Published online Apr 14, 2011. doi: 10.3748/wjg.v17.i14.1817
Revised: January 4, 2011
Accepted: January 11, 2011
Published online: April 14, 2011
AIM: To analyze the radiological features of multiple primary carcinoma (MPC) in the upper gastrointestinal (GI) tract, study its biological characteristics and evaluate X-ray examination in its diagnosis.
METHODS: Hypotonic double-contrast GI radiography was performed in 59 multiple primary carcinoma cases, pathologically proved by surgery or endoscopy biopsy. Radiological findings were analyzed.
RESULTS: Of the 59 cases, esophageal MPC (EMPC) was seen in 24, esophageal and gastric MPC (EGMPC) in 27 and gastric MPC (GMPC) in 8. Of the 49 lesions found in 24 EMPC, hyperplastic type was seen in 23, medullary type in 9. The lesions were located at the upper (n = 17), middle (n = 19) or lower (n = 13) segment of the esophagus. In 27 EGMPC, the esophageal lesions were located at the middle (n = 16) or lower (n = 11) segment of the esophagus, while the gastric lesions were located at the gastric cardia (n = 16), fundus (n = 1), body (n = 3) and antrum (n = 7). The esophageal lesions were mainly of the hyperplastic type (n = 12) or medullary type (n = 7), while the gastric lesions were mainly of the hyperplastic type (n = 18). A total of 119 lesions in the 59 patients with synchronous multiple carcinoma were proved by surgery or endoscopy biopsy, and preoperative upper radiographic examination detected 100 of them (84.03% sensitivity). Eighteen (52.94%) of the T1 lesions were found during preoperative diagnosis by radiographic examination. Moreover, only 3 (3.53%) of the T2-4 lesions were misdiagnosed.
CONCLUSION: Hypotonic double-contrast upper gastrointestinal examination, providing accurate information about lesion morphology, location and size, can serve as a sensitive technique for the preoperative diagnosis of MPC.
- Citation: Yang ZH, Gao JB, Yue SW, Guo H, Yang XH. X-ray diagnosis of synchronous multiple primary carcinoma in the upper gastrointestinal tract. World J Gastroenterol 2011; 17(14): 1817-1824
- URL: https://www.wjgnet.com/1007-9327/full/v17/i14/1817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i14.1817
Although malignant tumors are currently detected more often than in the past due to continued and vast improvements in medical modalities, the incidence of synchronous multiple primary carcinoma (MPC), defined as two or more primary carcinomas occurring in an individual simultaneously, is low[1]. Most of these synchronous cancers are in the head and neck region. Other frequently reported sites of synchronous cancer associated with esophageal cancer are the stomach, lung, and urinary bladder[2,3]. The mucous epithelium of the head and neck, lung, and esophagus is exposed to common carcinogenic agents, leading to multiple carcinomas in these regions. The etiology of synchronous multiple primary carcinomas is still unclear, strong epidemiologic evidence implicates tobacco as the main carcinogen and alcohol as a promoter of carcinogenesis[4]. Other potential risk factors include hot beverages[5], nutritional deficiencies[6], pickled vegetables, nitrosamine-rich food[7], and some genetic factors[8,9]. Radiation therapy is also associated with esophageal cancer[8,10]. Their coexistence can be problematic for surgeons, oncologists and pathologists, as regards diagnosis, treatment and follow-up.
Multiple primary malignant neoplasms in a single patient have been well documented with regard to the frequency of occurrence of primary multiple carcinomas, identification of high-risk groups, early diagnosis, treatment methods, and prognostic factors over the past hundred years[11]. For some reason, synchronous multiple primary carcinomas of the upper gastrointestinal (GI) tract can often be overlooked at the time of diagnosis, and so far, there has not been a consensus on how best to diagnose mucosal lesions of multiple primary carcinomas in the upper GI tract: by endoscopy or barium radiography? The double-contrast upper GI examination is a safe, noninvasive, inexpensive and cost-effective test for the work-up of a host of GI diseases. However, reports exclusively on evaluating X-ray examination in the diagnosis of synchronous MPC of the upper gastrointestinal tract have been few. In this study, we retrospectively analyzed 59 synchronous MPC cases proved by surgery or endoscopy biopsy: each of the patients underwent radiographic examination. The aim was to analyze the radiological features of synchronous MPC in the upper GI tract, study its biological characteristics, evaluate X-ray examination in its diagnosis, and seek ways to improve its preoperative diagnosis.
Patients with MPC were selected from January 1988 to December 2008 at the First Affiliated Hospital of Zhengzhou University. Of the 59 patients aged 42-85 years (mean: 61.20 years), 44 were men and 15 were women. Main clinical manifestations were choking or swallowing difficulties, some cases were associated with anemia, weight loss, abdominal pain and other upper GI signs or symptoms. Specimens for pathologic confirmation of the 59 cases were obtained by endoscopy biopsy alone in 13 patients, by endoscopy biopsy and surgery in 27, and by surgery alone in 19. Thirteen patients did not have surgery, either because they were not considered surgical candidates or because they chose to be treated elsewhere.
