Published online Apr 14, 2011. doi: 10.3748/wjg.v17.i14.1825
Revised: January 11, 2011
Accepted: January 18, 2011
Published online: April 14, 2011
AIM: To study the effect of breviscapine (Bre) on activity of protein kinase Cα (PKCα) and nuclear factor (NF)-κB in pancreas, and the mechanism of Bre attenuating acute pancreatitis (AP).
METHODS: One hundred and eight rats were randomly divided into acute necrotizing pancreatitis (ANP) group, Bre group (ANP + Bre group) and sham operation (SO) group, 36 rats in each group. ANP model was induced by a retrograde injection of 4% sodium deoxycholate into the bilio-pancreatic duct. Fifteen minutes after the ANP model was induced, the rats in Bre group were intraperitoneally injected with Bre (0.4 mg/100 g body weight or 0.1 mL/100 g body weight). Survival time and mortality of rats were calculated. Serum amylase and malondialdehyde levels were measured, volume of ascites was recorded and morphology of pancreas and lung was evaluated at 1, 5 and 10 h, after the ANP model was induced, respectively. Expressions of PKCα and subunit p65 of NF-κB in pancreas were detected by immunohistochemistry and Western blotting.
RESULTS: The life span of rats was longer and the mortality was lower in Bre group than in ANP group 13.51 ±5.46 vs 25.36 ± 8.11 (P < 0.05). The amylase and MDA levels as well as the volume of ascites were lower and the pathological changes in pancreas and lung were less in Bre group than ANP group (P < 0.05), indicating that the pancreatitis is less severe in Bre group than ANP group. The activation of PKCα and NF-κB p65 in pancreas was induced rapidly and reached their peak at 1 h or 5 h after ANP, but their activity in Bre group was significantly inhibited.
CONCLUSION: Bre exerts its therapeutic effect on AP by inhibiting the activation of PKCα and NF-κB p65 in pancreas.
- Citation: Zhang H, Cai CZ, Zhang XQ, Li T, Jia XY, Li BL, Song L, Ma XJ. Breviscapine attenuates acute pancreatitis by inhibiting expression of PKCα and NF-κB in pancreas. World J Gastroenterol 2011; 17(14): 1825-1830
- URL: https://www.wjgnet.com/1007-9327/full/v17/i14/1825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i14.1825
The complete mechanism of acute pancreatitis (AP) has not been established so far. The initial events in this disorder occur in pancreatic acinar cells, including activation of zymogens in acinar cells and release of inflammatory cytokines. It has been shown that nuclear factor (NF)-κB is a key regulator of the expression of many inflammatory molecules[1,2]. In experimental pancreatitis, NF-κB activation in acinar cells is one of the early events[3], and the inhibition of NF-κB activation attenuates inflammatory response and the severity of AP[4,5]. However, the signaling mechanisms mediating NF-κB activation are unclear.
One candidate for mediating NF-κB activation in pancreatic acinar cells is protein kinase C[6,7]. Protein kinase C (PKC) is a member of serine/threonine kinases comprising 10 isoforms that differ in their structures and regulations[8]. These isoforms can be subdivided into conventional PKC, novel PKC, and atypical PKC isoforms based on their molecular structure and mode of activation. PKCα belongs to conventional PKC isoforms which can mediate pathological secretory processes in experimental pancreatitis and regulate expression of inflammatory mediators[9]. PKC inhibitors significantly reduce pancreatic and pulmonary levels of myeloperoxidase (MPO) and pancreatic protease activity, and further ameliorate inflammatory injury of organs[10].
Breviscapine (Bre), a commercially available plant extract from the herb erigeron breviscapus, can inhibit PKC activation[11,12]. Bre possesses comprehensive pharmacological effects and has been widely used in treatment of disorders in blood supply to the heart and brain, as well as ischemic diseases[12,13]. Bre can elevate the activity of ATPase and superoxide dismutase SOD and reduce the malondialdehyde (MDA) level in brain mitochondria of rats after trauma[14]. MDA as a product of thiobarbituric acid reactant, is considered a good indicator of lipid peroxidation and one of the useful makers for AP[15]. It was reported Bre is effective against AP[16]. However, its therapeutic mechanism is still unclear[17]. In an attempt to further explore its mechanism in treatment of AP, a rat model of acute necrotizing pancreatitis (ANP) was induced to observe whether Bre exerts its protective effect against ANP by inhibiting the activation of PKCα and NF-κB.
