ACTIVE DISEASE
The general principles for treating active disease are to consider activity, site and behavior (inflammatory, stricturing, fistulating) of disease[1]. The choice of treatment is also influenced by the previous response to treatment, side effect profile of medication, presence of extraintestinal complications and the course of disease.
The activity of CD can be assessed clinically and endoscopically, or more formally by different indices[6]. The most established way for clinical trials is through the Crohn’s disease activity index (CDAI), which includes symptoms and objective criteria such as anemia and body weight[7]. Index values of 150 and below are associated with quiescent disease; values above that indicate active disease, and values above 450 indicate extremely severe disease. Other markers of activity such as erythrocyte sedimentation rate, C-reactive protein (CRP), or platelet count should also be taken into account. Fecal lactoferrin and calprotectin are highly sensitive and specific markers for detecting intestinal inflammation. These markers are similarly useful for both CD and ulcerative colitis to monitor the therapeutic response to treatments[8-10].
Endoscopic evaluation is unnecessary in every exacerbation, but helps when there is a disparity between symptoms and objective markers of inflammation, or when it is necessary to re-evaluate disease localization. The American College of Gastroenterology has characterized the different disease activities in clinical practice as follows[11]: mild to moderately active disease as “ambulatory patients able to tolerate oral alimentation without manifestations of dehydration, toxicity (high fevers, rigors, and prostration), abdominal tenderness, painful mass, obstruction or > 10% weight loss”. In contrast, moderate to severe disease can be recognized in “patients who have failed to respond to treatment for mild to moderate disease, or those with more prominent symptoms such as fever, significant weight loss, abdominal pain or tenderness, intermittent nausea or vomiting (without obstructive findings), or significant anemia”. Severe disease refers to “patients with persisting symptoms despite the introduction of steroids as outpatients, or individuals presenting with high fever, persistent vomiting, evidence of intestinal obstruction, rebound tenderness, cachexia, or evidence of an abscess”.
Aminosalicylates
No debate regarding the therapy for CD has been as longstanding and controversial as whether 5-aminosalicylic acid (5-ASA)-containing drugs in CD are justified or not. Numerous studies have been performed over the last 25 years, but the data from those currently available do not unequivocally support either point of view. This is partly because differences in study design and dosage render comparison of the outcomes rather difficult. Sulfasalazine is the parent compound, consisting of 5-ASA linked by an azo-bond to sulfapyridine which is split off by bacterial azo-reductase in the colon. The efficacy of sulfasalazine can therefore be expected to be limited to colonic disease. Furthermore, up to 50% of patients are unable to tolerate sulfasalazine at a dose of 4 g/d, due to nausea, headache, vomiting, or epigastric pain. These side effects are usually caused by the sulfapyridine moiety. Therefore, other 5-ASA formulations (mesalazine formulations and the pro-drugs olsalazine and balsalazide) without sulfapyridine have been introduced with different pharmacodynamic and pharmacokinetic profiles. These different preparations are best considered to be different and not interchangeable.
Sulfasalazine is significantly better than placebo in randomized clinical trials for inducing remission in active CD[12-14]. As anticipated, subgroup analyses suggest that patients with isolated colonic disease benefit most from sulfasalazine therapy[11,12], whereas patients treated previously with prednisone fail to respond[11]. Treatment with prednisolone is likely to be a marker of disease severity, rather than a generic modification of the response to sulfasalazine. Sulfasalazine has not been shown to have steroid-sparing properties[13-15]. The European Crohn’s and Colitis Organisation (ECCO) consensus on the management of CD states that active colonic CD can be treated with sulfasalazine if only mildly active, but that it cannot be recommended as first-line therapy because of the high incidence of side effects[1]. It may, however, be appropriate in selected patients, such as those with arthropathy. Once 5-ASA was identified as the active moiety in sulfasalazine, it is not surprising that other 5-ASA-containing formulations (such as mesalazine) were tested in CD.
Different pharmacological preparations allow release of the active drug in different parts of the intestine. Therefore, mesalazine, in contrast to sulfasalazine, might conceivably be used in CD affecting the small bowel. The studies on induction of remission in active CD with mesalazine, however, have yielded conflicting data. Six placebo-controlled trials with different dosages of mesalazine for treating active CD have so far been published. Two studies did not detect a benefit of mesalazine over placebo for inducing remission[16,17]. Tremaine observed a significantly greater number of patients with a response (defined as a decrease in CDAI ≥ 70 and/or CDAI < 150), but this benefit was minute (9 patients with mesalazine treatment vs 4 patients in the placebo group). However, there was no significant difference for clinical remission alone[18]. Singleton et al[15] conducted three separate trials with mesalazine (Pentasa) that have been combined in a meta-analysis, even though two of the three trials were never published in full[19]. This analysis observed a statistically significant benefit of mesalazine over placebo, but the benefit was technical (a greater reduction in CDAI of 18 points compared to placebo in the intention-to-treat-analysis), and of debatable clinical significance. Consequently, if mesalazine is used to treat active CD, the physician must be aware that it is little more effective than placebo. On the other hand, treatment with placebo is not the same as no treatment at all!