After 6-8 h of fasting, patients first received 20 mg hypotonic drugs as well as anisodamine (654-2) intravenous injection. Ten minutes later, the patient swallowed 3 g aerogenic agents, and then, as fast as possible, 150 mL of resuspended barium sulfate at 200% weight for volume (w/v). Three to four spot radiographs were obtained in sequence at different levels of the esophagus, and double-contrast esophagograms were obtained with the patient in the upright LPO position. Multi-position dynamic observation of the stomach and duodenum was performed for all cases, and all patients were pathologically confirmed by endoscopic biopsy or surgery.
According to the principle of Warren and Gates, multiple primary carcinomas are defined as follows[12]: (1) Each lesion is histopathologically malignant; (2) Each lesion is separated by the normal mucosa; and (3) Each lesion is not the result of local extension or metastasis of another lesion. Multiple primary cancers may be synchronous or metachronous depending on the interval between their diagnosis. Synchronous carcinomas were diagnosed simultaneously or within an interval of about 6 mo, and metachronous carcinomas were secondary cancers that developed more than 6 mo after the diagnosis of primary cancers usually after treatment of primary lesions. In our series of 59 cases, all were synchronous multiple primary carcinoma.
The radiographs were reviewed retrospectively by two authors to determine the location, size, morphology and interval distance of these tumors. Lesions were classified morphologically as hyperplastic, medullary, infiltrative, ulcerative or mixed type. Pathologic records were also reviewed to determine the postoperative histological classification, depth of invasion and number of lesions being misdiagnosed in this group.
We evaluated the clinicopathological differences of esophageal lesions between esophageal MPC (EMPC) and esophageal-gastric MPC (EGMPC), and of gastric lesions between gastric MPC (GMPC) and EGMPC. Statistical analyses were performed using software from SPSS for Windows 17.0. The statistical methods used included χ2 test for categorical variables and t test for continuous variables. P < 0.05 was taken to indicate statistical significance.
