Published online Apr 14, 2011. doi: 10.3748/wjg.v17.i14.1866
Revised: December 17, 2010
Accepted: December 24, 2010
Published online: April 14, 2011
AIM: To investigate the role of glucose transporter 1 (GLUT1) expression in colorectal carcinogenesis and evaluate the correlation with clinicopathological parameters and apoptosis-activating factor-1 (Apaf-1) expression in colorectal adenocarcinomas.
METHODS: We used tissue microarrays consisting of 26 normal mucosa, 50 adenomas, 515 adenocarcinomas, and 127 metastatic lesions. Medical records were reviewed and clinicopathological analysis was performed.
RESULTS: GLUT1 expression was absent in normal mucosa and low or moderately apparent in 19 cases (38.0%) of 50 adenomas. However, GLUT1 expression was detected in 423 (82.1%) of 515 adenocarcinomas and in 96 (75.6%) of 127 metastatic lesions. GLUT1 expression was significantly correlated with female gender (P = 0.009), non-mucinous tumor type (P = 0.045), poorer differentiation (P = 0.001), lymph node metastasis (P < 0.001), higher AJCC and Dukes stage (P < 0.001 and P < 0.001, respectively). There was a significant inverse correlation between GLUT1 expression and Apaf-1 expression (P = 0.001). In univariate survival analysis, patients with GLUT1 expression demonstrated poor overall survival and disease-free survival (P = 0.047 and P = 0.021, respectively, log-rank test).
CONCLUSION: GLUT1 expression was frequently increased in adenocarcinomas and metastatic lesions. GLUT1 expression was significantly correlated with poorer clinicopathologic phenotypes and survival of patients with colorectal adenocarcinomas.
- Citation: Jun YJ, Jang SM, Han HL, Lee KH, Jang KS, Paik SS. Clinicopathologic significance of GLUT1 expression and its correlation with Apaf-1 in colorectal adenocarcinomas. World J Gastroenterol 2011; 17(14): 1866-1873
- URL: https://www.wjgnet.com/1007-9327/full/v17/i14/1866.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i14.1866
Colorectal cancer is the second leading cause of cancer-related death in men and women in the industrialized nations[1,2]. There have been marked advances in the understanding of the carcinogenesis in colorectal cancer and cancer biology; however, the specific therapeutic problem continues to persist[3]. Most patients with colorectal malignancy, except for the advanced stage, undergo curative resection. Stage II patients with obstruction, perforation or certain tumor markers, and stage III patients, receive adjuvant chemotherapy after surgical resection[4]. However, there is no appropriate targeted therapy to further improve the clinical outcome. The molecular prognostic factors associated with a distinct prognostic outcome would be of great help for patients who are likely to benefit from adjuvant therapies, leading to an improvement in prognosis[5].
The transition from normality to malignancy through the adenoma-carcinoma carcinogenesis sequence is accompanied by various alterations in the expression of a number of genes associated with the maintenance of cellular homeostasis[6]. Previous studies have revealed an enhancement of glycolytic metabolism in malignant tumors. Increased glucose uptake and use is one of the major characteristics found in many malignant tumors. This process is mediated by the glucose transporters (GLUTs) which are membrane proteins responsible for the transport of glucose across cellular membranes[7].
Among seven cloned glucose transporters, GLUT1 is an isoform that is expressed in erythrocytes, the blood-tissue barriers such as the blood-brain and blood-nerve barriers, and the placenta[8]. GLUT1 protein expression can be altered by a number of different conditions, including cellular differentiation and transformation, and also can be altered under the influence of growth factors, insulin, glucose, and even stress[9]. The increased expression of GLUT1 mRNA and protein has been demonstrated in various cancer tissues which indicates that GLUT1 may play an important role in glucose uptake by various cancers and that GLUT1 expression could be useful as a marker for malignant transformation[10-15].
In this study, we immunohistochemically determined the GLUT1 expression in a large series of 26 normal mucosa, 50 tubular adenomas, 515 adenocarcinomas, and 127 metastatic lesions. This was to investigate the membranous GLUT1 expression in colorectal carcinogenesis and to evaluate the correlation between GLUT1 expression and the clinicopathological parameters and between GLUT1 expression and cytoplasmic apoptosis-activating factor-1 (Apaf-1) expression, as well as its effect on survival of patients with colorectal adenocarcinomas.
Our study enrolled a consecutive series of 515 patients with colorectal adenocarcinoma. All patients were diagnosed and treated at the Hanyang University Hospital (Seoul, Korea) between January 1991 and August 2001. There were 293 male and 222 female patients. The mean age of patients was 58 years. The tumor growth pattern was fungating in 239 cases and infiltrative in 276 cases. The tumors consisted of 489 non-mucinous adenocarcinomas and 26 mucinous adenocarcinomas. The tumors were located in the cecum (n = 18), ascending colon (n = 75), hepatic flexure (n = 12), transverse colon (n = 25), splenic flexure (n = 4), descending colon (n = 25), sigmoid colon (n = 107), and rectum (n = 249). The mean tumor size was 5.7 cm. The mean follow-up interval was 6.0 years. One hundred and eighty (35%) patients died and 335 (65%) patients survived. Twenty-six cases of normal mucosa, 50 cases of tubular adenomas, 127 metastatic lesions (lymph nodes and distant organs) were randomly selected to evaluate the role of GLUT1 in the multistep carcinogenesis.