Budesonide
The introduction of the topically acting steroid budesonide has become an attractive option for treating patients with CD located in the terminal ileum or right colon. Due to rapid metabolism by cytochrome P-450 enzymes in the liver, budesonide has less systemic bioavailability than conventional corticosteroids. Budesonide has been shown to be effective in inducing remission of active CD in several controlled studies[20-26], with remission rates ranging from 51% to 69% of patients over a period of 8 to 12 wk. A Cochrane Systematic Review combined data from 12 published studies investigating budesonide in comparison to placebo, 5-ASA and systemic corticosteroids[27], and showed that budesonide is more effective at inducing short-term remission, within 8 wk of treatment, in moderately active CD than placebo [relative risk (RR): 1.96, 95% confidence interval (CI): 1.19 to 3.23] or mesalamine (RR: 1.63; 95% CI: 1.23 to 2.16). Budesonide was significantly less effective than conventional steroids for induction of remission (RR: 0.86, 95% CI: 0.76 to 0.98), particularly among patients with severe disease (CDAI > 300) (RR: 0.52, 95% CI: 0.28 to 0.95). Fewer adverse events occurred in those treated with budesonide compared to conventional steroids (RR: 0.64, 95% CI: 0.54 to 0.76) and budesonide was better able to preserve adrenal function (RR for abnormal ACTH test 0.65, 95% CI: 0.55 to 0.78). The recommended dose of budesonide is 9 mg/d, usually for 6 wk, and then tapered 3 mg every 2-4 wk unless continued therapy with budesonide is considered (see below). The ECCO Consensus recommends budesonide 9 mg daily as the preferred treatment for mildly active localized ileocecal CD[1].
Systemic corticosteroids
The effect of systemic steroids for remission induction in CD has been studied in several uncontrolled[2-4] and controlled trials[5,6]. Overall, the clinical response rate achieved varies from 60% to 97% over a period of 1 to 5 mo. In active CD, corticosteroids have been shown to be superior to sulfasalazine, azathioprine and placebo[11,12]. Remarkably, no dose-finding studies have been performed. Reported doses range from 30 mg/d up to 1 mg/kg per day, but most clinicians start with prednisolone 40-60 mg/d, although some vary this according to body weight (1 mg/kg), especially in children. A Cochrane Systematic Review by Benchimol et al[7] included data from two placebo-controlled and six 5-ASA-controlled studies. Systemic steroids were significantly more effective (RR: 1.99, 95% CI 1.51-2.64, P < 0.00001) than placebo and significantly more effective than 5-ASA (RR: 1.65, 95% CI: 1.33-2.03, P < 0.00001). Across the different studies, systemic steroids have been shown to be effective in mild to severely active CD of any localization.
However, the short-term (i.e. 7 to 18 wk) high remission rate induced by systemic corticosteroids does not last, as 16% to 36% of patients become steroid-dependent, and less than half of the initially responding patients will still be in remission 1 year after the initial treatment with steroids[2,12]. In a multi-center, randomized, open-label study comparing a conventional treatment algorithm (steroids followed by immunomodulators in case of relapse after tapering off steroids) vs early combined immunosuppression [three infusions of infliximab (IFX) and concomitant immunomodulators], 74% of patients in the conventional arm were receiving immunomodulators at week 104[13], again underlining the poor long-term outcome of remission induced with systemic steroids. Comparing the two arms with respect to the primary end-point, steroid-free remission, 40/65 (61.5%) in the early combined immunosuppression arm vs 27/64 (42.2%) in the conventional arm were in remission at week 54 (absolute difference 19.3%, 95% CI: 2.4-36.3, P = 0.0278). However, beyond 1 year of follow-up, there was no difference in the steroid-free remission rate between the two groups. It is thus currently unclear whether replacing steroids by anti-tumour necrosis factor (TNF) agents in first-line therapy for active CD will lead to better long-term outcomes. There are no data available offering a direct comparison of treatments other than IFX (e.g. immunomodulators) with corticosteroids as first-line therapy for remission induction.
