Published online Apr 14, 2011. doi: 10.3748/wjg.v17.i14.1858
Revised: December 22, 2010
Accepted: December 29, 2010
Published online: April 14, 2011
AIM: To investigate whether potent acid inhibition is effective in non-erosive reflux disease (NERD) refractory to standard rabeprazole (RPZ) treatment.
METHODS: We treated 10 Japanese patients with NERD resistant to standard dosages of RPZ: 10 mg or 20 mg od, 20 mg bid, or 10 mg qid for 14 d. All patients completed a frequency scale for symptoms of gastroesophageal reflux disease questionnaire frequency scale for the symptoms of GERD (FSSG); and underwent 24 h pH monitoring on day 14.
RESULTS: With increased dosages and frequency of administration of RPZ, median intragastric pH significantly increased, and FSSG scores significantly decreased. With RPZ 10 mg qid, potent acid inhibition was attained throughout 24 h. However, five subjects were refractory to RPZ 10 mg qid, although the median intragastric pH in these subjects (6.6, range: 6.2-7.1) was similar to that in the remaining five responsive subjects (6.5, range: 5.3-7.3). With baseline RPZ 10 mg od, FSSG scores in responsive patients improved by > 30%, whereas there was no significant decrease in the resistant group.
CONCLUSION: NERD patients whose FSSG score fails to decrease by > 30% after treatment with RPZ 10 mg od for 14 d are refractory to higher dosage.
- Citation: Sugimoto M, Nishino M, Kodaira C, Yamade M, Uotani T, Ikuma M, Umemura K, Furuta T. Characteristics of non-erosive gastroesophageal reflux disease refractory to proton pump inhibitor therapy. World J Gastroenterol 2011; 17(14): 1858-1865
- URL: https://www.wjgnet.com/1007-9327/full/v17/i14/1858.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i14.1858
Gastroesophageal reflux disease (GERD) is defined as the presence of acid-reflux-related symptoms, or esophageal mucosal damage, caused by the abnormal reflux of gastric contents into the esophagus[1]. The diagnosis of GERD is therefore relatively easy when patients complain of typical acid-reflux-related symptoms (i.e. heartburn and regurgitation), and/or esophageal mucosal breaks are seen by gastroduodenal endoscopy. Recently, non-erosive gastroesophageal reflux disease (NERD) has been defined as the presence of acid-reflux-related symptoms without esophageal mucosal breaks[2]. NERD is classified into two types: grade M and grade N. Grade M is characterized by minimal mucosal changes, such as erythema, without sharp demarcation, whitish turbidity, and/or translucency in the lower esophageal mucosa; and grade N reveals no endoscopic abnormality[3]. The clinical characteristics of patients with NERD-that they are less likely to smoke or have an esophageal hiatal hernia, and more likely to be female, underweight, and have Helicobacter pylori (H. pylori) infection-differ from those of erosive GERD patients[2,4,5]. Furthermore, the fact that esophageal mucosal sensitivity in NERD patients tends to be higher than those with erosive GERD is another important clinical characteristic of patients with NERD[6]. These findings suggest that NERD is not simply a milder type of erosive GERD, and that the pathophysiology of erosive GERD differ from that of NERD[7].
Proton pump inhibitors (PPIs), which potently inhibit gastric acid secretion, improve acid-reflux heartburn symptoms and esophageal mucosal breaks[8-12]. Meta-analyses of treatment for erosive GERD patients have shown that PPIs are much more effective in curing esophageal erosions and acid-reflux-related symptoms than are H2 receptor antagonists (H2RAs) or prokinetics[13,14]. However, improvement of heartburn associated with NERD using standard PPI dosages are lower (around 30%-60%) than for erosive GERD[2,15,16]. This raises the question whether PPI-resistant NERD is an acid-related disease. Although PPIs potently inhibit acid secretion, standard PPI dosages do not sufficiently control intragastric pH throughout 24 h[17-19]. For patients with NERD refractory to a standard PPI dosage, therefore, treatment with potent acid inhibition will be required to determine whether PPI-resistant NERD is caused by insufficient acid inhibition.
To the best of our knowledge, no earlier studies have investigated whether acid-related symptoms in patients with NERD refractory to standard PPI dosages improve when sufficient acid inhibition is attained using PPI qid therapy. In this study, we investigated the effects of frequent PPI dosing on subjects with PPI-resistant NERD, with the aim of determining the clinical characteristics of subjects with PPI-refractory NERD that was resistant to potent acid inhibition.
After obtaining written informed consent, we invited 15 Japanese NERD patients with acid-reflux symptoms more than once a week to participate in our study. They underwent testing for CYP2C19 genotyping and gastroduodenal endoscopy. Endoscopy was performed in all subjects after fasting overnight, and the presence of esophageal mucosal breaks was assessed according to the Los Angeles classification (grade A-D)[20]. In addition, grade M NERD was defined as mucosal findings of redness, edema or white granules in the esophagocardial junction (EC) junction, and grade N as normal mucosa, in subjects with acid-reflux-related symptoms. Subjects were administered a standard PPI dosage (rabeprazole 10 mg od) for 4 wk. Of the 15 enrolled subjects, 10 H. pylori-negative subjects with a score higher than 8 on the Frequency Scale for the Symptoms of GERD (FSSG) questionnaire (Table 1) were diagnosed with PPI-resistant NERD, and were enrolled in the study proper[21,22]. However, because a score on the FSSG questionnaire in the remaining five NERD patients decreased to < 7 after PPI treatment (PPI-responded NERD), we did not enroll them in the study.