In our series of 59 cases, all were synchronous multiple primary carcinoma, and mostly occurred in males: male/female ratio = 2.93/1, 71.19% of the patients were older than 55 years, and average age was 61.2 years old. Table 1 shows the age and sex distribution of these 59 patients.
| Gender | Age(yr) | |||||
| n | Male | Female | Range | Mean | ≥ 55 | |
| Esophageal MPC | 24 | 13 | 11 | 44-85 | 61.63 | 15/24 (62.5%) |
| Esophageal and gastric MPC | 27 | 24 | 3 | 42-72 | 59.89 | 20/27 (74.07%) |
| Gastric MPC | 8 | 7 | 1 | 48-76 | 64.38 | 7/8(87.5%) |
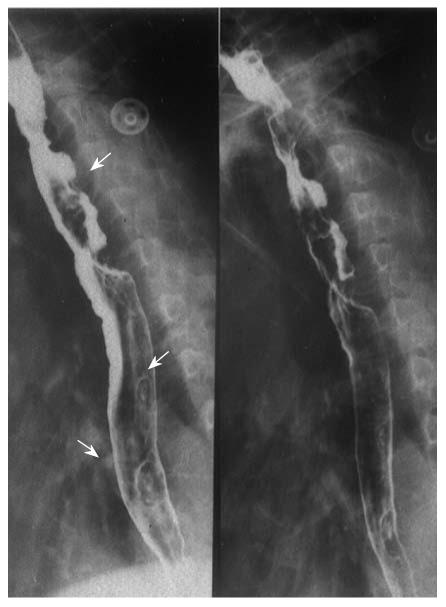
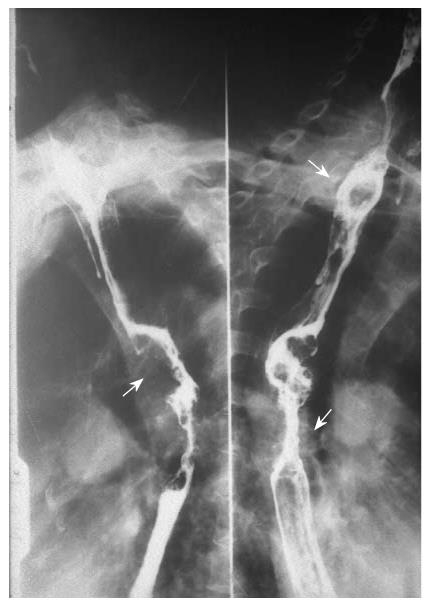
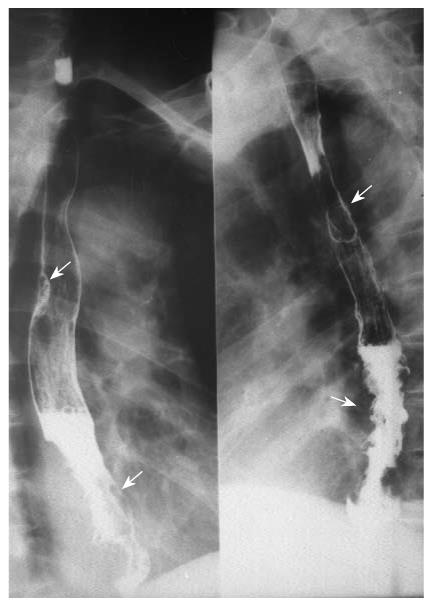
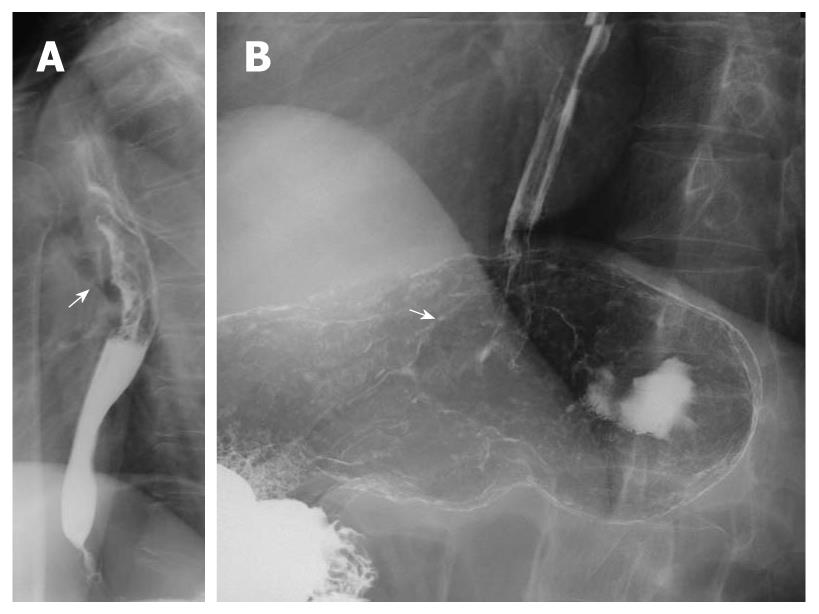
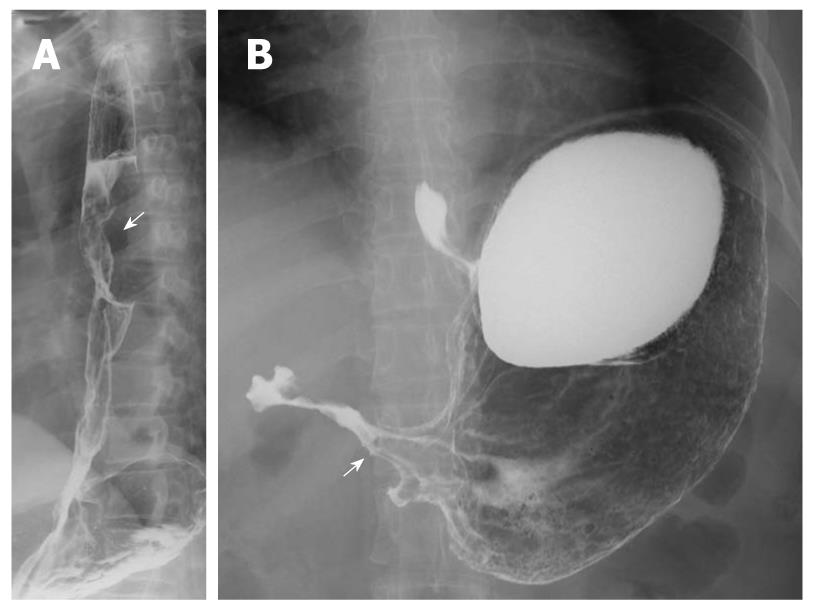
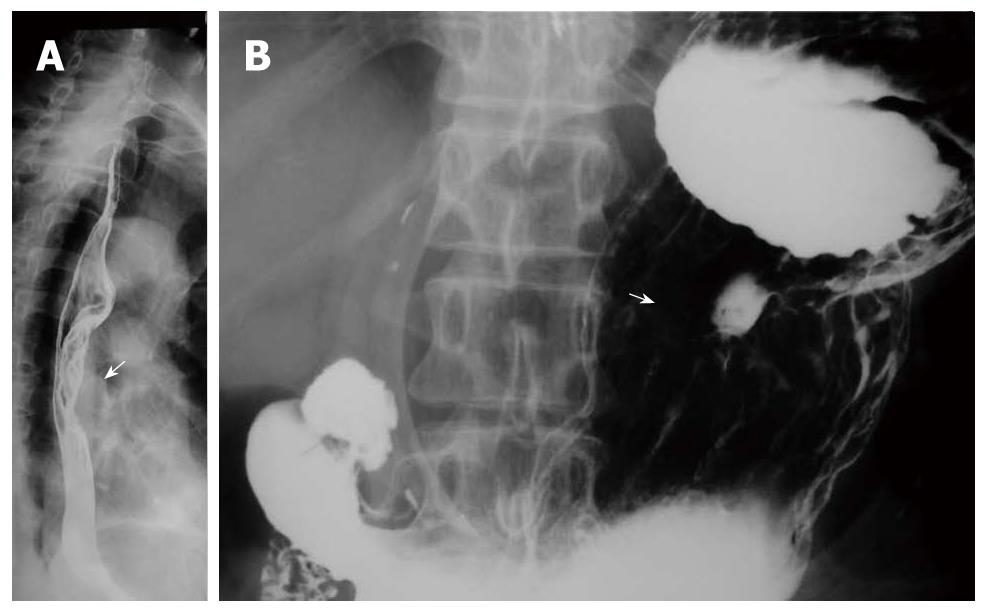
Multiple esophageal carcinoma was seen in 24, and 49 lesions were found. One case was a triple lesion (Figure 1), and the remaining 23 cases were double lesions (Figures 2, 3, 4). The distance between every two esophageal lesions ranged from 5 to 13 cm, and was 8.21 cm on average (Table 2). Of the 49 lesions, proliferative lesions made up a total of 23, followed by medullary type (n = 9). The size of the proliferative lesions ranged from 1.1 to 5.4 cm (average, 2.7 cm), medullary lesions ranged from 5.2 to 9.6 cm (average, 7.6 cm). There were 17 lesions located at the upper, 19 at the middle and 13 at the lower esophagus. Table 3 shows the largest dimension of the first lesions (3.63 ± 1.96 cm), which were significantly smaller than second ones (5.00 ± 2.67 cm). Regarding the depth of invasion, T1 lesions were more frequent in first lesions, especially in the upper region. The X-ray features of esophageal multiple primary carcinomas were the same as those of generally solitary ones. All esophageal-gastric carcinoma, and all were double lesions. Esophageal lesions concentrated in the middle (n = 16) (Figure 5A) and lower segment (n = 11). Of them, 11 were proliferative lesions (Figures 6A and 7A), 7 were medullary lesions. The gastric lesions were mainly located at the gastric cardia (n = 16) (Figure 5B), followed by antrum (n = 7) (Figure 6B), body (n = 3) (Figure 7B) and fundus (n = 