Published online Apr 14, 2011. doi: 10.3748/wjg.v17.i14.1889
Revised: September 25, 2010
Accepted: October 2, 2010
Published online: April 14, 2011
AIM: To identify the clinicopathological risk factors correlated with residual tumor in hepatocellular carcinoma (HCC) patients after resection.
METHODS: From January 2001 to April 2007, 766 HCC patients who had undergone resection were included in this research. Lipiodol angiography was performed within 2 mo after surgery and followed by post-Lipiodol computed tomography (CT) 4 wk later for all 766 patients to monitor tumor in the remnant liver. Tumor detected within the first 3-mo postoperative period was defined as residual tumor. Patients were divided into 2 groups: disease or disease-free within the first 3 mo after surgery. Risk factors for residual tumor were investigated among various clinicopathological variables.
RESULTS: A total of 63 (8.22%) patients were found to have residual tumor after surgery. Three independent factors associated with residual tumor were identified by multivariate analysis: preoperative serum α -fetoprotein (AFP) level [odds ratio (OR) = 1.68 (95% confidence interval (CI): 1.20-2.36)], tumor size [OR = 1.73 (95% CI: 1.29-2.31)] and microvascular invasion [OR = 1.91 (95% CI: 1.12-3.24)].
CONCLUSION: Residual tumor is related to AFP level, tumor size and microvascular invasion. Patients at high risk should undergo closer follow-up and could be candidates for multimodality therapy.
- Citation: Chen XH, Zhang BH, Xin Y, Ren ZG, Fan J, Qiu SJ, Zhou J. Risk factors for residual tumor after resection of hepatocellular carcinoma. World J Gastroenterol 2011; 17(14): 1889-1894
- URL: https://www.wjgnet.com/1007-9327/full/v17/i14/1889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i14.1889
Hepatocellular carcinoma (HCC) accounts for 80%-90% of primary liver cancer and is a global health problem[1,2]. About 55% of all HCC incidences are identified in China[1]. Surgical resection has a major role in the treatment for HCC and offers a chance of cure for patients[3,4]. Although resection is complete, there is still a possibility of early recurrence which occurs mainly because of pre-existing microscopic tumor foci that are undetected by imaging modalities before or during operation[5]. Tumor detected soon after resection is thought to be related to the presence of residual tumor, and it is associated with a poor prognosis.
Determination of factors predicting residual tumor may allow for identification of patients who are more likely to have this problem after so-called radical resection. In view of Lipiodol angiography with its post-Lipiodol computed tomography (CT) being regarded as the most sensitive means to confirm the presence of tumor, we identify in this study the risk factors correlated with residual tumor in HCC patients who received Lipiodol angiography within 2 mo after resection followed up by CT scan 4 wk later. Knowledge of these risk factors could be useful for clinicians to assess patient prognosis and determine treatment strategies, for the purpose of improving the long-term outcome.
From January 2001 to April 2007, pathologically confirmed HCC patients who underwent their first radical hepatic resection at the Liver Cancer Institute & Zhongshan Hospital of Fudan University were recruited for this study if they met the following entry criteria: (1) received no preoperative anticancer treatment; (2) age between 18-80 years; (3) liver function classified as Child Pugh Grade A or B; (4) recovered within 6 wk of the operation; and (5) general health satisfactory for toleration of the Lipiodol angiography within 2 mo after resection.
We defined radical resection in this study as: (1) complete removal of all tumor nodules and the cut surface being free of cancer by histological examination; (2) no cancerous thrombus found in the portal vein (main trunk or two major branches), hepatic veins, or bile duct by imaging and histological examination; (3) no demonstrable evidence of residual disease in the remnant on intraoperative ultrasonographic examination; (4) the number of tumor nodules not exceeding three[6]; and (5) no extrahepatic metastasis found.
There were 766 patients who satisfied the selection criteria. This study was approved by the Research Ethics Committee of Zhongshan Hospital.