Tissue microarrays were constructed from archival formalin-fixed, paraffin-embedded tissue blocks using a manual tissue arrayer (Quick-Ray Manual Tissue Microarrayer, Unitma Co, Ltd, Seoul, Korea). As described previously[16], for each sample, areas rich in tumor cells were identified by light microscopic examination of hematoxylin-eosin-stained sections and then selected for use in tissue microarrays. Tissue cylinders with a diameter of 2 mm were punched from the previously marked tumor area of each block (donor block) and then transferred to a recipient paraffin block. This resulted in a 6 × 10 array for 60 cases.
For immunohistochemical staining, multiple 4 μm sections were cut with a Leica microtome. Sections were transferred to adhesive-coated slides. Tissue microarray (TMA) slides were dewaxed by heating at 55°C for 30 min and by three washes, 5 min each, with xylene. Tissues were rehydrated by a series of 5 min washes in 100%, 90% and 70% ethanol and phosphate buffered saline (PBS). Antigen retrieval was performed by microwaving the samples for 4 min 20 s at full power in 250 mL of 10 mmol/L sodium citrate (pH 6.0). Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxidase for 20 min. The primary mouse monoclonal GLUT1 antibody (ab40084, Abcam, Cambridge, UK) was diluted 1:100 using goat serum and the primary polyclonal rabbit Apaf-1 antibody (Novocastra Laboratories, Newcastle upon Tyne, UK) was diluted 1: 200 using goat serum and incubated at room temperature for 1 h. After three washes, 2 min each with PBS, the sections were incubated with a biotinylated goat anti-mouse secondary antibody for 30 min (DAKO, Carpinteria, CA, USA). After three washes, 2 min each with PBS, horseradish peroxidase-streptoavidin (DAKO, Carpinteria, CA, USA) was added to the sections for 30 min, followed by another three washes, 2 min each with PBS. The samples were developed with 3,3’-diaminobenzidine substrate (Vector Laboratories, Burlington, Ontario, Canada) for 1 min and counterstained with Mayer’s hematoxylin. Then, the slides were dehydrated following a standard procedure and sealed with coverslips. Negative controls were performed by omitting the GLUT1 and Apaf-1 antibodies during the primary antibody incubation.
The GLUT1 and Apaf-1 expression was evaluated semi-quantitatively by two independent pathologists (Jang SM and Paik SS) without prior knowledge of the clinical follow-up data for each case. The GLUT1 immunostaining was semi-quantitated by grading the proportion of cells that were GLUT1-positive, as described previously[7,11]; grade 0: negative (positive cells are 0%), grade 1: low positive (positive cells are less than 10%), grade 2: moderate positive (positive cells are 10%-50%) and grade 3: high positive (positive cells are more than 50%). For purposes of statistical analysis, a cut-off value of 50% was adopted. Each tissue section was classified as either < 50% or > 50% GLUT1-positive. Apaf-1 expression was evaluated based on the staining intensity and staining extent, as described previously[17]. Staining intensity for Apaf-1 was scored as 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). Staining extent was scored as 0 (0%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%) and 4 (76%-100%) according to the percentage of positive-stained cells. The sum of intensity and extent scores was used as the final staining score. All cases were divided into four expression groups according to their sum scores which were as follows: 0 = negative; 1-3 = weak; 4-5 = moderate; and 6-7 = strong. If the sum scores were moderate or strong, cases were classified as Apaf-1-positive. If the sum scores were negative or weak, cases were classified as Apaf-1-negative. In cases of discrepant assessments, slides were reinvestigated by both pathologists under a multi-head microscope and an agreement was obtained.
Statistical analysis was performed using SPSS software (version 12.0, SPSS, Chicago, IL, USA). The chi-square test for linear trend, Fisher exact test, and one-way ANOVA test were used to examine the association between the GLUT1 expression and clinicopathological parameters including age, gender, tumor location, tumor size, tumor gross, tumor type, differentiation, TNM category, AJCC stage, Dukes stage, and Apaf-1 expression. The Kaplan-Meier method was used to calculate overall survival and disease-free survival curves. Univariate survival analysis with the log-rank test was used to compare the difference between the survival rates of the patient subgroups. Multivariate survival analysis with the Cox proportional hazards regression model was used to evaluate the independent prognostic factors. A difference of P < 0.05 between groups was considered significant.