1). Seventeen proliferative lesions were mainly located at the gastric cardia and the fundus, numbers of ulcerative type and infiltrating type were 6 and 4 respectively, found mainly at the antrum. In this series, one case of esophageal lesion was pathologically confirmed as sarcoma with a size of 9.1 cm × 5.3 cm × 5.2 cm, the others were squamous cell carcinoma. Twenty-three cases of gastric lesions were adenocarcinoma, and the other four cases were squamous cell carcinoma located at the cardia. From Table 3, we see that the largest dimension of esophageal lesions in EGMPC (5.56 ± 2.34 cm) was significantly larger than that of EMPC (4.33 ± 2.35 cm), and T1 esophageal lesions were more frequent in EGMPC, especially in the middle region.
| Esophageal MPC | n (%) | Interval distance (cm) | |
| Range | Mean | ||
| Upper-middle | 12 (50.00) | 5-12.5 | 8.36 |
| Upper-lower | 5 (20.83) | 8-13 | 11.20 |
| Middle-lower | 7 (29.17) | 5-9 | 5.83 |
| EMPC | EGMPC | |||
| Total (n = 49) | First lesion | Second or third lesion | (n = 27) | |
| Location | ||||
| Upper | 17 | 17 | 0 | 0 |
| Middle | 19 | 7 | 12 | 16 |
| Lower | 13 | 0 | 13 | 11 |
| Largest dimension of lesions (cm) | ||||
| mean ± SD1 | 4.33 ± 2.35 | 3.63 ± 1.96 | 5.00 ± 2.67 | 5.56 ± 2.34 |
| 95% CI | 3.63-5.02 | 2.80-4.45 | 3.90-6.10 | 4.63-6.48 |
| Morphology | ||||
| Hyperplastic | 23 | 11 | 12 | 11 |
| Medullary | 9 | 4 | 5 | 7 |
| Ulcerative | 7 | 5 | 2 | 5 |
| Infiltrative | 7 | 3 | 4 | 2 |
| Mixed | 3 | 0 | 3 | 2 |
| Histology | ||||
| Squamous cell carcinoma | 49 | 24 | 25 | 26 |
| Sarcoma | 0 | 0 | 0 | 1 |
| PT stage2 | ||||
| T1 | 12 | 9 | 3 | 13 |
| T2-4 | 37 | 15 | 22 | 14 |
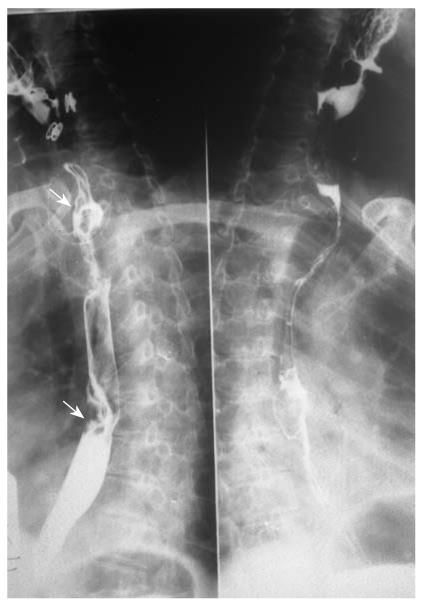
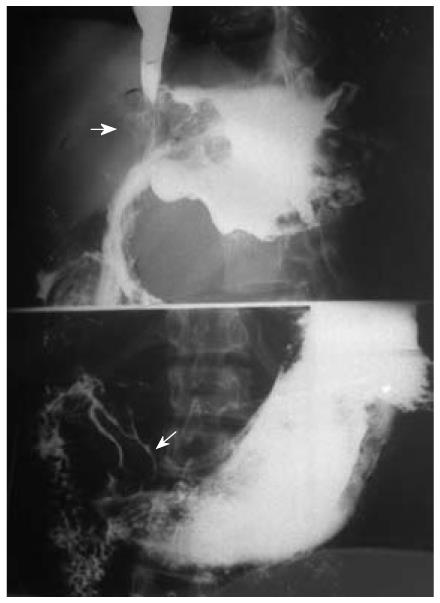
Eight patients (13.56%) were found to have synchronous multiple gastric carcinoma, all were double lesions and divided into 2 categories: main lesions (larger or advanced lesion) and additional lesions. The additional lesions were mostly located in the cardia; of them, two cases had evidence of invasion of the gastric fundus (Figure 8). Four main lesions were located in the antrum, and three at the gastric body. Thirteen (81.25%) cases were proliferative lesions, of which five cases