Lipiodol angiography was performed within 2 mo after resection in all these 766 patients. The hepatic artery supplying the liver remnant was selectively catheterized via the femoral artery under fluoroscopic guidance. An approximately 3 mL suspension of iodized oil was injected into the hepatic artery. A contrast CT scan of the liver was performed an average of 4 wk later. If there was early local lesion, it would be shown as dense foci of Lipiodol uptake, or as enhancing nodule not present in the preoperative CT.
The injection of Lipiodol into the hepatic artery is effective for aiding in the diagnosis of tumor by post-Lipiodol CT and it can detect the lesion in its earliest period. In this study, patients received Lipiodol angiography within 2 mo after resection, followed up by CT scan 4 wk later to monitor tumor in the remnant liver; so, if there was no demonstrable evidence of lesion within the first 3 mo after resection, the surgery could be regarded as a truly curative resection. Therefore, residual tumor was defined as tumor detected within the first 3-mo postoperative period. We divided the 766 patients into two groups: Group 1 = disease detected within 3 mo after surgery; Group 2 = no disease detected within 3 mo after surgery.
Follow-up cutoff date was April 2008. All surviving patients had a minimum follow-up of 12 mo, 16 patients were lost during follow-up. The median follow-up period of all 766 patients was 26 mo (range: 3-87 mo). Statistically significant differences between categorical variables were examined using the Chi-square or Fisher’s exact test where appropriate. The logistic regression model was applied to evaluate the risk factors related to residual tumor. Diagnostic accuracy of predictive risk factors was evaluated using receiver operating characteristic (ROC) analysis. Survival analysis was studied by the Kaplan-Meier method with a log-rank test to detect the statistical difference. The statistical analysis was performed using Stata 7.0. P values < 0.05 were considered statistically significant.
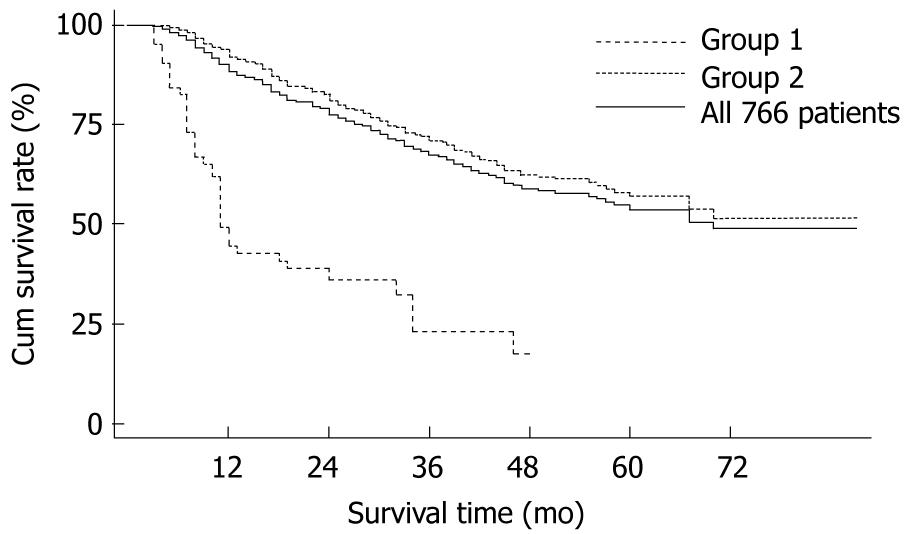
Tumor was detected in 314 (314/766, 40.99%) patients during the follow-up period. Of the 766 patients, 63 patients were found to have residual tumor (Group 1), whereas the other 703 patients showed no lesion detected within 3 mo after resection (Group 2). Respective cumulative survival rates at 1-, 2-, 3-, 4-, 5-years in Group 1 were 44.44%, 35.90%, 23.08%, 17.31% and 0.00% compared with 92.18%, 80.99%, 71.08%, 62.48% and 56.95% in Group 2 (P < 0.0001 ) (Figure 1).