One hundred and eight Sprague-Dawley rats weighing 200-250 g, obtained from Experimental Animal Center of Fourth Military Medical University (Grade SPF Certificate No.2005-005), were fasted for 12 h with free access to water. Antibodies against PKCα and NF-κB P65 were purchased from Santa Cruz Biotechnology Company (CA, USA). Secondary antibody and kits for immunohistochemistry were provided by Zhongshan Company (Beijing, China). Bre injection was purchased from Feixia Pharmacology Company (Harbin, China). Kits for amylase and MDA were obtained from Nanjing Jiancheng Biotechnology Company (Nanjing, China). All other chemicals were those of the highest purity.
The rats were randomly divided into acute necrotizing pancreatitis (ANP) group, Bre group (ANP + Bre group) and sham operation (SO) group, 36 rats in each group. A rat model of ANP was induced by retrograde injection of 4% sodium deoxycholate (0.1 μL/100 g body weight) into the bilio-pancreatic duct (BPD) as previously described[18]. Briefly, a small median laparotomy was performed to exposed the pancreas, the BPD was temporarily closed at the hilum of liver with a small soft bulldog clamp to prevent reflux of the infused material into the liver, and then 4% sodium deoxycholate (100 μL/100 g body weight) was injected into the distal BPD at a pressure of 50 cmH2O. The clamp was removed 5 min after the injection of sodium deoxycholate. The rats in SO group only underwent laparotomy. Finally, the abdomen was closed with a silk suture. Fifteen minutes after the model was induced, the rats in Bre group were intraperitoneally injected with Bre (0.4 mg/100 g body weight or 0.1 mL/100 g body weight) and those in the other two groups were given the same volume of normal saline.
Twelve rats in each group were observed during 72 h, their survival time and mortality in 24 h were recorded. The rats were sacrificed at 1, 5 and 10 h, respectively, after the rat AP model was induced. Whole blood samples were centrifuged at 4°C, and serum was stored at -80°C for amylase and MDA analysis. Ascites volume was recorded, and pancreas and lung morphology was evaluated. Expressions of PKCα and subunit p65 of NF-κB in pancreas were detected by immunohistochemistry or Western blotting.
Pancreas and lung were removed at 1, 5 or 10 h for morphological analyses after the model was induced, immediately immersed in 4% neutral phosphate-buffered paraformaldehyde for 12 h, embedded in paraffin, and cut into 5-μm thick sections which were stained with H&E to observe the morphological changes under a light microscope. The severity of AP was blindly graded by a semi-quantitative assessment of vacuolization, edema, inflammatory cell infiltration and acinar cell necrosis as previously described[19]. Ten microscopic fields were randomly chosen to observe them in each rat. Histological scoring of pancreatic tissue was performed to grade the extent of acinar cell vacuolization (0: none, 1: < 20% acini with vacuoles, 2: < 50% acini, 3: > 50% acini), edema (0: no edema, 1: interlobular edema, 2: intralobular edema 3: interacinar edema), inflammation (0: no inflammation, 1: inflammatory cells present at intralobular, 2: inflammatory cells present at intralobular 3: inflammatory cells present at interacini) and acinar cell necrosis (0: no necrosis, 1: < 10% necrosis, 2: < 40% necrosis, 3: > 40% necrosis).