were early gastric protruded lesions. All lesions were adenocarcinoma, pathologically proved. Table 4 shows that the main lesions were significantly larger than the accessory lesions (P = 0.001). Regarding the depth of invasion, T1 lesions were more frequent in accessory lesions, especially in the cardia. Moreover, the largest dimensions of gastric lesions in EGMPC were significantly larger than those of GMPC (P = 0.007), and also showed significant differences in the depth of invasion.
| GMPC | EGMPC | |||
| Total (n =16) | Main lesion | Additional lesion | (n = 27) | |
| Location | ||||
| Cardia | 8 | 1 | 7 | 16 |
| Fundus | 0 | 0 | 0 | 1 |
| Body | 3 | 3 | 0 | 3 |
| Antrum | 5 | 4 | 1 | 7 |
| Largest dimension of lesions (cm) | ||||
| Mean ± SD1 | 2.94 ± 1.39 | 3.93 ± 1.21 | 1.94 ± 0.62 | 4.94 ± 2.63 |
| 95% CI | 2.20-3.68 | 2.93-4.95 | 1.42-2.46 | 3.90-5.98 |
| Morphology | ||||
| Hyperplastic | 13 | 5 | 8 | 17 |
| Ulcerative | 1 | 1 | 0 | 6 |
| Infiltrative | 2 | 2 | 0 | 4 |
| Histology | ||||
| Squamous cell carcinoma | 0 | 0 | 0 | 4 |
| Adenocarcinoma | 16 | 8 | 8 | 23 |
| PT stage2 | ||||
| T1 | 6 | 1 | 5 | 3 |
| T2~4 | 10 | 7 | 3 | 24 |
A total of 119 lesions in the 59 patients with synchronous multiple carcinoma were proved by surgery or endoscopy biopsy, and preoperative upper radiographic examination detected 100 of them (84.03% sensitivity). Thus, 19 (15.97%) lesions were missed out of a total of 119 lesions. Regarding the depth of invasion, 18 (52.94%) of the T1 lesions were accurately found out of a total of 119 lesions during preoperative diagnosis by radiographic examination, Moreover, only 3 (3.53%) of the T2-4 lesions were misdetected. Table 5 summarizes the clinicopathological characteristics of 19 misdetected lesions in the 19 patients with MPC. By retrospectively reviewing the missed radiographs, we found four lesions mistaken for bubbles, uneven coating of barium appearance, and two lesions in the gastric cardia and lower esophagus were misdiagnosed as invasion. Of the 19 missed lesions, 16 lesions were microscopic early stage and T1 lesions. Moreover, missed gastric lesions showed a tendency to be of the flat type. The mean size of the missed lesions was 2.40 ± 1.01 cm.