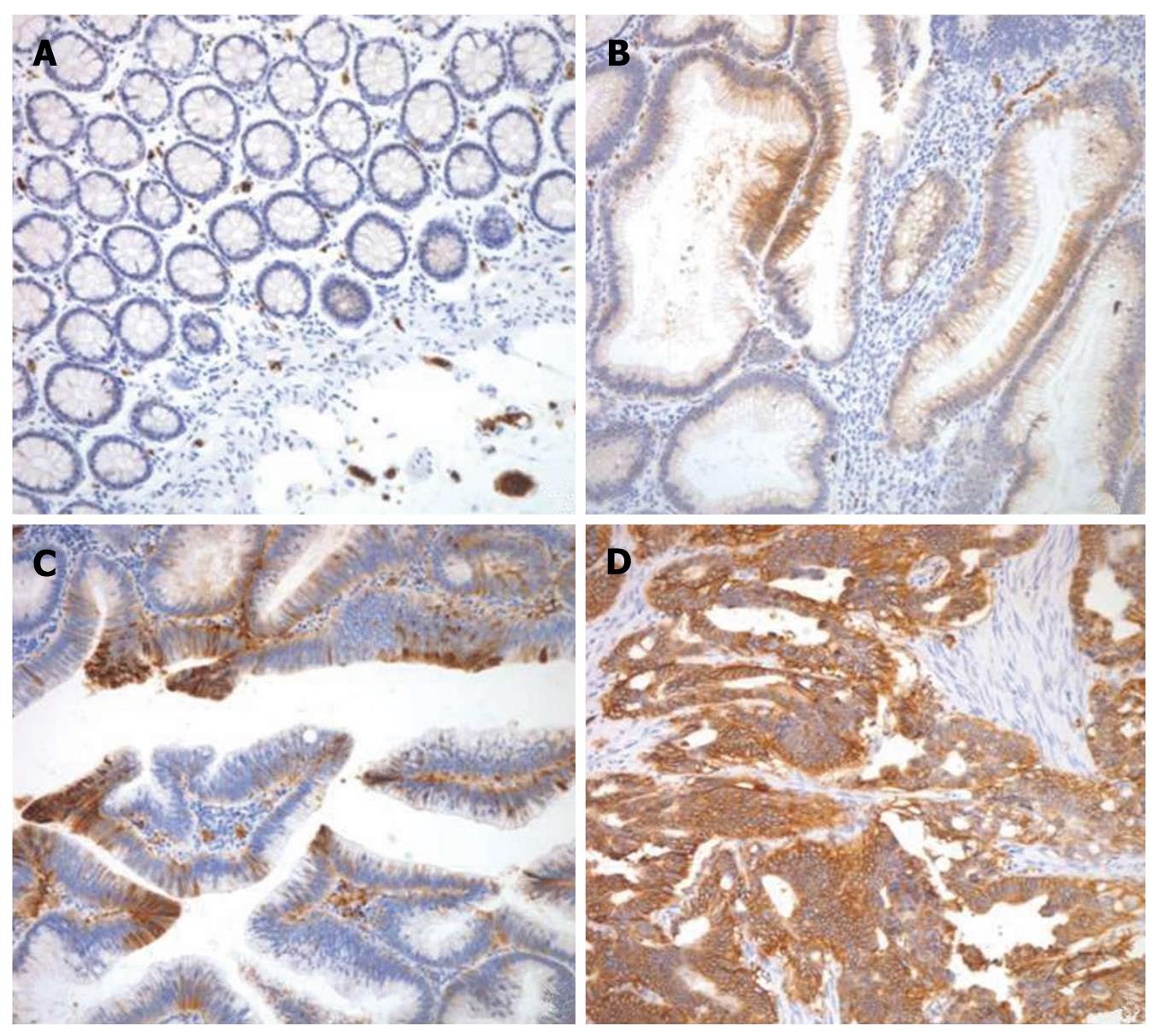
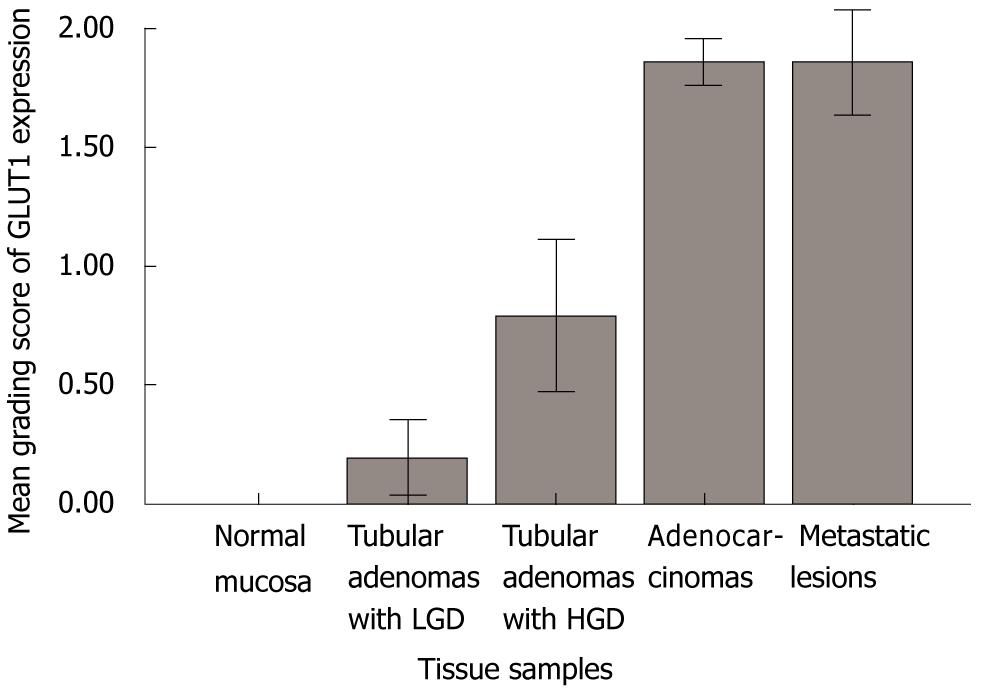
The GLUT1 expression was evaluated in 26 normal mucosa, 50 tubular adenomas, 515 adenocarcinomas, and 127 metastatic lesions. As expected, erythrocyte membranes were strongly GLUT1-positive. Various grades of membranous GLUT1 expression were observed in the included tissue samples. The representative photomicrographs of GLUT1 immunostaining are shown in Figure 1. All 26 normal mucosa specimens (100%) were negative for GLUT1 expression with no exception. Twenty-one (80.8%) of 26 tubular adenomas with low grade dysplasia were negative for GLUT1 expression and only 5 cases (19.2%) revealed a low grade of GLUT1 expression. Ten (41.7%) of 24 tubular adenomas with high grade dysplasia were negative for GLUT1 expression, 9 cases (37.5%) revealed a low grade of GLUT1 expression, and 5 cases (20.8%) showed a moderate grade of GLUT1 expression. However, in colorectal adenocarcinomas, 187 (36.3%) of 515 cases revealed a high grade of GLUT1 expression and 161 cases (31.2%) and 75 cases (14.6%) showed moderate grade and low grade of GLUT1 expression, respectively. Only 92 (17.9%) of 515 cases were negative for GLUT1 expression. In metastatic lesions, 59 (46.5%) of 127 cases showed high grade of GLUT1 expression and 22 cases (17.3%) and 15 cases (11.8%) showed moderate grade and low grade of GLUT1 expression, respectively. Thirty-one cases (24.4%) were negative for GLUT1 expression. A significant difference in the GLUT1 expression among normal mucosa, tubular adenomas, adenocarcinomas, and metastatic lesions was observed (Table 1 and Figure 2).
| Tissue samples | n | Expression of GLUT1 | P value | |||
| Negative (%) | Low (%) | Moderate (%) | High (%) | (χ2-test) | ||
| Normal mucosa | 26 | 26 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | < 0.0011 |