| Question | Never | Occasionally | Sometimes | Often | Always | |
| 1 | Do you get heartburn? | 0 | 1 | 2 | 3 | 4 |
| 2 | Does your stomach get bloated? | 0 | 1 | 2 | 3 | 4 |
| 3 | Does your stomach ever feel heavy after meals? | 0 | 1 | 2 | 3 | 4 |
| 4 | Do you sometimes subconsciously rub your chest with your hand? | 0 | 1 | 2 | 3 | 4 |
| 5 | Do you ever feel sick after meals? | 0 | 1 | 2 | 3 | 4 |
| 6 | Do you get heartburn after meals? | 0 | 1 | 2 | 3 | 4 |
| 7 | Do you have an unusual (e.g. burning) sensation in your throat? | 0 | 1 | 2 | 3 | 4 |
| 8 | Do you feel full while eating meals? | 0 | 1 | 2 | 3 | 4 |
| 9 | Do some things get stuck when you swallow? | 0 | 1 | 2 | 3 | 4 |
| 10 | Do you get bitter liquid (acid) coming up into your throat? | 0 | 1 | 2 | 3 | 4 |
| 11 | Do you burp a lot? | 0 | 1 | 2 | 3 | 4 |
| 12 | Do you get heartburn if you bend over? | 0 | 1 | 2 | 3 | 4 |
All subjects were administered the four different regimens in the following order: Rabeprazole (Pariet®; Eisai Co. Ltd., Tokyo, Japan) 10 mg od [RPZ(10)] at 08:00 h, rabeprazole 20 mg od [RPZ(20)], rabeprazole 20 mg bid [RPZ(20*2)] at 08:00 and 19:00 h, and rabeprazole 10 mg qid [RPZ(10*4)] at 07:00, 13:00, 19:00 and 0:00 h for 14 d each. On day 14 of each regimen, subjects filled in the FSSG questionnaire and underwent 24 h intraesophageal and intragastric pH monitoring. All subjects were provided three meals a day (breakfast at 07:00 h, lunch at 13:00 h, and dinner at 19:00 h). Mineral water was allowed ad libitum, but no other beverages (e.g. grapefruit juice) were permitted. There was a washout period of at least 2 wk between the study periods. There were no rescue drugs during and between the study periods. No subjects drank alcohol or smoked. No subjects had taken any medications for at least 1 mo prior to the study, nor were they allowed during the study. All protocols for each subject were completed within 6-10 mo.
The protocol was approved in advance by the Human Institutional Review Board of the Hamamatsu University School of Medicine. Written informed consent was again obtained from each subject before participation in each of the four trial phases.
Intraesophageal and intragastric pH readings were recorded using a Digitrapper pH 400 (Medtronic Functional Diagnostic A/S, Skovlunde, Denmark). On day 14 of each trial phase, after fasting overnight, an antimony pH catheter (Medtronic Inc., Minneapolis, MN, USA) was inserted transnasally under local anesthesia and placed 5 cm distal to the gastric cardia, and pH readings were recorded for 24 h.
GERD-related symptoms were evaluated using the FSSG, which includes seven acid-reflux-related questions and five dysmotility related questions[21,22]. Subjects answered questions about the frequency of their symptoms, scoring them as follows: never, 0; occasionally, 1; sometimes, 2; often, 3; and always, 4. We calculated the acid reflux, dysmotility and total scores, with a total score of ≥ 8 considered to indicate probable GERD/NERD.
CYP2C19 genotyping PPIs are mainly metabolized by hepatic CYP2C19, and there are genetic differences in the activity of this enzyme[17-19]. In poor metabolizers (PMs) of CYP2C19, the plasma PPI concentrations are markedly increased and the pharmacodynamic effects of PPIs are enhanced in comparison with those in rapid metabolizers (RMs) or intermediate metabolizers (IMs). Therefore, we tested CYP2C19.
DNA was extracted from each subject’s leukocytes using a commercially available kit (IsoQuick; ORCA Research Inc., Bothell, WA, USA). Genotyping procedures for identifying the CYP2C19 wild-type (*1) gene and the two mutated alleles, CYP2C19*2 (*2) and CYP2C19*3 (*3), were performed using an allele-specific primers-polymerase chain reaction method with allele-specific primers[23]. CYP2C19 genotypes were classified into three groups, RMs (*1/*1), IM (*1/*2 or *1/*3), and PM (*2/*2, *3/*3 or *2/*3).
Differences between different regimens and groups were determined using Wilcoxon’s signed rank test, when significant differences were obtained using Friedman’s test. All P values were two-sided, and P < 0.05 was taken to indicate statistical significance.
Japanese subjects with NERD resistant to standard PPI dosages enrolled in this study exhibited no demographic differences in age, body weight, CYP2C19 genotype status, median intragastric and intraesophageal pH, or baseline FSSG score between subjects with NERD grade N and grade M (Table 2). No severe adverse events occurred with any of the study regimens, and all regimens were well tolerated by all subjects.