Immunohistochemical analysis was performed. In brief, frozen pancreas was cut into 8-μm sections which were air-dried and treated sequentially with acetone at 4°C for 10 min, three times of phosphate-buffered saline (PBS) at pH 7.4, 5% bovine serum album (BSA) in PBS at pH7.4 for 30 min, PKCα (Santa Cruz Biotechnology Inc. sc-8393) diluted at 1:100 or NF-κB P65 (Santa Cruz Biotechnology Inc. sc-8008) diluted at 1:80 in a humid chamber overnight, three times of 0.02% Tween 20 in PBS for 10 min each time, biotinylated secondary antibody diluted at 1/500 for 40 min. The bound peroxidase was visualized by reaction for 2-5 min in a solution containing 50 mg of 3, 3-diaminobenzidine (DAB), counterstained with hematoxylin, dehydrated and embedded. For PKCα in common status, the cytoplasm of positive cells was stained, and translocation of positive cells to membrane from cytoplasm meant activation of PKCα. For NF-κB P65 in common status, the cytoplasm of positive cells was stained, and translocation of positive cells to nuclei from cytoplasm meant activation of NF-κB P65. The slides were observed under a light microscope.
For Western blotting analysis, cellular proteins were prepared from pancreas with standard methods. Protein concentration was measured (Bio-Rad protein assay) and adjusted to 4 ug/μL with loading buffer. The samples were boiled at 95°C for 5 min before they were loaded 10 uL, subjected to SDS-polyacrylamide gel electrophoresis, and blotted to PVDF membranes. The membranes were probed with anti-PKCα diluted at 1/1000 (Santa Cruz Biotechnology Inc. sc-8393) and anti-NF-κB P65 diluted at 1/1000 (Santa Cruz Biotechnology Inc. sc-8008). A low molecular weight protein marker was used to determine the size of bands detected by Western blotting.