The distribution of selected clinical and pathological characteristics between Group 1 and Group 2 is shown in Table 1. No significant differences were observed regarding sex, age, HBsAg-positive rate, cirrhotic nodules, liver function status, serum alanine aminotransferase (ALT) level, tumor number, cell differentiation grade and the percentage of incomplete/absent capsule. However, serum gamma-glutamyl transferase (GGT) level (χ2 = 4.6062, P = 0.032) and α-fetoprotein (AFP) level (χ2 = 16.0745, P < 0.0001) were statistically different between the two groups. The ratio of large tumor size in Group 1 was significantly higher than that in Group 2 (χ2 = 23.3257, P < 0.0001), and there was a significantly higher incidence of microvascular invasion (χ2 = 9.7556, P = 0.002) noted in Group 1 compared with that in Group 2. As for tumor staging, there was a significantly poorer degree of pTNM staging (χ2 = 15.1735, P = 0.001) in Group 1; BCLC classification also showed a trend towards significance (χ2 = 3.4501, P = 0.063) between the two groups.
| Characteristics | Group 1 | Group 2 | χ2 | P-value | |
| Sex | Female | 11 (17.46) | 96 (13.66) | 0.6964 | > 0.05 |
| Male | 52 (82.54) | 607 (86.34) | |||
| Age (yrs) | ≤ 30 | 3 (4.76) | 17 (2.42) | 1.2490 | > 0.05 |
| 30-60 | 48 (76.19) | 549 (78.09) | |||
| > 60 | 12 (19.05) | 137 (19.49) | |||
| Child-Pugh score | Class A | 62 (98.41) | 692 (98.44) | 0.0002 | > 0.05 |
| Class B | 1 (1.59) | 11 (1.56) | |||
| Cirrhotic nodule | No | 7 (11.11) | 121 (17.21) | 1.5462 | > 0.05 |
| Yes | 56 (88.89) | 582 (82.79) | |||
| HBsAg | Negative | 8 (12.70) | 90 (12.80) | 0.0006 | > 0.05 |
| Positive | 55 (87.30) | 613 (87.20) | |||
| ALT level1, U/L | ≤ 80 | 57 (90.48) | 621 (88.34) | 0.2605 | > 0.05 |
| > 80 | 6 (9.52) | 82 (11.66) | |||
| GGT level1, U/L | ≤ 60 | 24 (38.10) | 367 (52.20) | 4.6062 | < 0.05 |
| > 60 | 39 (61.90) | 336 (47.80) | |||
| AFP level, ng/mL | ≤ 20 | 8 (12.70) | 259 (36.84) | 16.0745 | < 0.01 |
| 20-400 | 20 (31.75) | 192 (27.31) | |||
| ≥ 400 | 35 (55.56) | 252 (35.85) | |||
| Tumor size2, cm | ≤ 2 | 3 (4.76) | 82 (11.66) | 23.3257 | < 0.01 |
| 2-5 | 19 (30.16) | 333 (47.37) | |||
| 5-8 | 15 (23.81) | 166 (23.61) | |||
| > 8 | 26 (41.27) | 122 (17.35) | |||
| Capsule | complete/present | 31 (49.21) | 390 (55.48) | 0.9183 | > 0.05 |
| incomplete/absent | 32 (50.79) | 313 (44.52) | |||
| Microvascular invasion | No | 30 (47.62) | 472 (67.14) | 9.7556 | < 0.01 |
| Yes | 33 (52.38) | 231 (32.86) | |||
| Tumor number | solitary nodule | 49 (77.78) | 597 (84.92) | 3.8413 | > 0.05 |
| 2 nodules | 13 (20.63) | 86 (12.23) | |||
| 3 nodules | 1 (1.59) | 20 (2.84) | |||
| Cell differentiation grade | GradeI-II | 41 (65.08) | 506 (71.98) | 1.3475 | > 0.05 |
| Grade III-IV | 22 (34.92) | 197 (28.02) | |||
| pTNM staging | Stage I | 21 (33.33) | 409 (58.18) | 15.1735 | < 0.01 |
| Stage II | 34 (53.97) | 250 (35.56) | |||
| Stage III A | 8 (12.70) | 44 (6.26) | |||
| BCLC classification | Stage A | 49 (77.78) | 607 (86.34) | 3.4501 | > 0.05 |
| Stage B | 14 (22.22) | 96 (13.66) | |||
A multivariate stepwise logistic regression model was constructed to predict risk factors for residual tumor including sex, age, HBsAg, cirrhosis nodules, Child-Pugh class, ALT, GGT and AFP levels, tumor size (diameter of the largest nodule was used as tumor size when multiple), tumor number, capsule, microvascular invasion, and cell differentiation grade. Only serum AFP level [odds ratio (OR) = 1.68 (95% confidence interval (CI): 1.20-2.36)], tumor size [OR = 1.73 (95% CI: 1.29-2.31)] and microvascular invasion [OR = 1.91 (95% CI: 1.12-3.24)] were revealed as independently predictive factors for residual tumor (Table 2).