| Grade n | Grade M | Total | P value | ||
| Number | 3 | 7 | 10 | ||
| Age | 22.3 ± 5.8 | 22.1 ± 0.7 | 22.2 ± 0.6 | 0.73 | |
| Height | 159.7 ± 3.2 | 156.7 ± 8.3 | 157.6 ± 7.0 | 0.31 | |
| Weight | 52.2 ± 2.5 | 52.7 ± 6.0 | 52.6 ± 5.0 | 0.91 | |
| CYP2C19 | RM/IM/PM | 1/2/0 | 3/2/2002 | 5/2/2003 | 0.41 |
| 24 h pH | Gastric pH | 2.6 (2.6-2.6) | 2.4 (1.5-2.8) | 2.5 (1.5-2.8) | 0.31 |
| Esophageal pH | 6.6 (6.4-7.6) | 6.6 (6.4-7.5) | 6.6 (6.4-7.6) | 0.75 | |
| FSSG | 19 (15-24) | 22 (19-31) | 21 (15-31) | 0.3 |
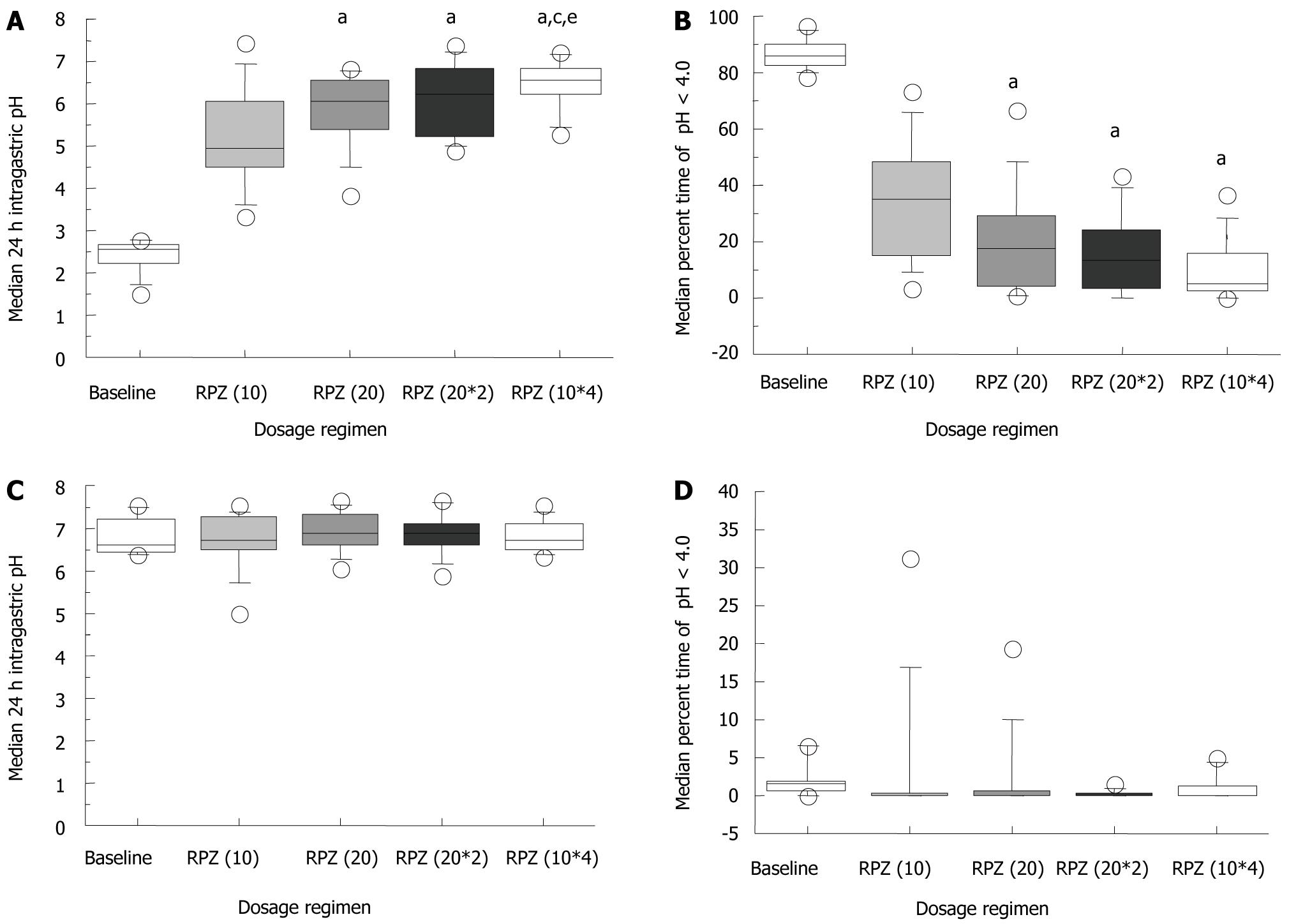
The median 24 h intragastric pH at baseline and on day 14 for the RPZ(10), RPZ(20), RPZ(20*2) and RPZ(10*4) regimens was 2.5 (range: 1.5-2.8), 5.0 (3.3-7.5), 6.1 (3.8-6.8), 6.2 (4.9-7.4) and 6.5 (5.3-7.3), respectively (Figure 1A). Median intragastric pH significantly increased in a dosage and dosing frequency-dependent manner, and was significantly higher with RPZ(10*4) than with RPZ(20*2), although the total daily dosage (40 mg) was the same (Figure 1A). The median percentage of intragastric pH < 4.0 in a day with the RPZ(20), RPZ(20*2) and RPZ(10*4) regimens was 17.2% (range: 0.8%-66.6%), 13.0% (0.0%-43.6%) and 5.2% (0.0%-36.8%), respectively, which was significantly lower than those at baseline [85.6% (78.3%-97.0%), P < 0.01] and with the RPZ(10) regimen [34.8% (3.5%-73.7%), P < 0.01] (Figure 1B).
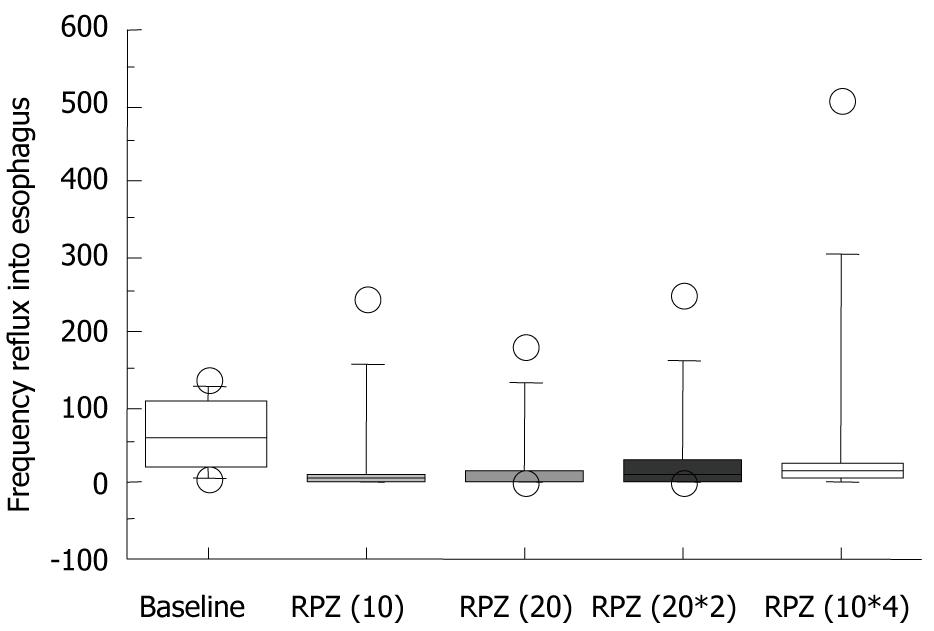
Median 24 h intraesophageal pH and the percentage of intraesophageal pH < 4.0 in a day were similar for the four different RPZ dosage regimens (Figure 1C and 1D). Most baseline measurements showed no abnormal acid reflux; defined as > 5% of patients with intraesophageal pH < 4.0 (Figure 1D). The median reflux frequency of gastric acid to the esophagus at baseline, RPZ(10), RPZ(20), RPZ(20*2) and RPZ(10*4) was 58.5 (range: 7-137), 5 (3-244), 4.5 (0-180), 13.5 (0-251) and 4.5 (0-506), respectively (Figure 2). Differences were not statistically significant in comparison with baseline.