| Type | Age | Gender | Largest dimension (cm) | Location of lesion | PT stage | ||
| Diagnosed | Missed | Diagnosed | Missed | ||||
| 1 EMPC | 49 | Female | 3.3 | 2.2 | Middle | Lower | T4/T1 |
| 2 EMPC | 58 | Male | 3.4 | 1 | Lower | Upper | T2/T1 |
| 3 EMPC | 67 | Male | 4.7 | 2.5 | Middle | Lower | T3/T1 |
| 4 EMPC | 73 | Female | 5.5 | 1.8 | Middle | Upper | T3/T1 |
| 5 EMPC | 64 | Male | 6.2 | 2 | Lower | Upper | T2/T1 |
| 6 EMPC | 57 | Male | 4.5 | 1,5 | Lower | Middle | T2/T1 |
| 7 EGMPC | 65 | Female | 6.5 | 2.2 | Lower | Body | T4/T2 |
| 8 EGMPC | 54 | Male | 5.5 | 2.5 | Lower | Fundus | T3/T1 |
| 9 EGMPC | 58 | Male | 4.5 | 1.8 | Cardia | Middle | T3/T1 |
| 10 EGMPC | 62 | Male | 3.2 | 2.8 | Cardia | Middle | T3/T1 |
| 11 EGMPC | 72 | Male | 3.5 | 5.7 | Cardia | Lower | T4/T3 |
| 12 EGMPC | 63 | Male | 4.2 | 3.2 | Lower | Antrum | T2/T1 |
| 13 EGMPC | 70 | Male | 2.8 | 2.5 | Cardia | Middle | T2/T1 |
| 14 EGMPC | 60 | Male | 4.2 | 2 | Antrum | Middle | T3/T1 |
| 15 EGMPC | 60 | Male | 4.2 | 2.2 | Cardia | Middle | T3/T1 |
| 16 EGMPC | 57 | Male | 4.5 | 3.5 | Lower | Cardia | T3/T3 |
| 17 GMPC | 52 | Male | 2.2 | 2.8 | Cardia | Antrum | T2/T1 |
| 18 GMPC | 57 | Male | 4.5 | 1.2 | Antrum | Cardia | T3/T1 |
| 19 GMPC | 64 | Male | 5.5 | 2.2 | Antrum | Cardia | T3/T1 |
Interest in multiple primary malignancies is long-standing, since Warren and Gates description in 1932, and several reports have indicated that the incidence of MPCs has been increasing in recent years. The incidence of synchronous cancers in patients with esophageal cancer varies from 3.6% to 27.1%[2,13]. In this series, we found that synchronous MPCs in the upper gastrointestinal tract mostly occurred in males: ratio of males/females = 3.21/1, 86.21% of the patients were older than 55 years, and average age was 62.7 years old. As shown in Table 1, 87.5% of patients with multiple gastric carcinomas were men older than 55 years. These findings are comparable to previous studies, and we conclude that elderly individuals aged 65 years or more are at significantly greater risk for multiple gastric carcinomas[14-16].
In our findings, X-ray characteristics of MPC in general were as follows: (1) Each lesion had a familiar X-ray appearance; (2) Normal X-ray appearance, namely continuous mucous membrane and soft margins, was seen between two lesions; (3) The main morphological feature of multiple primary carcinoma lesions, regardless of whether in the esophagus or stomach, was proliferative in 34 and 30, respectively, total 64 (53.78%); (4) The size of proliferative esophageal MPC lesions was limited, with an average of 2.7 cm; while the size of medullary lesions was relatively extensive, with an average of 7.6 cm; and (5) The distance between every two esophageal lesions ranged from 5 to 13, 8.21 cm on average.
Lesions of esophageal MPC were more commonly located at the upper (17/49) segment close to their predilection sites, which suggests we should pay more attention to observing lesions of the upper segment rather than the middle and lower segments. As to synchronous esophageal-gastric carcinoma, we found that the esophageal lesions were concentrated in the lower segment, while the gastric lesions were concentrated in the cardia, and this finding indicates that the occurrence of the tumor had a concentric trend. Concerning the depth of invasion, there were significant differences between the first lesion and the second lesion in esophageal MPC; T1 lesions were more frequent in first lesions (75%), especially in the upper segment. Furthermore, from Table 3 we see that esophageal lesions of synchronous esophageal-gastric carcinoma tend to be less invasive than esophageal MPC (P = 0.036). These findings are in accordance with Nagasawa et al[3], and support the concept that the second malignancy occurred late after carcinogenesis of the main carcinoma lesion or was less aggressive to invade[17]. In addition, we found that in EMPC the sizes of the first lesions were significantly smaller than second ones; the mean size of esophageal lesions in EMPC was 4.33 ± 2.35 cm, significantly smaller than that of EGMPC (5.56 ± 2.34 cm).
Only 8 patients (13.56%) were found to have synchronous multiple gastric carcinoma, the additional lesions mostly located in the cardia. Of them, 2 cases had evidence of invasion of the gastric fundus. Four main lesions were located in the antrum, and 3 at the gastric body. Lee et al[18], however, believed that the distribution of location was not significantly different between the main and accessory lesions, and the fact that our sample size was very small should be taken into account. Nevertheless, all assessments indicated the main lesions were significantly larger than the accessory lesions. Regarding the depth of invasion, T1 lesions were more frequent in accessory lesions, especially in the cardia. Thus, more attention should be paid to this multi-positional location, and detailed and thorough examination is particularly critical. In addition, the largest dimensions of gastric lesions in EGMPC were significantly larger than those of GMPC (P = 0.007), and also showed significant differences in the depth of invasion. These findings are in accordance with Lee et al[18], and support the “collision theory”; that is, that early multiple gastric lesions “fuse” together to form single, advanced gastric cancer lesions[15].