| Tubular adenomas with LGD | 26 | 21 (80.8) | 5 (19.2) | 0 (0.0) | 0 (0.0) | |
| Tubular adenomas with HGD | 24 | 10 (41.7) | 9 (37.5) | 5 (20.8) | 0 (0.0) | |
| Adenocarcinomas | 515 | 92 (17.9) | 75 (14.6) | 161 (31.2) | 187 (36.3) | |
| Metastatic lesions | 127 | 31 (24.4) | 15 (11.8) | 22 (17.3) | 59 (46.5) | |
To assess the clinicopathologic significance of GLUT1 expression, we evaluated the correlation between GLUT1 expression and the clinicopathologic parameters in 515 colorectal adenocarcinomas. We found that a higher expression of GLUT1 correlated with more aggressive phenotypes of colorectal adenocarcinomas. GLUT1 expression was significantly correlated with female gender (P = 0.009), non-mucinous tumor type (P = 0.045), poorer differentiation (P = 0.001), frequent lymph node metastasis (P < 0.001), higher AJCC and Dukes stage (P < 0.001 and P < 0.001, respectively). There was a significant inverse correlation between GLUT1 expression and Apaf-1 expression (P = 0.001) (Table 2).
| Factors | n | Expression of GLUT1 | ||
| < 50% (%) | ≥ 50% (%) | P value | ||
| (n = 328) | (n = 187) | |||
| Age (yr) | 0.0691 | |||
| < 58 | 244 | 164 (67.2) | 80 (32.8) | |
| ≥ 58 | 271 | 164 (60.5) | 107 (39.5) | |
| Gender | 0.0091 | |||
| Male | 293 | 200 (68.3) | 93 (31.7) | |
| Female | 222 | 128 (57.7) | 94 (42.3) | |
| Tumor location | 0.5061 | |||
| Colon | 266 | 169 (63.5) | 97 (36.5) | |
| Rectum | 249 | 159 (63.9) | 90 (36.1) | |
| Tumor size (cm) | 0.5071 | |||
| < 5.7 | 282 | 180 (63.8) | 102 (36.2) | |
| ≥ 5.7 | 233 | 148 (63.5) | 85 (36.5) | |
| Tumor gross | 0.2161 | |||
| Fungating | 239 | 157 (65.7) | 82 (34.3) | |
| Infiltrative | 276 | 171 (62.0) | 105 (38.0) | |
| Tumor type | 0.0451 | |||
| Non-mucinous | 489 | 307 (62.8) | 182 (37.2) | |
| Mucinous | 26 | 21 (80.8) | 5 (19.2) | |
| Differentiation | 0.0012 | |||
| Well | 21 | 19 (90.5) | 2 (9.5) | |