Median 24 h intraesophageal and intragastric pH, the percentage of intraesophageal and intragastric pH < 4.0 in a day, and the frequency of reflux of gastric acid to the esophagus were similar between patients with different CYP2C19 genotype status (data not shown).
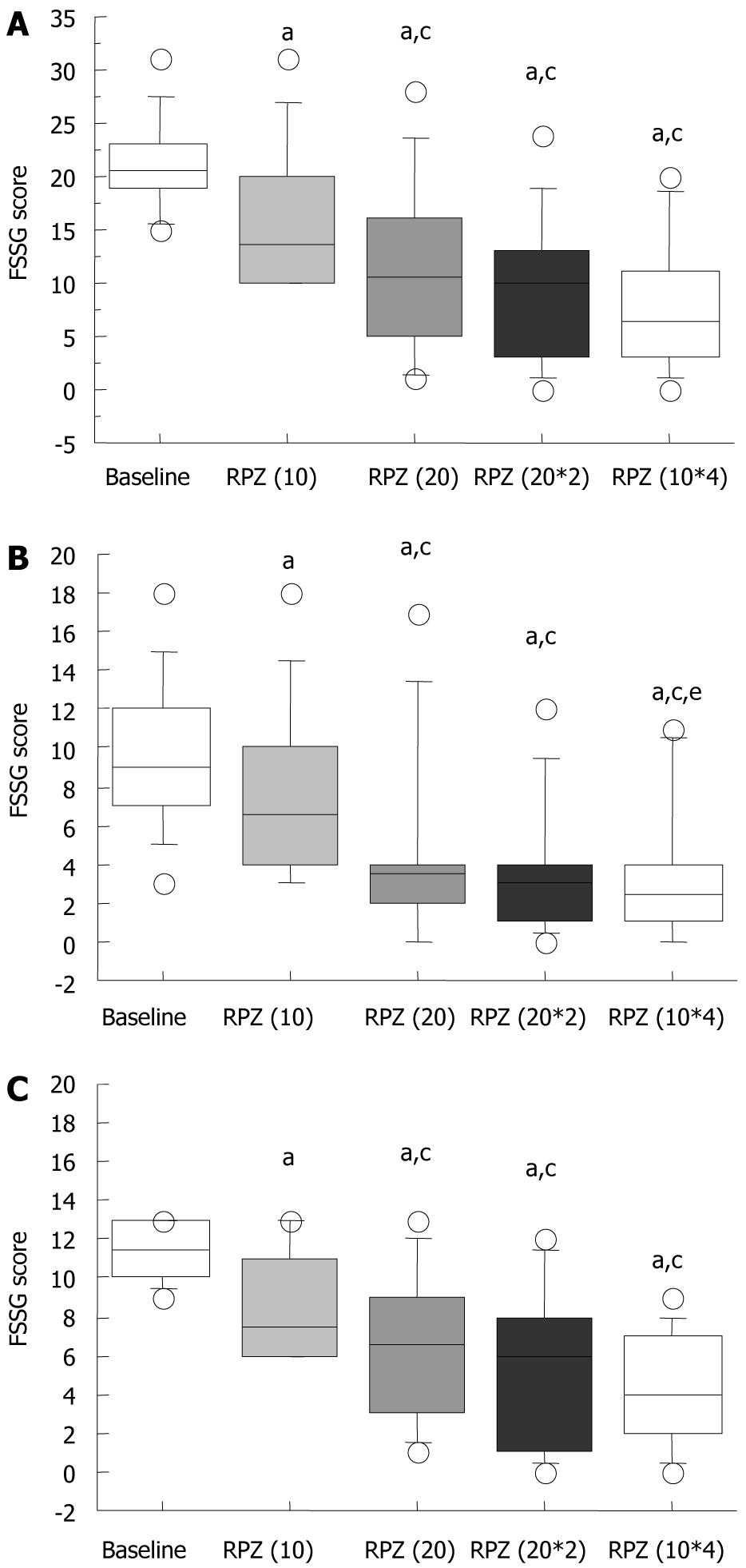
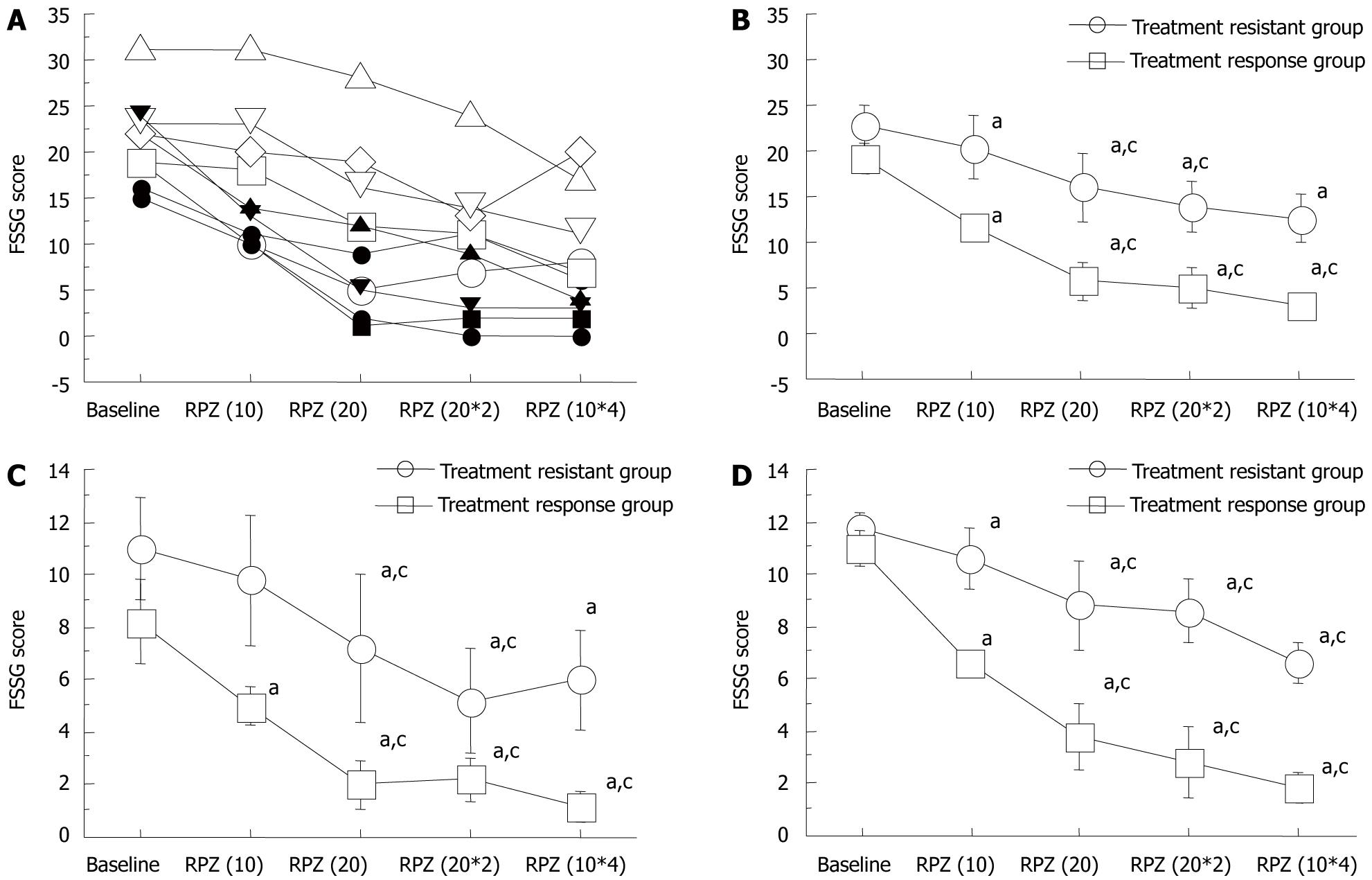
FSSG scores improved significantly in a dose-dependent manner (Figure 3A). The median FSSG score at baseline was 20.5 (range: 15-31), which significantly decreased with each RPZ dosage regimen [RPZ(10), 13.5 (10-31); RPZ(20), 10.5 (1-28); RPZ(20*2), 10.0 (0-24); and RPZ(10*4), 6.5 (0-20), P < 0.05] (Figure 3A). When patients with NERD were administered RPZ 10 mg od, their FSSG scores were all > 8. FSSG scores with the RPZ(20), RPZ(20*2) and RPZ(10*4) regimens were significantly lower than that with the RPZ(10) regimen (Figure 3A).
When FSSG scores were subdivided into acid reflux and dysmotility scores (Table 1), these were seen to decrease significantly with changes in RPZ dosage schemes in acid reflux scores (Figure 3B and C). The FSSG acid reflux scores with the RPZ(20*2) [3.5 (0-17)], RPZ(20*2) [3 (0-12)] and RPZ(10*4) [2.5 (0-11)] regimens were significantly lower than that with RPZ(10) [6.5 (3-18)] (P < 0.05).
Also in dysmotility scores, these were seen to decrease significantly with changes in RPZ dosage schemes (Figure 3C). The FSSG acid reflux scores with the RPZ(20*2) [6.5 (1-13)], RPZ(20*2) [6 (0-12)] and RPZ(10*4) [4 (0-9)] regimens were significantly lower than that with RPZ(10) [7.5 (6-13)] (P < 0.05) (Figure 3C). The FSSG dysmotility score with the RPZ(10*4) regimen was significantly lower than that with RPZ(20*2), although the total daily dosage was the same (40 mg) (Figure 3C).