| Risk factor | Coefficient | OR (95% CI) | P-value | |
| AFP level (ng/mL) | ≤ 20 = 0 | 0.52 | 1.68 (1.20-2.36) | < 0.01 |
| 20-400 = 1 | ||||
| > 400 = 2 | ||||
| Tumor size (cm) | ≤ 2 = 0 | 0.55 | 1.73 (1.29-2.31) | < 0.01 |
| 2-5 = 1 | ||||
| 5-8 = 2 | ||||
| > 8 = 3 | ||||
| Microvascular invasion | No = 0 | 0.65 | 1.91 (1.12-3.24) | 0.02 |
| Yes = 1 | ||||
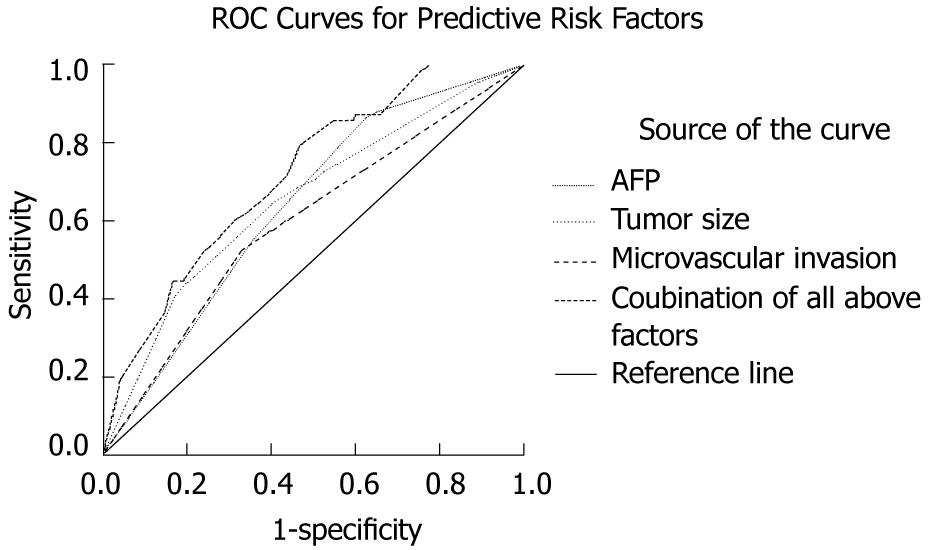
Analysis of the prevalence of these three parameters among the entire population of 766 patients showed that the incidence of residual tumor was very low (nil, 0/24) when none of these three factors was present, and the simultaneous presence of the risk factors increased the probability of residual tumor: this rate increased to 28.15% (9/32) when AFP > 400 ng/mL, tumor size > 8 cm and the presence of microvascular invasion were present at the same time. ROC curves for the combination of these three risk factors showed an area under the curve (AUC) of 0.717 (95% CI: 0.657-0.778) to predict residual tumor. ROC curves for serum AFP level had an AUC of 0.640 (95% CI: 0.575-0.704), for tumor size had an AUC of 0.655 (95% CI: 0.582-0.728), and for microvascular invasion had an AUC of 0.598 (95% CI: 0.523-0.672). Significant difference was observed among these four curves (P < 0.0001) (Figure 2).
