscularis mucosa and the glands. In contrast, IpostC treatment ameliorated severe gastric damages (Figure 3A-C). According to the Yong-Mei Zhang scores, 3 h gastric ischemia followed by 6 h reperfusion resulted in severe acute gastric lesions. Quantitative analysis showed dramatically increased scores in the I/R group compared with the sham-operated group and decreased scores in the IpostC group compared with the I/R group (Figure 3D).
Photomicrographs of HIF-1α staining in the gastric tissues of all groups are shown in Figure 4A-C. The thickness of gastric specimens containing HIF-1α-positive gastric cells was determined at a 6-h time point. HIF-1α was seen in the glandular epithelial cytoplasm and the vascular endothelial cytoplasm or nucleus of normal gastric tissues, but expression increased in density and intensity with progression to GI-RI (Figure 4D). Compared with group I/R, HIF-1α expression level was decreased significantly in the glandular epithelial cytoplasm, and the vascular endothelial cytoplasm and nucleus of gastric tissues of the IpostC group (Figure 4D).
Changes of MDA content, SOD, XOD and MPO activities and expression of HIF-1α are shown in Figures 2 and 4. MDA content, XOD and MPO activities increased, and SOD activity decreased. Consequently, the expression of HIF-1α significantly increased in the I/R group; and when MDA content, XOD and MPO activities decreased and SOD activity increased, the expression of HIF-1α was also decreased in the IpostC group.
The ischemia of lower extremities is one of the most common clinical problem. The ischemia reperfusion injury (IRI) of extensive muscle tissue mass and the sensitive vascular tissues and endothelium often leads to systemic complications with distant organ damage (e.g. lung, liver, gastrointestinal mucosa and kidney), and even systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS)[17,18]. Therefore, limb ischemia can result in IRI of the gastric mucosa, which is an important clinical condition with undesirable outcomes concerning patients’ morbidity and mortality. In recent years, studies on GI-RI have revealed that reactive oxygen species (ROS), microvascular dysfunction, polymorphonuclear leukocyte (PMN) infiltration and gastric acid secretion during reperfusion may play a role in the pathogenesis of gastric mucosal injury induced by IR. Additionally, oxidative stress, due to free radicals and/or ROS, is known to cause organ injury and plays an important role during ischemia reperfusion injury in the gastrointestinal tract[1]. HIF-1α is a nuclear transcription factor and is critical for initiating cellular response to hypoxia. Many studies demonstrated that there was a very close relationship between the expression of HIF-1α and the formation of ROS in cancer and ischemia[19]. This is the first study to investigate the relationship between the formation of ROS and the expression of HIF-1α of gastric tissue following GI-RI. In this study, we investigated the oxidative stress and HIF-1α expression of gastric tissue resulting from LI-RI, and found that IpostC can reduce post-ischemic oxidative stress and HIF-1α expression of gastric tissue, and that ROS can modulate HIF-1α expression under gastric ischemic condition.
In the present study, ischemia-reperfusion (3 + 24 h) insult was sufficient to attain a considerable degree of gastric injury. IpostC prevented this deleterious effect. Some studies suggested that IpostC may reduce the post-ischemic oxidative damage through its antioxidant action[20]. MDA and XOD are regarded as indexes for mucosal injuries from ROS. Scarcity of MDA and low activity of XOD were detected in normal mucosa. MDA is an important product of lipid peroxidation that causes cell injury and death[21]. XOD exists in nonischemic tissue predominantly as xanthine dehydrogenase (XDH) and converts to oxygen radical-producing XOD with ischemia[22], which is derived from XDH and is capable of generating ROS. Oxygen radicals derived from XOD are important mediators of the cellular injury associated with reperfusion of ischemic intestine, stomach, liver, kidney, and pancreas. Our study showed that gastric MDA content and XOD activity were also significantly increased, whereas administration of IpostC reversed this change. These data indicated that IpostC against GI-RI may be related to the decreased lipid peroxidation caused by oxidative stress.
The primary ROS produced in aerobic organisms is superoxide, which is a highly reactive cytotoxic agent. Superoxide is converted to H2O2 by SOD. H2O2, in turn, is converted to water and molecular oxygen by either catalase (CAT) or glutathione peroxidase (GSH-Px). Accordingly, their deficiencies can cause oxidative stress. The overproduction of oxygen-derived free radicals (OFRs) during IRI brings about a consumption and depletion of these endogenous scavenging antioxidants. Concurrently, a member of endogenous antioxidant system SOD was found to be attenuated in I/R group, reflecting the over-production of OFRs. SOD is an enzyme that exists in cells removing oxyradicals, whose activity variation may represent the degree of tissue injury. In this study, compared with the I/R group, IpostC showed significantly increased antioxidase activities as well as the activities of SOD. MPO is an enzyme located mainly in the primary granules of neutrophils, thus tissue MPO levels may suggest neutrophil accumulation in the site of inflammation and generate reactive oxygen and nitrogen species and proteases[23,24]. Polymorphonuclear neutrophil infiltration is characteristic of acute inflammation and has the collective action of chemotactic mediators. Once neutrophils migrate into the ischemic area, they release ROS, proteases, elastase, MPO, cytokines, and various other mediators, all of which are involved in tissue injury. According to our findings, MPO activity, an index of tissue neutrophil infiltration, was increased by GI-RI, whereas IpostC inhibited neutrophil infiltration and protected the tissue against further injuries. These results indicated that the protective effects of IpostC against GI-RI may be related to the improvement in the endogenous antioxidant system and anti-inflammatory action.