Data were expressed as mean ± SD, compared by non paired Student t test and one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
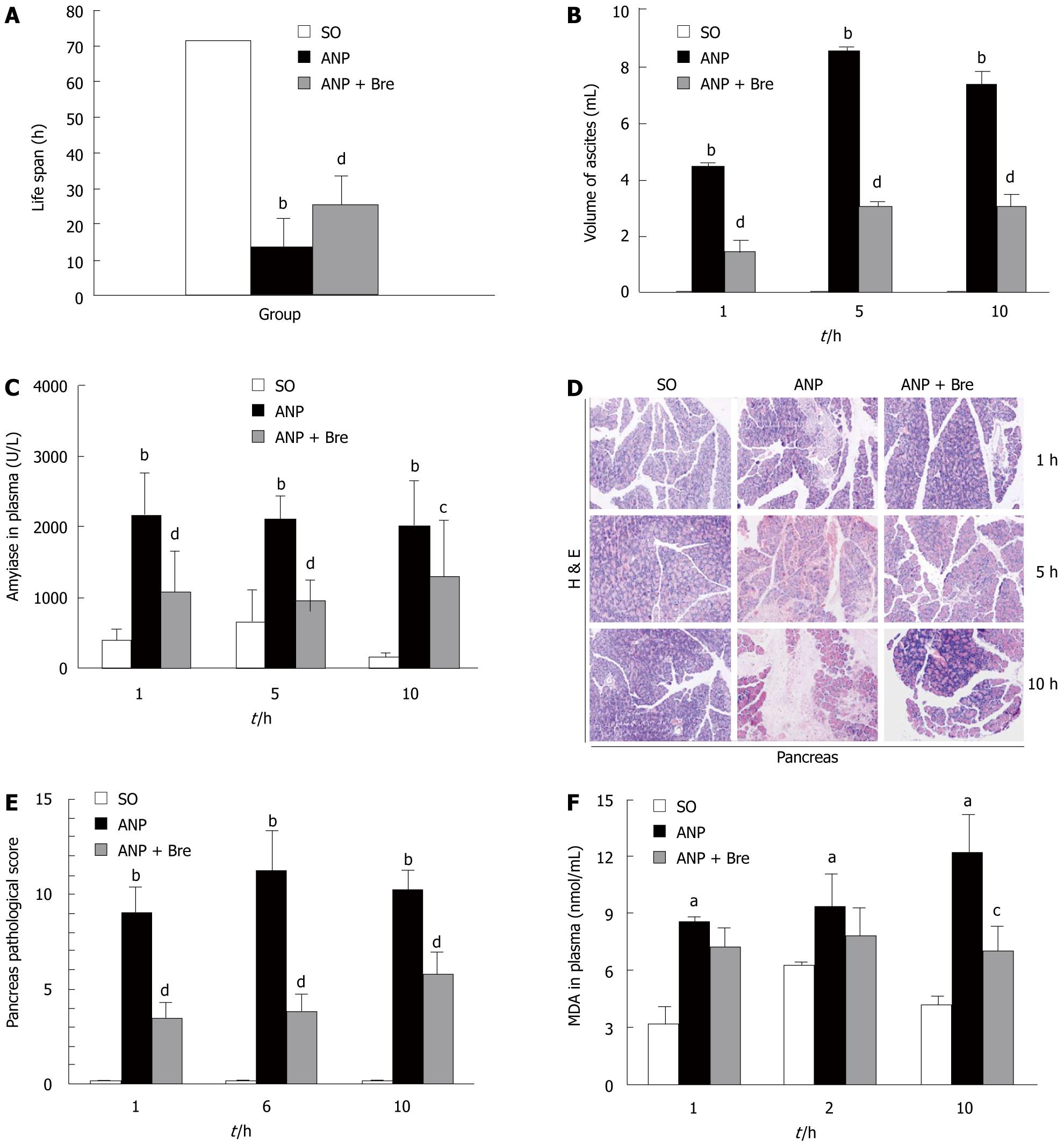
No rat in SO group died in 72 h. The mortality of rats in ANP group was 100% in 24 h with a life span of 13.51 ± 5.46 h, while the mortality of rats in Bre group was 50% with a life span of 25.36 ± 8.11 h (P < 0.01, Figure 1A).
Ascites was not produced in SO group at any time points. The volume of ascites was 4.46 ± 1.62 mL at 1 h and 8.57 ± 2.38 mL at 5 h after the model was induced in ANP group. The volume of ascites was smaller in Bre group than in ANP group (P < 0.01, Figure 1B).
The serum amylase level was significantly higher at 1, 5, and 10 h after the model was induced in ANP group than in SO group (P < 0.01). The amylase activity was lower in Bre group at each time point than in ANP group (P < 0.05, Figure 1C).
The pancreas and lung morphology was significantly different in rats with AP. Sodium deoxycholate caused mild interlobular edema at 1 h, and inflammatory cell infiltration, hemorrhage and acinar cell necrosis at 5 and 10 h after the model was induced. The morphological change of pancreatic tissue was milder (Figure1 D) and the morphological score was lower in Bre group than in ANP group (P < 0.01, Figure 1E). In contrast to the rats in SO group, those in ANP group exhibited severe inflammation of the lungs as indicated by alveolar fluid accumulation and progressive thickening of the interalveolar tissue, while those in Bre group showed milder morphological changes in lungs.
The MDA increased significantly with the development of AP in ANP group. The MDA level was significantly lower in Bre group than in ANP group at 1, 5, and 10 h after the model was induced (P < 0.05, Figure 1F).