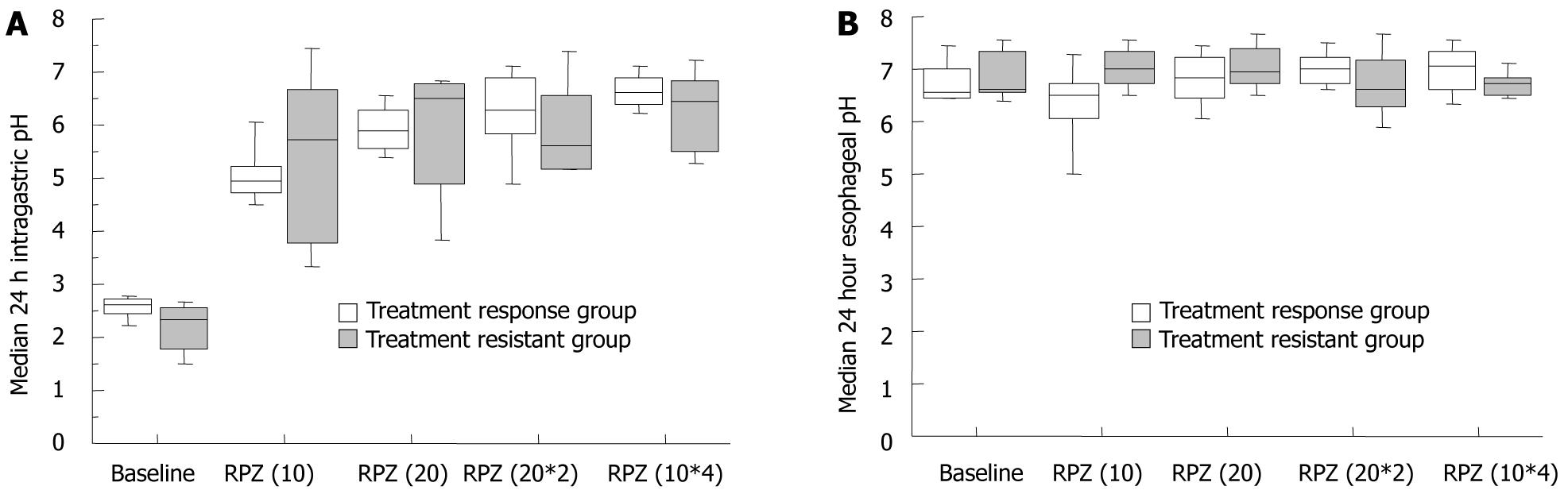
When patients were classified into a responsive (FSSG score: < 8, n = 5) and resistant (FSSG score: > 8, n = 5) group for the RPZ(10*4) regimen, the median intragastric pH was similar for the two groups [responsive group: 6.5 (5.3-7.3) and resistant group: 6.6 (6.2-7.1)] (Figure 4), which indicated that sufficient acid inhibition was attained in both subjects with PPI-resistant as well responsive NERD. Median total, acid reflux and dysmotility FSSG scores significantly decreased in a dose-dependent manner in subjects who responded to, or were refractory to, the RPZ(10*4) regimen (Figure 5A-D). Acid reflux and dysmotility FSSG scores both differed significantly between the two groups (Figure 5A-D). FSSG scores in subjects with NERD responsive to the RPZ(10*4) regimen improved by > 30% in comparison with baseline scores with RPZ 10 mg od. On the other hand, in subjects with NERD refractory to the RPZ(10*4) regimen, FSSG scores decreased by < 30% compared with the baseline with RPZ 10 mg od.
Recently, the rising prevalence of NERD worldwide[24], and its adverse impact on quality of life[25], have led to the acceptance of NERD as a gastrointestinal disease that requires treatment. Intraesophageal and intragastric pH directly correlate with the degree of esophageal mucosal damage and the degree of sensitivity[26], therefore, treatment strategies for NERD recommend that pH levels in both the esophagus and stomach should be maintained above 4.0 by optimal use of acid inhibitory agents[27]. We previously have reported that it is difficult to maintain the intragastric pH above 4 throughout 24 h with standard PPI dosages (omeprazole 20 mg, lansoprazole 30 mg and rabeprazole 10 mg)[17-19], but that PPI qid therapy[18] and H2RA + PPI combination therapy[28] provide profound inhibition of gastric acid secretion. These therapies may be the main strategies for treating PPI-resistant, acid-related GERD with/without nocturnal acid breakthrough, as well as peptic ulcers and H. pylori infection. Complete remission of endoscopic esophageal mucosal injury and reflux-related symptoms are achieved in around 30%-60% of patients with NERD using standard PPI dosages[2,15,16], which suggests that PPI-resistant NERD may be due to insufficient acid inhibition by standard PPI dosages. In this study, we demonstrated that the qid RPZ regimen maintained intraesophageal and intragastric pH above 4.0 throughout 24 h, and that our subjects with PPI-resistant NERD responded to varying degrees. Accordingly, we assume that cases of PPI-resistant NERD are caused by insufficient acid inhibition, and that the pathogenesis of NERD may be related to weak acid reflux into the esophagus, and not observed as abnormal reflux and not inhibited by standard PPI dosages. In subjects with NERD responsive to potent acid inhibition by RPZ 10 mg qid, FSSG scores decreased by > 30% compared with baseline after RPZ 10 mg od for 14 d. Therefore, NERD patients whose FSSG reduces by > 30% by a standard dosage of PPI will respond to a higher dose of PPI, although the clinical effects of standard PPI dosages on their symptoms are limited.
Cases of NERD that are resistant to high PPI dosages appear to be acid-independent, and are considered to have been caused by other factors, such as esophageal hypersensitivity and/or functional heartburn. In fact, the prevalence of NERD grade N patients with abnormal acid reflux into the esophagus (> 5% with intraesophageal pH < 4.0) is reported to be only 11.8% in Japanese[29] and 33%-50% in Caucasians[30,31]. In this study, also, no abnormal acid reflux was detected in most of the baseline trials in subjects with NERD. Moreover, in some patients, NERD resembles postprandial distress syndrome type functional dyspepsia, which is characterized by dysmotility symptoms, and there is considerable overlap between NERD and functional dyspepsia[32,33]. It is not easy to distinguish NERD patients from functional heartburn using either the Montreal definition or the Rome III criteria[34].
Although double-dosage PPI therapy is recommended for patients with PPI-resistant NERD, this study shows that additional PPI therapy is not indicated for all NERD patients refractory to standard-dosage PPI. In patients with NERD resistant to potent acid inhibition by PPIs, FSSG scores improve < 30% with a standard PPI dosage compared with baseline. We can therefore easily select PPI-resistant patients using the FSSG questionnaire; the simplest and time-saving method of diagnosing GERD/NERD refractory to higher PPI dosages[21,22]. Recently, Futagami et al[35] have reported that combination therapy with a PPI and mosapride citrate significantly improves acid reflux symptoms in patients with PPI-resistant NERD. It may therefore be better to treat PPI-resistant patients with other drugs, such as prokinetics, rather than with increased PPI dosages.
Patients with grade M NERD are more likely to have pathological acid reflux than those with grade N disease, and acid reflux symptoms in patients with grade M disease are more likely to be attributable to acid reflux[29]. However, there have been a number of reports of similar rates of complete resolution of heartburn, clinical features and quality of life scores with PPI therapy in patients with grade M and N NERD[16,29,36,37]. Also in this study, there were no significant differences in FSSG scores and intraesophageal pH values between patients with grade M and grade N NERD (data not shown). The pathophysiological differences between the two types of NERD are unclear, and further studies are required to clarify the pathogenesis of NERD.