It is apparent that gastric tissue is vulnerable to remote organs or tissue IRI. Some studies found that generation of ROS through xanthine oxidase, lipid peroxidation and Ca++-dyshomeostasis trigger secondary release of leukotriene (LTB4) and platelet activating factor (PAF) and promote PMN sequestration within stomach that initiates an amplification loop via further liberation of ROS (oxidative burst) and proteolytic enzymes[23,25]. In our study, MDA, SOD, XOD and MPO were used as indexes to mucosal injuries from ROS, and ROS was found to cause direct damage to cellular membranes as well as proteins and induce lipid peroxidation, leading to GI-RI that may be complicated by mucosal edema, microcirculation disturbance, epithelia hemorrhagic erosions and impaired function.
We also found that the expression of HIF-1α protein significantly increased in I/R group, and when ROS decreased, the expression of HIF-1α also decreased in IpostC group. Besides, hypoxia obviously induced the expression of HIF-1α in the gastric epithelial cells (GECs) and the vascular endothelial cells (VECs) of gastric tissue. Thus, we suggested that ROS might contribute to the initiation and progression of gastric ischemic injury, and oxidative stress resulting from gastric oxidative damage can induce the expression of HIF-1α in gastric ischemia induced by LI-RI.
HIF-1α is a nuclear transcription factor that mediates adaptive responses to hypoxia in mammalian cells. It is now recognized that HIF-1α is central to the regulation of genes involved in angiogenesis, vasomotor regulation and regulations of cell proliferation. HIF-1α plays a critical role in limb ischemia[26]. Some studies demonstrated that HIF-1α can stimulate neovascularization of vessels of ischemia tissue that respond to vascular endothelial growth factor (VEGF) and placental growth factor (PLGF)[27], and stimulate the recovery of blood flow in operative models of hindlimb ischemia[28]. ROS is generated from a number of sources including the mitochondrial electron transport system, xanthine oxidase, the cytochrome p450, the NADPH oxidase, uncoupled NOS and MPO, including superoxide radical (O2_), hydrogen peroxide (H2O2), and hydroxyl radical. They are closely related to the oxygen content in cells and extensive biologic activities. Exogenous ROS stimulate induction of VEGF by various cell types, and promote cell proliferation and migration, cytoskeletal reorganization and tubular morphogenesis in endothelia cells (ECs). Some studies demonstrated that ROS was also involved in physiological repair processes such as ischemia-induced angiogenesis and wound healing in vivo[29,30]. Therefore, ROS also play an important role in neovascularization during limb ischemia .
According to some studies, ROS can regulate the expression of HIF-1α via some pathways. Firstly, HIF-1α expression is regulated in terms of not only its stability but also its transcriptional and translational activities. The extracellular signal regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/Akt (protein kinase B, PKB) signaling pathways are involved in the transcription and translation of HIF-1α and can be activated by ROS. The PI3K/PKB signaling pathway phosphorylates the components that regulate translation, and provokes the accumulation of HIF-1α in response to growth factors, hormones, and cytokines[31]. Secondly, HIF-1α is a highly phosphorylated protein in vivo and this phosphorylation of HIF-1α induces strong changes in the HIF-1α’s migration pattern. Activation of the p42 and p44 mitogen-activated protein kinase (MAPK) pathway in quiescent cells induced the phosphorylation and shift of HIF-1α, which was abrogated in presence of the MEK inhibitor. This interaction between HIF-1α and p42/p44 MAPK suggests a cooperation between hypoxic and growth factor signals that ultimately leads to the increase in HIF-1-mediated gene expression[32]. Some studies suggested that ROS induced HIF-1α expression by decreasing the activity of prolyl hydroxylases (PHDs) in cancer and ischemia[19] at high concentrations, and ROS would upregulate HIF-1α independently and then PHD2 would be upregulated by ROS, leading to HIF-1αdownregulation[33]. So, ROS as a signaling molecule, can stimulate HIF-1α protein synthesis via activation of the PI3K/AKT and p42/p44MAPK pathways, and ROS may also have the potential to interfere with prolyl hydroxylase activity to regulate HIF-1α expression.