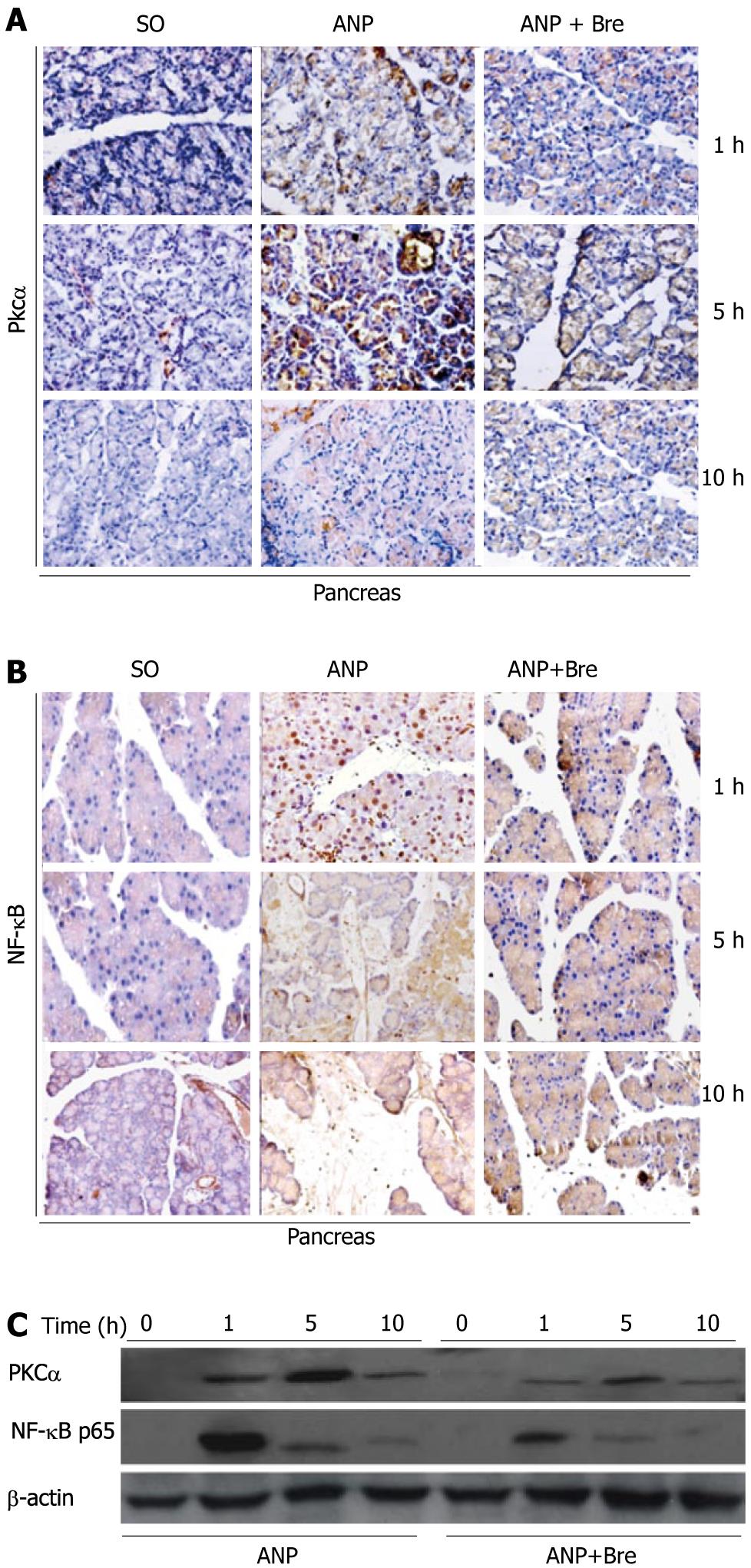
Immunohistochemical analysis showed no PKCα and NF-κB P65 expression in acinar cells of rats in SO group at any time points. PKCα was detected at 1 h after the model was induced and localized in cytoplasm of acini (Figure 2A). However, the PKCα expression increased in cytoplasm at 5 h after the model was induced and could be detected on the membrane of acini. Only little staining of acini was observed at 10 h after the model was induced. The expression of NF-κB P65 was detected at 1 h after the model was induced and localized in cytoplasm or nuclei of acini and inflammatory cells (Figure 2B). The NF-κB P65 expression level in pancreas was lower at 5 and 10 h than at 1 h after the model was induced AP (P < 0.05). Western blotting showed that the expression level of PKCα and NF-κB P65 in pancreatic tissue was significantly lower in Bre group than in ANP group (P < 0.05, Figure 2C).
PKC can mediate NF-κB activation in pancreatic acinar cells[6,7] and pathological secretory processes in experimental pancreatitis, and regulate the expression of inflammatory mediators[9]. In our study, ANP was induced with sodium deoxycholate and the expression of PKCα and NF-κB P65 in frozen sections of pancreas tissue was observed by immunohistochemical analysis. PKCα was detected only in acini, while NF-κB P65 was localized in acini and inflammatory cells. The expression of PKCα and NF-κB P65 increased in the early stage of ANP, and both PKCα and NF-κB P65 played an important role in the development of ANP.