| Moderate | 386 | 252 (65.3) | 134 (34.7) | |
| Poor | 108 | 57 (52.8) | 51 (47.2) | |
| T category | 0.2262 | |||
| Tis, T1 | 16 | 13 (81.3) | 3 (18.8) | |
| T2 | 35 | 22 (62.9) | 13 (37.1) | |
| T3 | 452 | 286 (63.3) | 166 (36.7) | |
| T4 | 12 | 7 (58.3) | 5 (41.7) | |
| N category | < 0.0012 | |||
| N0 | 226 | 170 (75.2) | 56 (24.8) | |
| N1 | 130 | 73 (56.2) | 57 (43.8) | |
| N2 | 159 | 85 (53.5) | 74 (46.5) | |
| M category | 0.1451 | |||
| M0 | 495 | 318 (64.2) | 177 (35.8) | |
| M1 | 20 | 10 (50.0) | 10 (50.0) | |
| AJCC stage | < 0.0012 | |||
| 0, I | 41 | 30 (73.2) | 11 (26.8) | |
| IIA, IIB | 183 | 139 (76.0) | 44 (24.0) | |
| IIIA, IIIB, IIIC | 271 | 149 (55.0) | 122 (45.0) | |
| IV | 20 | 10 (50.0) | 10 (50.0) | |
| Dukes stage | < 0.0012 | |||
| A | 12 | 10 (83.3) | 2 (16.7) | |
| B1, B2 | 208 | 156 (75.0) | 52 (25.0) | |
| C1, C2 | 275 | 152 (55.3) | 123 (44.7) | |
| D | 20 | 10 (50.0) | 10 (50.0) | |
| Apaf-1 | 0.001 | |||
| Negative | 401 | 241 (60.1) | 160 (39.9) | |
| Positive | 114 | 87 (76.3) | 27 (23.7) | |
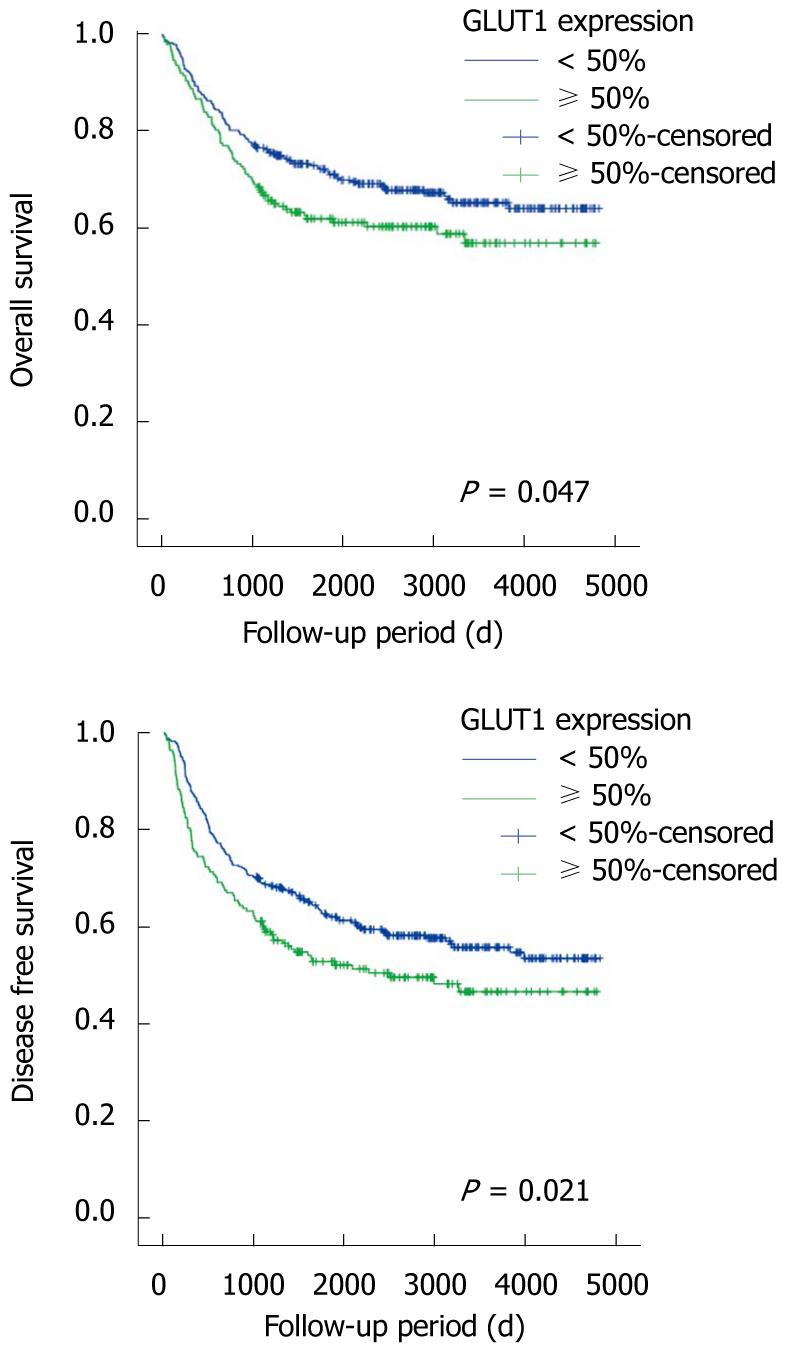
We examined the impact of GLUT1 expression on patient survival. As we expected, a significant prognostic influence of patient age, tumor differentiation, AJCC stage, and vascular invasion on overall and disease-free survival was found in univariate and multivariate analyses (Table 3). Notably, GLUT1 expression was significantly correlated with poor overall survival (P = 0.047, log-rank test) and disease-free survival (P = 0.021, log-rank test) in univariate analysis. However, in multivariate survival analysis with the Cox proportional hazards model, GLUT1 expression was not an independent prognostic factor for overall survival and disease-free survival (P = 0.534 and P = 0.416, respectively). Kaplan-Meier survival curves showed a significant difference in patient survival according to GLUT1 expression (Figure 3).