Some reports[34,35] found that a variety of non-hypoxic stimuli including growth factors, hormones, vasoactive peptides and metal ions can induce HIF-1α in normoxia, and many of these factors can stimulate ROS production as part of their signaling cascades. These indicate that ROS regulates HIF stability and transcriptional activity in well oxygenated cells, as well as under hypoxic conditions.
Our study showed that the expression of HIF-1α protein significantly increased in gastric ischemia, and when ROS was reduced, the expression of HIF-1α also decreased. It is shown that, at least in GECs and VECs, ROS may control the levels of HIF-1α expression and the proliferation of GECs and VECs through regulating HIF-1α expression. The effects of ROS on HIF-1α can be attributed to three factors: the degree of hypoxia, the form and intracellular location of ROS produced, and the molecular microenvironment of the cell.
In conclusion, our findings suggest that LI-RI can markedly induce oxidative stress and the expression of gastric tissue HIF-1α, and ROS controls the expression of HIF-1α probably as a signaling molecule and has the potential to interfere with prolyl hydroxylase activity under gastric ischemic condition. Thus, ROS plays an important role in the regulation of HIF-1α expression in gastric ischemia. The possible mechanisms of how ROS interacts with the HIF-1 pathway and alters HIF-1α expression might be related to the activation of the PI3K/AKT, p42/p44MAPK pathways. Further understanding of these mechanisms will be undoubtedly a major contribution to the studies in the pathogenesis of GI-RI.
The transcription factor hypoxia-induced factor (HIF) plays a critical role in the mammalian response to oxygen (O2) levels. HIF-1 transcriptionally activates hundreds of genes associated with angiogenesis in cancer, ischemia, as well as energy metabolism, nutrient transport, cell cycle, and cell migration. HIF-1αand HIF-1βmake up the HIF-1 heterodimer. The intracellular HIF-1αrapidly accumulates in the cell nucleus and triggers gene expression under hypoxia conditions. Concomitantly, the study of reactive oxygen species (ROS) and the interest in antioxidants as potential dietary supplements for prevention of cancer, cardiac dysfunction, and neurodegeneration has grown rapidly. Oxidant stress, due to free radicals and/or reactive oxygen species, is known to cause organ injury. Although, some studies have shown that increased HIF-1αexpression contributed to mitochondrial activity, and especially the ROS formation in hypoxia. However, other studies have demonstrated a decrease of HIF-1αwith increasing ROS, and related observations seem to be conflicting.
HIF-1α is a key determinant of oxygen-dependent gene regulation in angiogenesis. HIF-1α overexpression may be beneficial in cell therapy of hypoxia-induced pathophysiological processes, such as ischemic heart disease. Oxidative stress plays an important role in the pathogenesis of many clinical conditions involving cardiovascular diseases, liver diseases, lung disease, gastrointestinal disorders, neurological disorders, muscle damage, diabetes, aging and ischemia reperfusion (I/R). These clinical evidence focuses on the role of oxidant stress in the mechanism of I/R injury and the use of antioxidant agents for its treatment.
The authors used a rat model of hindlimb ischemic reperfusion to observe oxidative stress and the expression of HIF-1α in the gastric injury induced by limb ischemic reperfusion. This study demonstrated that limb ischemia reperfusion injury markedly induced oxidative damage resulting from ROS and the expression of HIF-1α of gastric tissue, and suggested that ROS controls the expression of HIF-1α under ischemic conditions.
HIF-1α overexpression may be beneficial in cell therapy of hypoxia-induced pathophysiological processes of many clinical conditions. The use of antioxidant agents for oxidant stress treatment in pathophysiological processes of many clinical diseases.
The authors examined oxidative stress and HIF-1α protein expression, and the relationship between ROS and HIF-1α in gastric ischemia induced by limb ischemia reperfusion. It revealed that limb ischemia reperfusion injury markedly induced oxidative damage and the expression of HIF-1α of gastric tissue, and ROS controls the expression of HIF-1α under ischemic conditions. The paper describes a very careful biochemical and histological documentation.