The distinction between recurrence after a radical resection and residual tumor after a palliative resection is crucial[8]. Tumor detected soon after resection is thought to be related to the presence of residual tumor. Residual tumor cells in the remnant liver can acquire more malignant characteristics, and may accelerate tumor progression and induce intrahepatic metastasis due to the enhanced growth and increased neovascularization after surgery[9]. The shorter is the disease-free interval time; the poorer is the prognosis[10]. In this study, we showed that postoperative patients with residual tumor carried a significantly poorer prognosis than those who did not (Figure 1).
Complete surgical excision is an important factor for long-term outcome. However, it is difficult to remove all tumor cells in most patients[11]. Even with so-called radical resection, there is still a possibility of residual tumor with a rate of up to 37.6%[12]. The curative operation for HCC is difficult to define and the presence of residual tumor influences the evaluation of radical resection for HCC. Determination of predictive factors for residual tumor can help the selection of patients suitable for more aggressive therapy to supplement surgery.
With regard to the definition of residual tumor, there is no consensus on this definition in the literature. Some authors have suggested the demonstration of intrahepatic disease found by imaging studies (ultrasonography, angiography, and post-Lipiodol CT) within one or two months after resection as residual tumor[10]. In this study, positive finding by angiography followed by Lipiodol-CT within the first 3-mo postoperative period was defined as residual tumor. We then investigated the risk factors for residual tumor among various pathologic and clinical factors.
Results suggested that only high preoperative level of serum AFP, large tumor size and the presence of microvascular invasion were significantly associated with residual tumor at the univariate and multivariate analysis. When none of these three factors was present, the incidence of residual tumor was nil and increased up to 28.15% when AFP > 400 ng/mL, tumor size > 8 cm and the presence of microvascular invasion were present in the same patient. ROC analysis suggested that combination of these three factors was more sensitive to predict residual tumor than any single factor.
HCC is characterized by its propensity for vascular invasion. Numerous previous studies have demonstrated that the presence of microvascular invasion is the risk factor for early tumor occurrence after resection of HCC[13-16]. In addition, tumor size, especially > 5 cm, also predicts a high risk of tumor recurrence after resection[15,17,18]. Large tumor size is always correlated with increased invasiveness, as demonstrated by a higher incidence of intrahepatic metastasis and portal venous invasion[13,15,17,19,20]. The larger the tumor is, the earlier the lesion occurs[21].
As for preoperative serum AFP level, Hanazaki et al[22] reported that AFP ≥ 1000 ng/mL was an independently significant factor for poor disease-free survival; Imamura et al[23] identified serum AFP level > 32 ng/mL as a factor for early (< 2 years) recurrence; and Furihata et al[24] also suggested that patients with AFP/volume > 20.0 were likely to experience recurrence within 6 mo after radical hepatectomy. Serum AFP level may represent a marker for either tumor bulk or aggressive tumor biology, such as tumor cell proliferation and spread[25]. An HCC patient with a high serum AFP concentration (≥ 400 ng/mL) tends to have greater tumor size, bilobar involvement, massive or diffuse type of recurrence, portal vein thrombosis[26,27]. All of these may be due to be the ability of AFP to elicit the escape of carcinoma cells from the host’s lymphocyte immune surveillance[28,29]. However, there are several studies which have shown no relation between serum AFP level and recurrence[30,31]. In this present study, we support the concept of elevated serum AFP level as a candidate for early tumor occurrence[7].
Here, our study showed tumor number was not a predictive factor for residual tumor. This might be because, in this study, we defined radical resection as the number of tumor nodules not exceeding three (since multiple tumors may be the sign of intrahepatic metastasis[16]). Accordingly, radical resection could be achieved as long as tumor number was no more than three.