| Variables | Significance univariate1 | Significance multivariate2 | Hazard ratio | 95% CI |
| Overall survival | ||||
| GLUT1 expression (< 50% vs≥ 50%) | 0.047 | 0.534 | 1.101 | 0.813-1.491 |
| Patient age (< 58 yr vs≥ 58 yr) | < 0.001 | < 0.001 | 1.884 | 1.389-2.555 |
| Differentiation (low vs high) | < 0.001 | < 0.001 | 1.846 | 1.344-2.536 |
| AJCC stage (0, I, II vs III, IV) | < 0.001 | < 0.001 | 2.715 | 1.909-3.863 |
| Vascular invasion (absent vs present) | 0.001 | 0.005 | 2.993 | 1.393-6.430 |
| Disease free survival | ||||
| GLUT1 expression (< 50% vs≥ 50%) | 0.021 | 0.416 | 1.118 | 0.855-1.461 |
| Patient age (< 58 yr vs≥ 58 yr) | 0.002 | 0.003 | 1.497 | 1.150-1.947 |
| Differentiation (low vs high) | < 0.001 | 0.002 | 1.593 | 1.193-2.126 |
| AJCC stage (0, I, II vs III, IV) | < 0.001 | < 0.001 | 2.940 | 2.161-3.999 |
| Vascular invasion (absent vs present) | 0.014 | 0.035 | 2.264 | 1.057-4.848 |
In the present study, we investigated the expression of GLUT1 in normal mucosa, tubular adenomas, adenocarcinomas and metastatic lesions and evaluated the correlation with the clinicopathologic parameters and patient survival in patients with adenocarcinomas. GLUT1 expression was absent in 26 normal mucosa specimens with no exception. Only 5 of 26 tubular adenomas with low grade dysplasia showed low GLUT1 expression and 14 of 24 tubular adenomas with high grade dysplasia revealed low or moderate GLUT1 expression. On the other hand, 423 of 515 adenocarcinomas and 96 of 127 metastatic lesions showed GLUT1 expression with variable grades. Furthermore, the GLUT1 expression was closely correlated with poor prognostic parameters.
The activation of GLUT1 gene expression is a molecular feature of malignant phenotype in a variety of cancers and has been shown to be associated with malignant transformation. Various malignant tumors, including colorectal cancers, show increased glucose metabolism and utilization[12-14]. Increased GLUT1 expression in neoplastic tissue reflects an increased glycolytic metabolism and is observed under conditions that induce greater dependence on glycolysis as an energy source, such as ischemia or hypoxia[7,18-20]. Previous studies suggest that GLUT1 expression may play an important role in the survival of tumor cells by promoting an adequate energy supply[21,22]. Two possible mechanisms were suggested to explain the activation of GLUT1 gene expression in cancers. Firstly, increased glycolysis and concomitant GLUT1 expression may be a constitutive feature of the malignant phenotype in many cancers. Secondly, local hypoxia in the tumor microenvironment may result in an adaptive increase in glycolytic metabolism and GLUT1 expression[11].
Sakashita et al[7] demonstrated that GLUT1 expression was positive in 18% of low-grade adenomas and in 63% of high-grade adenomas. In our results, GLUT1 expression was demonstrated in 19.2% (5/26) of tubular adenomas with low grade dysplasia and in 58.3% (14/24) of tubular adenomas with high grade dysplasia. Haber et al[11] demonstrated GLUT1 expression in 101 (90%) of 112 colorectal adenocarcinomas. GLUT1 expression was undetected in 11 cases (9.8%) and detected in < 10% of the tumor cells in 39 cases (34.8%), in 10%-50% of the tumor cells in 42 cases (37.5%), and in > 50% of the tumor cells in 20 cases (17.9%). In our study, GLUT1 expression was demonstrated in 423 (82.1%) of 515 colorectal adenocarcinomas and undetectable in 92 cases (17.9%). Furthermore, we evaluated GLUT1 expression in 127 metastatic lesions, including lymph nodes and distant organs. The GLUT1 expression was significantly different between normal mucosa, tubular adenomas with low grade dysplasia, tubular adenomas with high grade dysplasia, adenocarcinomas and metastatic lesions (Table 1, P < 0.001). Our findings indicate that GLUT1 expression may play an important role at the late stage in the adenoma-carcinoma carcinogenesis sequence.