The short-term outcome of a first course of steroids shows that approximately 50%-60% of patients have a complete response, 30% a partial response, and about 10%-20% have no response. However, at 1 year, only a third of patients will have a durable response, while another third become corticosteroid-dependent and cannot be withdrawn from treatment without a relapse of symptoms, and another third develop steroid resistance[27,28]. Corticosteroid-dependence is a particular concern due to well-established systemic and metabolic toxicities associated with long-term corticosteroid use. Recent data from a referral centre in France identified the need for corticosteroids during the first flare as a predictor of a disabling disease course over the subsequent 5 years[29].
Tapering should be performed according to improvement of clinical symptoms and is usually done in steps of 5-10 mg/wk. At lower dosages, tapering might be reduced to 2.5-5 mg/wk. Intravenous steroids are frequently used when oral treatment has not been effective, but whether this has an advantage over oral therapy in acute severe flares is unclear. The ECCO Consensus on CD management recommends that systemic corticosteroids should be used initially in patients with moderate to severe disease[1].
Corticosteroids are undoubtedly effective in relieving symptoms in CD[12,13]; however, high-dose corticosteroids induce complete mucosal healing in only 13% of patients and a proportion of patients who achieve clinical remission on corticosteroids may in fact have worsened disease at the mucosal level[30].
Azathioprine/mercaptopurine
The most commonly used immunomodulators are the thiopurines, mercaptopurine (MP) and its prodrug azathioprine (AZA). A number of clinical trials have studied the efficacy of these immunomodulators in active CD. The most convincing data were obtained in the early trial by Present where 67% patients in the MP group achieved remission compared to 8% given placebo[31]. Other trials have not observed a significant difference of AZA compared to placebo[11,32], but this partly reflects trial design, dose and duration of therapy for this drug, which takes up to three months to be effective.
Despite these conflicting data, a recent Cochrane analysis evaluated 8 randomized placebo-controlled trials of AZA and 6-MP therapy in adult patients: five dealt with active disease and three had multiple therapeutic arms. The odds ratio (OR) of a response to AZA or 6-MP therapy compared with placebo in active CD was 2.43 (95% CI: 1.62 to 3.64). This corresponded to a number needed to treat (NNT) of about 5 to observe an effect of therapy in one patient. When the two trials using 6-MP in active disease were excluded from the analysis, the OR was 2.06 (95% CI: 1.25 to 3.39). Treatment > 17 wk increased the OR to 2.61 (95% CI: 1.69 to 4.03). A steroid-sparing effect was seen with an OR of 3.69 (95% CI: 2.12 to 6.42), corresponding to a NNT of about 3 to observe steroid-sparing in one patient. Adverse events requiring withdrawal from a trial, principally allergy, leukopenia, pancreatitis and nausea, were increased with active therapy with an OR of 3.44 (95% CI: 1.52 to 7.77). The NNT to observe one adverse event in one patient treated with azathioprine or 6-mercaptopurine was 14[33].
Because thiopurines are slow-acting drugs they are used less frequently to induce remission and more commonly to maintain remission. A combination of prednisolone and AZA was superior to prednisolone monotherapy in one study[34]. Consequently, the main role for thiopurines is as a steroid-sparing therapy and they should be started for corticosteroid-dependent or corticosteroid-refractory patients.
Most evidence is available to support the dose-escalating method: AZA may be started at 50 mg daily and the dose increased by 25 mg every 1-2 wk to a target dose of 2.0-3.0 mg/kg along with monitoring for leukopenia and other potential adverse events. Similarly, 6-MP may be started at 50 mg daily and the dose increased by 25 mg every 1-2 wk to a target dose of 1.0-1.5 mg/kg along with similar monitoring for leukopenia and other potential adverse events. In a recent survey study, most gastroenterologists escalated the dose of AZA or 6-MP relatively rapidly, generally within 4 wk, reporting weight-based target dosing[35].
However, benefits of this therapy are offset by higher treatment-related risk of lymphoproliferative disorders. In particular, it has been shown recently[36] that a multivariate-adjusted hazard ratio of lymphoproliferative disorder between patients receiving thiopurines and those who had never received the drugs was 5.28 (95% CI: 2.01 to 13.9, P = 0.0007).
The ECCO Consensus recommends that AZA/MP be added to corticosteroids for severe CD in the event of relapse[1]. Thiopurines are capable of achieving mucosal healing. AZA heals the mucosa in up to 58% of patients at 1 year and 70% at 2 years, and is superior to budesonide (mucosal healing rate 15%)[37,38]. For those who had been on AZA for longer than 3.5 years and who were in clinical remission, complete mucosal healing was seen in 36% and absence of ulcers in 53%[12].