In this study, we demonstrated the effectiveness of RPZ on both dyspeptic and acid-reflux-related symptoms, which improved in a dose-dependent manner in subjects with PPI-resistant NERD. Miner et al[8] and Kusano et al[22] previously have reported that RPZ significantly improves dyspeptic symptoms in patients with NERD. It is reasonable to conclude that dyspeptic symptoms in patients with NERD can be expected respond to PPI treatment.
The limitations of this study included low sample power and a lack of placebo effect. However, in this study the most important point was to prove that NERD patients who were refractory to a standard PPI dosage, caused by insufficient acid inhibition, were improved by a greater dosage of PPI. Moreover, we analyzed intragastric and intraesophageal pH with each regimen in each subject, and were able to demonstrate the characteristics of subjects with PPI-resistant NERD using the FSSG. We believe that the FSSG score after RPZ 10 mg od for 14 d can be used to predict whether a patient with NERD refractory to a standard dosage of a PPI will respond to a higher dosage of that PPI (e.g. RPZ 10 mg qid).
In conclusion, we demonstrated that patients with NERD with a > 30% decrease in their FSSG score with RPZ 10 mg od responded to a higher PPI dosage, although they appeared to be refractory to the standard PPI dosage. On the other hand, symptoms in patients completely resistant to a standard dosage of a PPI (FSSG reduction < 30% after RPZ 10 mg od) do not resolve even if the PPI dosage is increased (e.g. RPZ 10 mg qid). We recommend that such patients should be treated with other agents such as prokinetics rather than increasing the PPI dosage[35]. The FSSG score after rabeprazole 10 mg od for 14 d shows promise in determining the optimal treatment for patients with NERD refractory to a standard PPI dosage. The clinical usefulness of the FSSG in the PPI treatment of NERD requires verification in further studies with larger subject numbers.
Half of patients with non-erosive reflux disease (NERD) are resistant to treatment with standard proton pump inhibitor (PPI) dosages, due to insufficient control of acid secretion throughout 24 h. However, no earlier studies have investigated whether acid-related symptoms in patients with NERD refractory to standard PPI dosages improve when sufficient acid inhibition is attained using PPI qid therapy.
PPIs are rapidly eliminated from the systemic circulation (t1/2: 2-3 h). H+,K+-ATPase newly generated or activated in gastric parietal cells after the rapid elimination of PPI can secrete gastric acid. Frequent PPI dosing sustains plasma PPI levels for a longer time to achieve sufficient acid inhibition over 24 h. Sugimoto et al have investigated the effects of frequent PPI dosing on subjects with PPI-resistant NERD, with the aim of determining the clinical characteristics of subjects with PPI-refractory NERD resistant to potent acid inhibition.
Sugimoto et al have highlighted the clinical characteristics of PPI-refractory NERD patients. In subjects with NERD that is responsive to potent acid inhibition, the Frequency Scale for the Symptoms of GERD (FSSG) scores decreased by > 30% compared with baseline after a standard dose of PPI. Therefore, NERD patients whose FSSG reduces by > 30% after a standard dosage of PPI will respond to a higher dose of PPI, although the clinical effects of standard PPI dosages on their symptoms are limited.
The FSSG score after PPI treatment may show promise in determining the optimal treatment for patients with NERD that is refractory to a standard PPI dosage; increasing the PPI dosage or other agents such as prokinetics. The clinical usefulness of the FSSG in the PPI treatment of NERD requires verification in further studies with larger subject numbers.
The study analyzed the problem of patients with symptoms of GERD but refractory to PPIs. This is a very important problem in the treatment of these patients. The study was well designed and the results are clearly described.
Peer reviewer: Elfriede Bollschweiler, Professor, Department of Surgery, University of Cologne, Kerpener Straße 62, 50935 Köln, Germany
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190-200. |
| 2. | Fass R. Epidemiology and pathophysiology of symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 2003;98:S2-S7. |
| 3. | Hongo M. Minimal changes in reflux esophagitis: red ones and white ones. J Gastroenterol. 2006;41:95-99. |
| 4. | Fujiwara Y, Higuchi K, Shiba M, Yamamori K, Watanabe Y, Sasaki E, Tominaga K, Watanabe T, Oshitani N, Arakawa T. Differences in clinical characteristics between patients with endoscopy-negative reflux disease and erosive esophagitis in Japan. Am J Gastroenterol. 2005;100:754-758. |
| 5. | Fujiwara Y, Kohata Y, Kaji M, Nebiki H, Yamasaki T, Sasaki E, Hayakawa T, Machida H, Tanigawa T, Watanabe K. Sleep dysfunction in Japanese patients with gastroesophageal reflux disease: prevalence, risk factors, and efficacy of rabeprazole. Digestion. 2010;81:135-141. |
| 6. | Miwa H, Minoo T, Hojo M, Yaginuma R, Nagahara A, Kawabe M, Ohkawa A, Asaoka D, Kurosawa A, Ohkusa T. Oesophageal hypersensitivity in Japanese patients with non-erosive gastro-oesophageal reflux diseases. Aliment Pharmacol Ther. 2004;20 Suppl 1:112-117. |
| 7. | Fass R, Ofman JJ. Gastroesophageal reflux disease--should we adopt a new conceptual framework? Am J Gastroenterol. 2002;97:1901-1909. |
| 8. | Miner P Jr, Orr W, Filippone J, Jokubaitis L, Sloan S. Rabeprazole in nonerosive gastroesophageal reflux disease: a randomized placebo-controlled trial. Am J Gastroenterol. 2002;97:1332-1339. |