Some studies have reported the correlation between GLUT1 expression and the clinicopathologic parameters in colorectal adenocarcinomas. Sakashita et al[7] reported that GLUT1 expression was significantly different between well differentiated and less differentiated groups (positivity of 67% vs 93%, P < 0.05). The rate of GLUT1 expression, both moderate and strong, was also significantly different between these two groups (49% vs 74%, P < 0.05). Ito et al[15] demonstrated that GLUT1 immunostaining was stronger in tumors with less differentiation in lung adenocarcinomas. However, Younes et al[10] and Haber et al[11] reported that there was no correlation between GLUT1 expression and histologic differentiation. In our results, GLUT1 expression was < 50% of tumor cells in 19 cases (90.5%) and > 50% of the tumor cells in 2 cases (9.5%) of well differentiated adenocarcinomas. In moderately differentiated adenocarcinomas, GLUT1 expression was < 50% of tumor cells in 252 cases (65.3%) and > 50% of the tumor cells in 134 cases (34.7%). In poorly differentiated adenocarcinomas, GLUT1 expression was < 50% of tumor cells in 57 cases (52.8%) and > 50% of the tumor cells in 51 cases (47.2%). There was a significant correlation between GLUT1 expression and the histologic differentiation (P = 0.001).
The relationship between GLUT1 expression and the depth of invasion has been reported in colorectal adenocarcinomas. Sakashita et al[7] reported that GLUT1 expression was significantly different between T1 and T2 groups (positivity of 61% vs 97%, P < 0.01). The rate of moderate and strong GLUT1 expression was also significantly different between these two groups (45% vs 74%, P < 0.01). However, Younes et al[10] demonstrated that there was no significant difference between GLUT1 expression and the depth of invasion. Our results revealed that there was no significant correlation between GLUT1 expression and the depth of invasion. Younes et al[10] documented that there was a close correlation between strong GLUT1 expression and the frequency of lymph node metastasis in colorectal adenocarcinomas. Sakashita et al[7] reported that the rate of GLUT1 expression in colorectal carcinomas with nodal metastasis was higher than that in those without, but the difference was not significant due to the small size of lymph node metastases-positive carcinomas. In our study, there was a close correlation between GLUT1 expression and the presence of lymph node metastasis (P < 0.001). This result indicates that GLUT1 may be important for maintaining the high-energy requirements of aggressive cancers. The immunohistochemical detection of GLUT1 in biopsies of colorectal cancers may be useful as a marker of aggressive biologic behavior, especially in lymph node metastasis[10] .
There has been no documented report as to the relationship between GLUT1 expression and tumor stages in colorectal adenocarcinomas. Haber et al[11] reported the association of GLUT1 staining status with Dukes stage; however, no statistical significance was revealed. Our results documented that there was a close correlation between GLUT1 expression and tumor stages, AJCC and Dukes stages (P < 0.001 and P < 0.001, respectively). The correlation between GLUT1 expression and survival in colorectal adenocarcinomas has been reported[11]. There was a significant increase in mortality in those patients whose tumors had more than 50% of GLUT1-positive cells (relative risk, 2.4; P = 0.02 by the log rank test). Our study showed that GLUT1 expression was significantly correlated with poor overall survival (P = 0.047) and disease-free survival (P = 0.021) in univariate analysis. However, in multivariate analysis with the Cox proportional hazards model, GLUT1 expression was not an independent prognostic factor of overall survival and disease-free survival (P = 0.534 and P = 0.416, respectively).
There are multiple interactions between the cellular machinery involved in glucose uptake and metabolism, and the cellular mechanism of programmed cell death or apoptosis. Glucose deprivation can promote apoptosis in a variety of cells. The induction of glucose uptake and metabolism can prevent or reduce apoptosis[23,24]. Enhanced GLUT1 expression has been shown to inhibit cytochrome c release and downstream caspase activation during hypoxia[25,26]. Vesely et al[27] documented that GLUT1 prevents hypoxia-induced apoptosis in vascular smooth muscle cells and cardiac myocytes largely via a mitochondrial, caspase 9-dependent pathway. In this study, we evaluated the correlation between GLUT1 expression and the expression of Apaf-1, one of the key regulators in the mitochondrial apoptotic pathway[4,28]. Our results revealed that there is a significant inverse correlation between GLUT1 expression and Apaf-1 expression (P = 0.001). The GLUT1 expression may prevent apoptosis through the suppression of Apaf-1 expression via a mitochondrial apoptotic pathway.
In conclusion, we tried to clarify the clinicopathologic significance of GLUT1 expression in a large cohort consisting of 26 normal mucosa, 50 tubular adenomas, 515 adenocarcinomas, and 127 metastatic lesions. The GLUT1 expression pattern suggested an important role in colorectal cancer development, especially at the late stage of the adenoma-carcinoma sequence, and GLUT1 expression was closely correlated with poor clinicopathologic factors in colorectal adenocarcinomas.
Colorectal cancer is the second leading cause of cancer-related death in men and women in the industrialized nations. There have been marked advances in the understanding of the carcinogenesis in colorectal cancer and cancer biology; however, the relevant therapeutic problem continues to persist. Previous studies revealed an enhancement of glycolytic metabolism in malignant tumors. The increased expression of glucose transporter 1 (GLUT1) mRNA and protein has been demonstrated in various cancer tissues which indicates that GLUT1 may play an important role in glucose uptake by various cancers and that GLUT1 expression could be useful as a marker for malignant transformation.
This study was to investigate the membranous GLUT1 expression in colorectal carcinogenesis and to evaluate the correlation between GLUT1 expression and the clinicopathological parameters, and between GLUT1 expression and cytoplasmic Apaf-1 expression, as well as its effect on survival of patients with colorectal adenocarcinomas.