So far, there has not been a consensus on how best to diagnose mucosal lesions of the upper GI tract. Despite the diagnostic advantages of upper endoscopy, it is more expensive and requires more staff and technological expertise than upper GI X-ray. In financial terms, the test is not as effective if the cost is high. The cost of upper endoscopy is 3 to 4-fold more expensive than that of upper GI X-ray in Japanese gastric cancer screening programs[19,20]. Furthermore, it is unlikely that upper endoscopy would be feasible as a mass screening program, even in highly developed countries such as Japan, because of a lack of experienced endoscopists[19,21]. It is also a more invasive procedure than the barium examination, and associated with a small but measurable risk of complications related to sedation on perforation of the upper gastrointestinal tract[22,23]. In addition, it is difficult to find lesions located in the upper and middle thirds of the stomach because of technical difficulties of forward-viewing endoscopy.
In a review of a large series of gastric cancers, malignant tumor was diagnosed or suspected by the use of double-contrast technique with a sensitivity of 96%[24]. This is comparable with the reported sensitivity of endoscopy and biopsy of 94% and 99%, respectively[25]. In the same study, only 4% of all patients had been recommended for endoscopy because of the unequivocal findings in double-contrast studies[24]. In our investigation, preoperative upper radiographic examination detected 100 out of 119 lesions (84.03% sensitivity). Regarding the depth of invasion, 18 (52.94%) of the T1 lesions were accurately detected during preoperative diagnosis by radiographic examination; moreover, only 3 (3.53%) of the T2-4 lesions were misdetected. As a result, double-contrast studies have a high sensitivity in the diagnosis of upper gastrointestinal carcinoma. As the double-contrast study is safer and less expensive than endoscopy, and also has high diagnostic sensitivity (84.03%), we believe that it is an excellent technique for the detection of synchronous multiple primary carcinomas of the upper GI tract. On the other hand, application of hypotonic measures to restrict the motility of the esophageal or stomach wall, which can better display the fine structure of the stomach, greatly improve the detection rate of smaller lesions and reduce the rate of misdiagnosis.
It is also important to recognize that barium studies are also operator dependent, and our experiences are as follows: (1) Radiologists should recognize the characteristics of multiple primary carcinomas of the upper GI tract, and special attention should be given to elderly male patients to avoid missing synchronous lesions; (2) In order to avoid misdiagnosis, when one lesion is found on barium images, the entire upper GI tract should be carefully evaluated for other synchronous lesions; (3) When esophagus or gastric cardia are seriously obstructed, measures such as hypotonic drugs, diluted barium, multi-position and delayed check should be taken; and (4) It should also be noted that adjacent multiple foci should be carefully observed to judge whether normal tissue exists between two lesions, in order not to automatically make a diagnosis of local extension or metastasis of another lesion.
As to early gastric cancer, the following points should be noted during double-contrast barium examination: (1) Good filling is essential for displaying abnormal gastric margins, 300-350 mL is an appropriate amount to extend the gastric body; (2) Middle or small volumes would be suitable for depressed lesions; (3) Mobile technology and thin-layer technology is necessary. After the lesion is found, local fine structure around the stained lesions should be repeatedly observed under flowing conditions; (4) For depression lesions of the greater curvature, the check bed can first be level and then gradually become erect. In the process, observe the barium slowly down from the upper stomach along the greater curvature, so the positive outlook of depressed lesions can be fully displayed; and (5) For lesions of the anterior, compression methods can best show the lesions.
The incidence of multiple synchronous upper gastrointestinal cancers is increasing gradually; early diagnosis, and thus screening of patients at risk, is key. We believe that hypotonic double-contrast upper gastrointestinal examination is a sensitive, safe, noninvasive and relatively inexpensive global examination, which can serve as the first choice for the screening and diagnosis of synchronous upper gastrointestinal MPC, especially in China. Furthermore, careful radiographic follow-up is also required to detect metachronous lesions at the earliest possible stage, which will substantially increase patient survival.
The incidence of multiple synchronous upper gastrointestinal (GI) cancers is increasing gradually, but for some reason, some synchronous primary lesions can often be overlooked at the time of diagnosis. Accurate diagnosis of these synchronous primary lesions before operation is crucial because it can significantly alter clinical management. However, so far, there has not been a consensus on how best to diagnose mucosal lesions of multiple primary carcinomas in the upper GI tract: endoscopy or barium radiography?
Compared with endoscopy, double-contrast upper GI examination is a safe, non-invasive, inexpensive and cost-effective test for providing accurate information about lesion morphology, location, size, and interval distance of these tumors. In addition, because of improved imaging modalities and application of hypotonic measures to restrict the motility of esophageal or stomach wall which can better display the fine structure of the GI tract, the detection rate of smaller lesions is greatly improved with a reduced rate of misdiagnosis.