| 9. | Fock KM, Teo EK, Ang TL, Chua TS, Ng TM, Tan YL. Rabeprazole vs esomeprazole in non-erosive gastro-esophageal reflux disease: a randomized, double-blind study in urban Asia. World J Gastroenterol. 2005;11:3091-3098. |
| 10. | Adachi K, Hashimoto T, Hamamoto N, Hirakawa K, Niigaki M, Miyake T, Taniura H, Ono M, Kaji T, Suetsugu H. Symptom relief in patients with reflux esophagitis: comparative study of omeprazole, lansoprazole, and rabeprazole. J Gastroenterol Hepatol. 2003;18:1392-1398. |
| 11. | Robinson M, Fitzgerald S, Hegedus R, Murthy A, Jokubaitis L. Onset of symptom relief with rabeprazole: a community-based, open-label assessment of patients with erosive oesophagitis. Aliment Pharmacol Ther. 2002;16:445-454. |
| 12. | Holtmann G, Bytzer P, Metz M, Loeffler V, Blum AL. A randomized, double-blind, comparative study of standard-dose rabeprazole and high-dose omeprazole in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:479-485. |
| 13. | Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798-1810. |
| 14. | Van Pinxteren B, Sigterman KE, Bonis P, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2010;CD002095. |
| 15. | Bate CM, Green JR, Axon AT, Murray FE, Tildesley G, Emmas CE, Taylor MD. Omeprazole is more effective than cimetidine for the relief of all grades of gastro-oesophageal reflux disease-associated heartburn, irrespective of the presence or absence of endoscopic oesophagitis. Aliment Pharmacol Ther. 1997;11:755-763. |
| 16. | Uemura N, Inokuchi H, Serizawa H, Chikama T, Yamauchi M, Tsuru T, Umezu T, Urata T, Yurino N, Tanabe S. Efficacy and safety of omeprazole in Japanese patients with nonerosive reflux disease. J Gastroenterol. 2008;43:670-678. |
| 17. | Furuta T, Ohashi K, Kosuge K, Zhao XJ, Takashima M, Kimura M, Nishimoto M, Hanai H, Kaneko E, Ishizaki T. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552-561. |
| 18. | Sugimoto M, Furuta T, Shirai N, Kajimura M, Hishida A, Sakurai M, Ohashi K, Ishizaki T. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004;76:290-301. |
| 19. | Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Yamade M, Ikuma M, Watanabe H, Ohashi K, Hishida A. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother. 2007;8:2701-2717. |
| 20. | Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85-92. |
| 21. | Kusano M, Shimoyama Y, Sugimoto S, Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H, Ino K. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888-891. |
| 22. | Kusano M, Shimoyama Y, Kawamura O, Maeda M, Kuribayashi S, Nagoshi A, Zai H, Moki F, Horikoshi T, Toki M. Proton pump inhibitors improve acid-related dyspepsia in gastroesophageal reflux disease patients. Dig Dis Sci. 2007;52:1673-1677. |
| 23. | Nakamura A, Furuta T, Shirai N, Sugimoto M, Kajimura M, Soya Y, Hishida A. Determination of mutations of the 23S rRNA gene of Helicobacter pylori by allele specific primer-polymerase chain reaction method. J Gastroenterol Hepatol. 2007;22:1057-1063. |
| 24. | Mishima I, Adachi K, Arima N, Amano K, Takashima T, Moritani M, Furuta K, Kinoshita Y. Prevalence of endoscopically negative and positive gastroesophageal reflux disease in the Japanese. Scand J Gastroenterol. 2005;40:1005-1009. |
| 25. | Wiklund I. Review of the quality of life and burden of illness in gastroesophageal reflux disease. Dig Dis. 2004;22:108-114. |
| 26. | Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med. 1999;159:649-657. |
| 27. | Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51 Suppl 1:59-67. |
| 28. | Sugimoto M, Furuta T, Shirai N, Nakamura A, Kajimura M, Hishida A, Ohashi K, Ishizaki T. Comparison of an increased dosage regimen of rabeprazole versus a concomitant dosage regimen of famotidine with rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotypes. Clin Pharmacol Ther. 2005;77:302-311. |
| 29. | Joh T, Miwa H, Higuchi K, Shimatani T, Manabe N, Adachi K, Wada T, Sasaki M, Fujiwara Y, Hongo M. Validity of endoscopic classification of nonerosive reflux disease. J Gastroenterol. 2007;42:444-449. |
| 30. | Fass R, Fennerty MB, Vakil N. Nonerosive reflux disease--current concepts and dilemmas. Am J Gastroenterol. 2001;96:303-314. |
| 31. | Watson RG, Tham TC, Johnston BT, McDougall NI. Double blind cross-over placebo controlled study of omeprazole in the treatment of patients with reflux symptoms and physiological levels of acid reflux--the "sensitive oesophagus". Gut. 1997;40:587-590. |
| 32. | Keohane J, Quigley EM. Functional dyspepsia and non-erosive reflux disease. A review. Minerva Gastroenterol Dietol. 2006;52:261-267. |
| 33. | Quigley EM. Functional dyspepsia (FD) and non-erosive reflux disease (NERD): overlapping or discrete entities? Best Pract Res Clin Gastroenterol. 2004;18:695-706. |
| 34. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. |
| 35. | Futagami S, Iwakiri K, Shindo T, Kawagoe T, Horie A, Shimpuku M, Tanaka Y, Kawami N, Gudis K, Sakamoto C. The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J Gastroenterol. 2010;45:413-421. |
| 36. | Kinoshita Y, Kobayashi T, Kato M, Asahina K, Haruma K, Shimatani T, Inoue S, Kabemura T, Kurosawa S, Kuwayama H. The pharmacodynamic effect of omeprazole 10 mg and 20 mg once daily in patients with nonerosive reflux disease in Japan. J Gastroenterol. 2006;41:554-561. |
| 37. | Miwa H, Sasaki M, Furuta T, Koike T, Habu Y, Ito M, Fujiwara Y, Wada T, Nagahara A, Hongo M. Efficacy of rabeprazole on heartburn symptom resolution in patients with non-erosive and erosive gastro-oesophageal reflux disease: a multicenter study from Japan. Aliment Pharmacol Ther. 2007;26:69-77. |