GLUT1 expression was significantly correlated with female gender, non-mucinous tumor type, poorer differentiation, lymph node metastasis, higher AJCC and Dukes stage. There was a significant inverse correlation between GLUT1 expression and Apaf-1 expression. Patients with GLUT1 expression demonstrated poor overall survival and disease-free survival in univariate survival analysis.
The authors evaluated the correlation between GLUT1 expression and expression of Apaf-1, one of the key regulators in the mitochondrial apoptotic pathway. The results revealed that there is a significant inverse correlation between GLUT1 expression and Apaf-1 expression. These results warrant further careful and well-designed studies of GLUT1 and Apaf-1 expression in colorectal cancers for clinical therapeutic application.
GLUTs are membrane proteins responsible for the transport of glucose across cellular membranes. GLUT1 is an isoform that is restricted to erythrocytes and blood-tissue barriers such as the blood-brain and blood-nerve barriers. Apaf-1 is one of the key regulators in the mitochondrial apoptotic pathway. Apaf-1 binds to a protein called cytochrome-c, which is released from mitochondria under the control of p53, and this complex activates caspase-9, which then triggers executioner caspases, leading to apoptosis.
The paper investigated the role of GLUT1 expression in colorectal carcinogenesis and evaluated the correlation with the clinicopathological parameters and Apaf-1 expression in colorectal adenocarcinomas. It is very interesting.
Peer reviewer: Kazuhiro Hanazaki, MD, Professor and Chairman, Department of Surgery, Kochi Medical School, Kochi University, Kohasu, Okohcho, Nankoku, Kochi 783-8505, Japan
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30. |
| 2. | Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376-388. |
| 3. | Walker J, Quirke P. Prognosis and response to therapy in colorectal cancer. Eur J Cancer. 2002;38:880-886. |
| 5. | Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465-8471. |
| 6. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. |
| 7. | Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Maeda S, Kasuga M. Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer. 2001;37:204-209. |
| 8. | Kim SJ, Lee HW, Kim DC, Rha SH, Hong SH, Jeong JS. Significance of GLUT1 expression in adenocarcinoma and adenoma of the ampulla of Vater. Pathol Int. 2008;58:233-238. |
| 9. | Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911-930. |
| 10. | Younes M, Lechago LV, Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res. 1996;2:1151-1154. |
| 11. | Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, Slater G, Weiss A, Burstein DE. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34-40. |
| 12. | Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993;72:2979-2985. |
| 13. | Mellanen P, Minn H, Grénman R, Härkönen P. Expression of glucose transporters in head-and-neck tumors. Int J Cancer. 1994;56:622-629. |
| 14. | Nagase Y, Takata K, Moriyama N, Aso Y, Murakami T, Hirano H. Immunohistochemical localization of glucose transporters in human renal cell carcinoma. J Urol. 1995;153:798-801. |
| 15. | Ito T, Noguchi Y, Satoh S, Hayashi H, Inayama Y, Kitamura H. Expression of facilitative glucose transporter isoforms in lung carcinomas: its relation to histologic type, differentiation grade, and tumor stage. Mod Pathol. 1998;11:437-443. |
| 16. | Park JM, Jung CK, Choi YJ, Lee KY, Kang JH, Kim MS, Hu HJ. The use of an immunohistochemical diagnostic panel to determine the primary site of cervical lymph node metastases of occult squamous cell carcinoma. Hum Pathol. 2010;41:431-437. |
| 17. | Paik SS, Jang KS, Song YS, Jang SH, Min KW, Han HX, Na W, Lee KH, Choi D, Jang SJ. Reduced expression of Apaf-1 in colorectal adenocarcinoma correlates with tumor progression and aggressive phenotype. Ann Surg Oncol. 2007;14:3453-3459. |
| 18. | Merrall NW, Plevin R, Gould GW. Growth factors, mitogens, oncogenes and the regulation of glucose transport. Cell Signal. 1993;5:667-675. |
| 19. | Camps M, Vilaro S, Testar X, Palacín M, Zorzano A. High and polarized expression of GLUT1 glucose transporters in epithelial cells from mammary gland: acute down-regulation of GLUT1 carriers by weaning. Endocrinology. 1994;134:924-934. |
| 20. | Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625-1632. |
| 21. | Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, Honma K, Suzuki T. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634-641. |
| 22. | Newsholme EA, Board M. Application of metabolic-control logic to fuel utilization and its significance in tumor cells. Adv Enzyme Regul. 1991;31:225-246. |
| 24. | Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899-5912. |
| 25. | Lin Z, Weinberg JM, Malhotra R, Merritt SE, Holzman LB, Brosius FC 3rd. GLUT-1 reduces hypoxia-induced apoptosis and JNK pathway activation. Am J Physiol Endocrinol Metab. 2000;278:E958-E966. |
| 26. | Loberg RD, Vesely E, Brosius FC 3rd. Enhanced glycogen synthase kinase-3beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. J Biol Chem. 2002;277:41667-41673. |
| 27. | Vesely ED, Heilig CW, Brosius FC 3rd. GLUT1-induced cFLIP expression promotes proliferation and prevents apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2009;297:C759-C765. |
| 28. | Yoshida H. The role of Apaf-1 in programmed cell death: from worm to tumor. Cell Struct Funct. 2003;28:3-9. |