It was reported that NF-κB can be activated by protein kinase C and intracellular Ca2+[6,7], suggesting that PKC is an upstream regulator of NF-κB activation in pancreatic acinar cells. In the present study, the effect of Bre, a PKC inhibitor, on ANP and activation of PKCα and NF-κB was observed, showing that Bre could decrease the mortality, the severity of AP, the ascites production, the serum amylase level in rats with ANP, and prolonged their survival time. The morphological changes in pancreas and lungs of rats with ANP after treated with Bre were milder, suggesting that PKC is related to AP and can alleviate the pathological injury by inhibiting PKC. Furthermore, the serum MDA level was lower in Bre group than in ANP group, suggesting that Bre can eliminate the production of oxygen-free radicals and further attenuate the harmful influence of peroxidation on pancreas and lungs[20,21]. The expression of PKCα and NF-κB P65 was significantly lower in Bre group than in ANP group, indicating that Bre can inhibit the activation of PKCα and NF-κB which are important for the release of many inflammatory molecules and inflammatory response[3,22,23].
In conclusion, PKC activation may contribute to inflammatory response and cell injury in pancreas and lungs. Bre exerts its protective effect against ANP by inhibiting PKC and NF-κB activation, which can explain the therapeutic mechanism of Bre for AP at molecular level.
Inflammatory response is important in the development of acute necrotizing pancreatitis (ANP). Nuclear factor (NF)-κB can regulate the expression of many inflammatory molecules. One candidate for mediating NF-κB activation in pancreatic acinar cells is protein kinase C (PKC). Breviscapine (Bre) extracted from erigeron can inhibit the activation of PKC.
Some studies demonstrated that PKC activation plays an important role in the development of acute pancreatitis (AP). PKC inhibitor can significantly reduce pancreatic protease activity and myeloperoxidase (MPO) level in pancreas or lungs, and further alleviate the inflammatory injury of organs.
As an inhibitor of PKC activation, Bre is effective against AP. However, its therapeutic mechanism is still unclear. In the authors’ present study, a rat model of ANP was induced to observe the effect and mechanism of Bre on ANP, showing that Bre exerts its protective effect against ANP by inhibiting PKC and NF-κB activation.
The results of this study suggest that PKC activation may contribute to inflammatory response and cause cell injury in pancreas and lungs, which may explain the therapeutic mechanism of Bre for AP at biological level and accelerate its applications in treatment of AP.
PKCs are a family of serine/threonine kinases comprising 10 isoforms that differ in their structures and regulations. These isoforms can be subdivided into three classes based on their molecular structure and mode of activation, namely conventional PKC isoforms, novel PKC isoforms, and atypical PKC isoforms. PKCα belongs to conventional PKC isoforms which can mediate pathological secretory processes in experimental pancreatitis and regulate the expression of inflammatory mediators.
In this study, the authors showed that Bre exerts its protective effect against ANP by inhibiting PKC and NF-κB activation. As a PKC inhibitor, Bre can significantly reduce pancreatic protease activity and MPO level in pancreas or lungs, and further alleviate the inflammatory injury of organs, and can thus be applied in treatment of AP.