High preoperative level of serum AFP, large tumor size or the presence of microvascular invasion may indicate an increased biological aggressiveness of tumor and a greater possibility of systemic diffusion. The simultaneous presence of these factors increases the risk of residual tumor, and patients presenting these three risk factors in association are prone to have residual tumor. This information is beneficial for better clinical decision-making and future trial design. Some aggressive therapies can be tested in selected patients, such as utilizing neoadjuvant or adjuvant therapy (including hepatic artery chemotherapy or chemoembolization, immunotherapy, targeted therapy and differentiation therapy, etc.) to supplement surgery for a better chance of a cure or at least a longer survival. Patients with high risk factors for residual tumor should be monitored very carefully for early detection, and the surveillance interval also needs to be shortened to have a chance to eradicate the residual tumor at its earlier period, because long-term survival after recurrence is still possible if appropriated therapy is adopted[32].
One limitation in the present study is the low discriminative power of the identified predictive factors (with the specificity < 75%). In other words, even if patients have all these three risk factors, many are unlikely to have residual tumor after surgery. To resolve these issues, a more discriminatory method is required, such as molecular analysis. In addition, in our hospital, Lipiodol angiography is usually recommended to each postoperative patient for the purpose of early detection of residual tumor. A patient can receive this examination as long as his general condition allows. It may be that there is a bias in patient selection, but considering the large sample size in this study and the eligible patients presenting various clinicopathologic characteristics, this bias should be reduced. Certainly, further studies to confirm the present results are still needed.
In conclusion, this study showed that factors reflecting tumor behavior were correlated with residual tumor. High preoperative serum AFP level, large tumor size and the presence of microvascular invasion were independent predictors associated with an increased risk of residual tumor detected by angiography followed by Lipiodol-CT. This finding may have clinical implications in determining rational strategies in surveillance, prevention and management of postoperative residual tumor.
Even with so-called radical resection for hepatocellular carcinoma (HCC), there is still a possibility of residual tumor. Determination of factors predicting residual tumor can help the selection of patients suitable for more aggressive therapy to supplement surgery. However, the definition of residual tumor and its prognostic factors have not been fully determined.
Complete surgical excision offers a chance of cure to HCC patients, while recurrence is common and is the main cause of death. A large number of studies have investigated the prognostic risk factors for recurrence appearing at different times.
Lipiodol angiography followed by post-lipiodol computed tomography (CT) is the most sensitive means to confirm the presence of tumor. In this study, high preoperative serum α-fetoprotein (AFP) level, large tumor size and the presence of microvascular invasion were independent predictors associated with an increased risk of residual tumor detected by angiography followed by Lipiodol-CT. The simultaneous presence of these factors increases the risk of residual tumor.
Patients with high risk factors for residual tumor should be monitored very carefully for early detection, and the surveillance interval also needs to be shortened to have a chance to eradicate the residual tumor at its earlier period.
Lipiodol angiography is an imaging technique for HCC using the injection of Lipiodol into the hepatic artery. Lipiodol-CT, which involves computed tomography after intrahepatic arterial injection of Lipiodol, is regarded as the most sensitive imaging modality for HCC.
This is a large experience with HCC treated with resection, with careful follow-up using sensitive imaging studies. Recurrence within the first 3 mo was appropriately considered as residual tumor. The three independent factors associated with residual tumor were AFP level, tumor size, and microscopic invasion.