Synchronous multiple primary carcinomas (MPC) in the upper GI tract mostly occurred in males, 86.21% of the patients were older than 55 years, and average age was 62.7 years old; the fact that elderly individuals aged 65 years or more are at greater risk for multiple gastric carcinomas is clinically significant. Esophageal MPC (EMPC) and esophageal-gastric MPC (EGMPC) are the main types of MPC in the upper GI tract. In esophageal lesions of EMPC/EGMPC, hyperplastic and medullary types are commonly seen; the lesions are mainly located at the middle and lower segment of the esophagus. Gastric lesions are usually located at the gastric cardia, and hyperplastic type is mostly seen. This series supports the concept that double-contrast upper GI examination is safer and less expensive than endoscopy, and also has high diagnostic sensitivity (84.03%); it can serve as an excellent technique for the detection of synchronous MPC of the upper GI tract.
Hypotonic double-contrast upper GI examination is a sensitive, safe, noninvasive and relatively inexpensive global examination, which can serve as the first choice for the screening and diagnosis of synchronous upper gastrointestinal MPC, especially in China.
Synchronous MPC is defined as two or more primary carcinomas occurring in an individual simultaneously. Most of these synchronous cancers are in the head and neck region; other frequently reported sites of synchronous cancer associated with esophageal cancer are the stomach, lung, and urinary bladder. Their coexistence can be problematic for surgeons, oncologists and pathologists with regard to diagnosis, treatment, and follow-up.
It is very interesting for the readers. It is well written, the data is very valuable and the conclusions applicable. It should be accepted for publication in the journal.
Peer reviewer: Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8:162-167. |
| 2. | Kumagai Y, Kawano T, Nakajima Y, Nagai K, Inoue H, Nara S, Iwai T. Multiple primary cancers associated with esophageal carcinoma. Surg Today. 2001;31:872-876. |
| 3. | Nagasawa S, Onda M, Sasajima K, Takubo K, Miyashita M. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226-230. |
| 4. | Castellsagué X, Quintana MJ, Martínez MC, Nieto A, Sánchez MJ, Juan A, Monner A, Carrera M, Agudo A, Quer M. The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Int J Cancer. 2004;108:741-749. |
| 5. | Cheng KK, Day NE. Nutrition and esophageal cancer. Cancer Causes Control. 1996;7:33-40. |
| 6. | Chainani-Wu N. Diet and oral, pharyngeal, and esophageal cancer. Nutr Cancer. 2002;44:104-126. |
| 7. | Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol. 2006;12:4296-4303. |
| 8. | Layke JC, Lopez PP. Esophageal cancer: a review and update. Am Fam Physician. 2006;73:2187-2194. |
| 9. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 10. | Roychoudhuri R, Evans H, Robinson D, Møller H. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004;91:868-872. |
| 11. | Song GQ, Wang Q. Synchronous Multiple Primary Gastric Carcinomas: A Case Report. Clin Oncol Cancer Res. 2010;7:64-66. |
| 12. | Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;51:1358-1414. |
| 13. | Kagei K, Hosokawa M, Shirato H, Kusumi T, Shimizu Y, Watanabe A, Ueda M. Efficacy of intense screening and treatment for synchronous second primary cancers in patients with esophageal cancer. Jpn J Clin Oncol. 2002;32:120-127. |
| 14. | Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol. 2005;11:22-26. |
| 15. | Otsuji E, Kuriu Y, Ichikawa D, Okamoto K, Hagiwara A, Yamagishi H. Clinicopathologic characteristics and prognosis of synchronous multifocal gastric carcinomas. Am J Surg. 2005;189:116-119. |
| 16. | Nitta T, Egashira Y, Akutagawa H, Edagawa G, Kurisu Y, Nomura E, Tanigawa N, Shibayama Y. Study of clinicopathological factors associated with the occurrence of synchronous multiple gastric carcinomas. Gastric Cancer. 2009;12:23-30. |
| 17. | Kuwano H, Ohno S, Matsuda H, Mori M, Sugimachi K. Serial histologic evaluation of multiple primary squamous cell carcinomas of the esophagus. Cancer. 1988;61:1635-1638. |
| 18. | Lee HL, Eun CS, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS, Koh DH. When do we miss synchronous gastric neoplasms with endoscopy? Gastrointest Endosc. 2010;71:1159-1165. |
| 19. | Tashiro A, Sano M, Kinameri K, Fujita K, Takeuchi Y. Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol. 2006;12:4873-4874. |
| 20. | Matsumoto S, Yamasaki K, Tsuji K, Shirahama S. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol. 2007;13:4316-4320. |
| 21. | Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279-287. |
| 22. | Shahmir M, Schuman BM. Complications of fiberoptic endoscopy. Gastrointest Endosc. 1980;26:86-91. |
| 23. | Reiertsen O, Skjøtø J, Jacobsen CD, Rosseland AR. Complications of fiberoptic gastrointestinal endoscopy--five years’ experience in a central hospital. Endoscopy. 1987;19:1-6. |
| 24. | Low VH, Levine MS, Rubesin SE, Laufer I, Herlinger H. Diagnosis of gastric carcinoma: sensitivity of double-contrast barium studies. AJR Am J Roentgenol. 1994;162:329-334. |
| 25. | Evans E, Harris O, Dickey D, Hartley L. Difficulties in the endoscopic diagnosis of gastric and oesophageal cancer. Aust N Z J Surg. 1985;55:541-544. |