Peer reviewer: Dr. José Liberato Ferreira Caboclo, Professor, RuaAntônio de Godoy, 4120 ,São José do Rio Preto, Brazil
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Sasaki M, Joh T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J ClinBiochemNutr. 2007;40:1-12. |
| 2. | Dorweiler B, Pruefer D, Andrasi TB, Maksan SM, Schmiedt W, Neufang A and Vahl CF. Ischemia-reperfusion injury pathophysiology and clinical implications. Eur J Trauma EmergSurg. 2007;33:600-612. |
| 3. | Abela CB, Homer-Vanniasinkham S. Clinical implications of ischaemia-reperfusion injury. Pathophysiology. 2003;9:229-240. |
| 4. | Liu L, Ning X, Han S, Zhang H, Sun L, Shi Y, Sun S, Guo C, Yin F, Qiao T. [Hypoxia induced HIF-1 accumulation and VEGF expression in gastric epithelial mucosa cell: involvement of ERK1/2 and PI3K/Akt]. MolBiol(Mosk). 2008;42:459-469. |
| 5. | Laderoute KR. The interaction between HIF-1 and AP-1 transcription factors in response to low oxygen. Semin Cell DevBiol. 2005;16:502-513. |
| 6. | Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830-5835. |
| 7. | Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485-490. |
| 8. | Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9 Suppl 5:10-17. |
| 9. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. ProcNatlAcadSci USA. 1995;92:5510-5514. |
| 10. | Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401-408. |
| 11. | Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J BiolChem. 2000;275:25130-25138. |
| 12. | Pedowitz RA, Gershuni DH, Schmidt AH, Fridén J, Rydevik BL, Hargens AR. Muscle injury induced beneath and distal to a pneumatic tourniquet: a quantitative animal study of effects of tourniquet pressure and duration. J Hand Surg Am. 1991;16:610-621. |
| 13. | Souza Filho MV, Loiola RT, Rocha EL, Simão AF, Gomes AS, Souza MH, Ribeiro RA. Hind limb ischemic preconditioning induces an anti-inflammatory response by remote organs in rats. Braz J Med Biol Res. 2009;42:921-929. |
| 14. | Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart CircPhysiol. 2003;285:H579-H588. |
| 15. | Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151:1099-1103. |
| 16. | Zhang YM, Wei EQ, Li L, Qiao WL, Wang L, Zhang JF. Extracellular signal-regulated kinase pathways may mediate the protective effect of electrical stimulation of the paraventricular nucleus against ischaemia-reperfusion injury of the gastric mucosa. ClinExpPharmacolPhysiol. 2007;34:742-752. |
| 17. | Yassin MM, Harkin DW, Barros D‘Sa AA, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26:115-121. |
| 18. | Eliason JL, Wakefield TW. Metabolic consequences of acute limb ischemia and their clinical implications. SeminVascSurg. 2009;22:29-33. |
| 19. | Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol Cell Biol. 2008;28:5106-5119. |
| 20. | Gyurkovics E, Aranyi P, Stangl R, Onody P, Ferreira G, Lotz G, Kupcsulik P, Szijarto A. Postconditioning of the Lower Limb-Protection Against the Reperfusion Syndrome. J Surg Res. 2009;Epub ahead of print. |
| 21. | Gaweł S, Wardas M, Niedworok E, Wardas P. [Malondialdehyde (MDA) as a lipid peroxidation marker]. WiadLek. 2004;57:453-455. |
| 22. | Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol. 1988;254:G768-G774. |
| 23. | Naito Y, Yoshikawa T, Matsuyama K, Yagi N, Arai M, Nakamura Y, Kaneko T, Yoshida N, Kondo M. Neutrophils, lipid peroxidation, and nitric oxide in gastric reperfusion injury in rats. Free RadicBiol Med. 1998;24:494-502. |
| 24. | Yoshikawa T, Naito Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radic Res. 2000;33:785-794. |
| 25. | Hayashi N, Tsujii M, Itoh T, Sakura H, Tsuji S, Tanimura H, Ogihara T, Yoshihara H, Kawano S, Sato N. Changes in the intracellular calcium ion concentration in the gastric mucosa in a rat ischemia-reperfusion model. Scand J GastroenterolSuppl. 1989;162:43-46. |
| 26. | Patel TH, Kimura H, Weiss CR, Semenza GL, Hofmann LV. Constitutively active HIF-1alpha improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovasc Res. 2005;68:144-154. |
| 27. | Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074-1081. |
| 28. | Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C, Goldberg MA, Akita GY, Gregory RJ, Isner JM. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation. 2000;102:2255-2261. |
| 29. | Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409-419. |
| 30. | Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347-2355. |
| 31. | Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-732. |
| 32. | Richard DE, Berra E, Gothié E, Roux D, Pouysségur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J BiolChem. 1999;274:32631-32637. |
| 33. | Alon U. An Introduction to SystemsBiology. Chapman & Hall/CRC Press, Boca Raton FL, 2006. . |
| 34. | BelAiba RS, Djordjevic T, Bonello S, Flügel D, Hess J, Kietzmann T, Görlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. BiolChem. 2004;385:249-257. |
| 35. | Park JH, Kim TY, Jong HS, Kim TY, Chun YS, Park JW, Lee CT, Jung HC, Kim NK, Bang YJ. Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells. Clin Cancer Res. 2003;9:433-440. |