Peer reviewer: Emmet B Keeffe, MD, Professor, Chief of Hepatology, Medical Director, Liver Transplant Program, Program Director, Gastroenterology Fellowship, Stanford University Medical Center, 750 Welch Road, Suite 210, Palo Alto, CA 94304, United States
S- Editor Sun H L- Editor Logan S E- Editor Ma WH
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 2. | Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143-154. |
| 3. | Bruix J, Hessheimer AJ, Forner A, Boix L, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene. 2006;25:3848-3856. |
| 4. | Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, Lau WY, Wu MC. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195-202. |
| 5. | Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376-381. |
| 6. | Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH, Wang L, Ren N, Zhuang PY, Zhu XD, Fan J. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol. 2007;47:684-690. |
| 7. | Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44-50. |
| 8. | Lai EC, Lo CM, Fan ST, Liu CL, Wong J. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998;133:183-188. |
| 9. | Rupertus K, Kollmar O, Scheuer C, Junker B, Menger MD, Schilling MK. Major but not minor hepatectomy accelerates engraftment of extrahepatic tumor cells. Clin Exp Metastasis. 2007;24:39-48. |
| 10. | Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229-235. |
| 11. | Li JQ, Zhang YQ, Zhang WZ, Yuan YF, Li GH. Randomized study of chemoembolization as an adjuvant therapy for primary liver carcinoma after hepatectomy. J Cancer Res Clin Oncol. 1995;121:364-366. |
| 12. | Lin Z, Ren Z, Xia J. [Appraisal of postoperative transcatheter arterial chemoembolization (TACE) for prevention and treatment of hepatocellular carcinoma recurrence]. Zhonghua Zhongliu Zazhi. 2000;22:315-317. |
| 13. | Kosuge T, Makuuchi M, Takayama T, Yamamoto J, Shimada K, Yamasaki S. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepatogastroenterology. 1993;40:328-332. |
| 14. | Vauthey JN, Klimstra D, Franceschi D, Tao Y, Fortner J, Blumgart L, Brennan M. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28-34; discussion 34-35. |
| 15. | Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71-76. |
| 16. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219-1222. |
| 17. | Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227:424-432. |
| 18. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. |
| 19. | Di Carlo V, Ferrari G, Castoldi R, Nadalin S, Marenghi C, Molteni B, Taccagni G, Castrucci M. Surgical treatment and prognostic variables of hepatocellular carcinoma in 122 cirrhotics. Hepatogastroenterology. 1995;42:222-229. |
| 20. | Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022-2031. |
| 21. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. |
| 22. | Hanazaki K, Kajikawa S, Koide N, Adachi W, Amano J. Prognostic factors after hepatic resection for hepatocellular carcinoma with hepatitis C viral infection: univariate and multivariate analysis. Am J Gastroenterol. 2001;96:1243-1250. |
| 23. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. |
| 24. | Furihata T, Sawada T, Kita J, Iso Y, Kato M, Rokkaku K, Shimoda M, Kubota K. Serum alpha-fetoprotein level per tumor volume reflects prognosis in patients with hepatocellular carcinoma after curative hepatectomy. Hepatogastroenterology. 2008;55:1705-1709. |
| 25. | Okuwaki Y, Nakazawa T, Shibuya A, Ono K, Hidaka H, Watanabe M, Kokubu S, Saigenji K. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: risk factors and patterns. J Gastroenterol. 2008;43:71-78. |
| 26. | Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302-308. |
| 27. | Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic study on the expressions of alpha-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128-1134. |
| 28. | Iida H, Honda M, Kawai HF, Yamashita T, Shirota Y, Wang BC, Miao H, Kaneko S. Ephrin-A1 expression contributes to the malignant characteristics of {alpha}-fetoprotein producing hepatocellular carcinoma. Gut. 2005;54:843-851. |
| 29. | Li MS, Ma QL, Chen Q, Liu XH, Li PF, Du GG, Li G. Alpha-fetoprotein triggers hepatoma cells escaping from immune surveillance through altering the expression of Fas/FasL and tumor necrosis factor related apoptosis-inducing ligand and its receptor of lymphocytes and liver cancer cells. World J Gastroenterol. 2005;11:2564-2569. |
| 30. | Ju MJ, Qiu SJ, Fan J, Zhou J, Gao Q, Cai MY, Li YW, Tang ZY. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for Child-Pugh A hepatocellular carcinoma after operation. J Gastroenterol. 2009;44:635-642. |