Peer reviewers: Naoaki Sakata, MD, PhD, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan; Giedrius Barauskas, Professor, Department of Surgery, Kaunas University of Medicine, Eiveniu str. 2, Kaunas, LT-50009, Lithuania; Dr. Ashok Kumar, MD, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, 226014, India
S- Editor Tian L L- Editor Wang XL E- Editor Ma WH
| 1. | Schmid RM, Adler G. NF-kappaB/rel/IkappaB: implications in gastrointestinal diseases. Gastroenterology. 2000;118:1208-1028. |
| 2. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. |
| 3. | Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197-G1208. |
| 4. | Gukovsky I, Reyes CN, Vaquero EC, Gukovskaya AS, Pandol SJ. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G85-G95. |
| 5. | Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253-258. |
| 6. | Gukovskaya AS, Hosseini S, Satoh A, Cheng JH, Nam KJ, Gukovsky I, Pandol SJ. Ethanol differentially regulates NF-kappaB activation in pancreatic acinar cells through calcium and protein kinase C pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G204-G213. |
| 7. | Han B, Logsdon CD. CCK stimulates mob-1 expression and NF-kappaB activation via protein kinase C and intracellular Ca(2+). Am J Physiol Cell Physiol. 2000;278:C344-C351. |
| 8. | Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429-L438. |
| 9. | Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR Jr, Shimosegawa T, Pandol SJ. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582-G591. |
| 10. | Shi C, Zhao X, Wang X, Zhao L, Andersson R. Potential effects of PKC or protease inhibitors on acute pancreatitis-induced tissue injury in rats. Vascul Pharmacol. 2007;46:406-411. |
| 11. | Zhang SJ, Song Y, Zhai WL, Shi JH, Feng LS, Zhao YF, Chen S. Breviscapine alleviates hepatic injury and inhibits PKC-mRNA and its protein expression in brain-dead BA-Ma mini pigs. Hepatobiliary Pancreat Dis Int. 2007;6:604-609. |
| 12. | Shuai J, Dong WW. Experimental research of PKC inhibitor, erigeron breviscapus on the ischemic/reperfusional brain injury. Zhongguo Yaolixue Tongbao. 1998;14:75-77. |
| 13. | Xu LX. The Effect of Breviscapine on 67 cases of cerebral infarction. Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:227-228. |
| 14. | ChengY , Chen X, Zhu ZA, Zhang H, Ma YB. Effects of Erigeron Breviscapus on the Activity of ATPase, SOD and the Content of MDA in Brain Mitochondria in Post-traumatic Rats‘ Brain. Shanghai Zhongyiyao Daxue Xuebao. 2004;18:58-60. |
| 15. | Tascilar O, Cakmak GK, Tekin IO, Emre AU, Ucan BH, Bahadir B, Acikgoz S, Irkorucu O, Karakaya K, Balbaloglu H. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol. 2007;13:6172-6182. |
| 16. | Huang DK. The effect of Breviscapine in serve pancreatitis. Zhongguo Shiyong Yiyao. 2006;4:11-12. |
| 17. | Wang YF, Huo L, Wu Y, Zhao YF, Zhang SJ. The protective effect of breviscapine on brain tissues in rats with acute hemorrhagic necrotizing pancreatitis. Zhongguo Putong Waike Zazhi. 2008;17:857-860. |
| 18. | Aho HJ, Suonpää K, Ahola RA, Nevalainen TJ. Experimental pancreatitis in the rat. Ductal factors in sodium taurocholate-induced acute pancreatitis. Exp Pathol. 1984;25:73-79. |
| 19. | Schmidt J, Lewandrowsi K, Warshaw AL, Compton CC, Rattner DW. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. Int J Pancreatol. 1992;12:41-51. |
| 20. | Chen SR. The Effect of Breviscapine of anti-oxidation and Hypercoagulability in patients with serve pancreatitis. Jiangsu Yiyao. 2001;27:62. |
| 21. | Li ZD, Ma QY, Wang CA. Effect of resveratrol on pancreatic oxygen free radicals in rats with severe acute pancreatitis. World J Gastroenterol. 2006;12:137-140. |
| 22. | Zhang XP, Xu HM, Jiang YY, Yu S, Cai Y, Lu B, Xie Q, Ju TF. Influence of dexamethasone on mesenteric lymph node of rats with severe acute pancreatitis. World J Gastroenterol. 2008;14:3511-3517. |
| 23. | Wang YJ, Sun JB, Li F, Zhang SW. Hyperlipidemia intensifies cerulein-induced acute pancreatitis associated with activation of protein kinase C inrats. World J Gastroenterol. 2006;12:2908-2913. |