In the same study, endoscopic scores correlated well with clinical activity indicators. In the postoperative setting, AZA can achieve complete mucosal healing of recurrent ileitis in 40% of patients and improvement in 93% after at least 6 mo of therapy[12]; however, endoscopy at 6 mo may be too early to assess the effect of AZA in de novo disease, as seen in the Study of Biologic and Immunomodulator-Naive Patients in CD (SONIC)[39].
AZA/6-MP treatment should be maintained for several years due to the high relapse rate of patients when these drugs are discontinued. Many studies have investigated the duration of maintenance of remission after AZA/6MP withdrawal in CD patients who were in long-term remission while on this therapy. Withdrawal of AZA/6-MP after up to a median of 6 years under treatment and long-standing remission was associated with a high risk of relapse, whatever the duration of remission under this treatment. Thus, AZA/6-MP withdrawal is not equivalent to continued therapy for maintenance of remission in patients with CD who have been in remission on this therapy. These data suggest that if AZA/6-MP is well tolerated, it should not be interrupted[40,41]. Younger age and a higher daily dose of 6-MP were associated with a higher rate of relapse[40].
Methotrexate
In a pivotal trial conducted by Feagan, methotrexate (MTX) given intramuscularly 25 mg once a week was more likely to induce remission than placebo. Steroid-sparing properties were noted[41]. However, side effects were more common with MTX therapy than with placebo. Other studies using low-dose MTX have not shown a significant benefit[42,43] and no benefit was observed when high-dose intravenous MTX was compared to oral AZA[43]. Like AZA/MP, MTX is only rarely used to treat acute exacerbations of CD, but much more frequently for persistently active CD[44]. Side effects of MTX (notably liver dysfunction and myelotoxicity) need to be monitored and it is contradicted during pregnancy. Consequently, it should be used very cautiously in women of child-bearing potential. MTX has the same indications for treating CD as the thiopurines (although neither are licensed for treating CD in most countries), but because of greater familiarity with thiopurines, most gastroenterologists reserve MTX for active or relapsing CD in patients refractory to, or intolerant of, AZA/MP.
Antibiotics
Although antibiotics are frequently used to treat CD, there is little substantive evidence from randomized trials. Nevertheless, increasing awareness of the importance of mucosal bacteria in the pathogenesis of CD provides a rationale for exploring antibiotic therapy[45]. Metronidazole (20 mg/kg per day) was superior to placebo at reducing the CDAI, but not at inducing remission, in one of the few randomized trials[46]. Furthermore, this benefit was only seen in patients with colonic or ileocolonic disease and no benefits were found with disease limited to the ileum. Similar findings were reported in another trial where a few patients with Crohn’s colitis showed an improvement[47]. On the other hand, another study reported no benefit of metronidazole compared to placebo[48], nor to sulfasalazine, in a 4 mo cross-over study. However, in the cross-over study, patients switched to metronidazole showed CDAI response, although there was no change in CDAI in those switched from metronidazole to sulfasalazine[49,50].
Ciprofloxacin is the other antibiotic used in clinical practice, commonly in combination with metronidazole. Ciprofloxacin was significantly better than placebo at inducing remission in a small trial[51] and similarly effective to mesalazine[52]. In contrast, corticosteroids resulted in higher rates of clinical remission compared to ciprofloxacin and metronidazole[53]. In patients with persistent disease activity given budesonide, the addition of metronidazole and ciprofloxacin was not superior over budesonide monotherapy, despite a trend towards benefit in patients with colonic CD[54]. Further studies are warranted to establish the role of antibiotics in the treatment of CD, but for the time being they cannot be recommended as standard therapy.
As stated by the ECCO, at present, antibiotics are only considered appropriate for septic complications, symptoms attributable to bacterial overgrowth, or perineal disease. Anti-mycobacterial therapy cannot be recommended on the evidence from controlled trials[1].
MEDICALLY-INDUCED REMISSION
5-ASA
Numerous randomized, placebo-controlled studies, including four meta-analyses, have tried to establish a role for 5-ASA in the maintenance of remission. Different study regimens, dosages and durations of therapy have been performed, while a substantial number of trials included small numbers of patients. The two most recent meta-analyses failed to show a benefit for mesalazine over placebo in the maintenance of medically-induced remission[57,58].
Azathioprine/mercaptopurine
Azathioprine or mercaptopurine is the treatment of choice for patients with a high risk of relapse. The effectiveness of AZA has been confirmed in the most recent meta-analysis which included 7 trials of AZA therapy and one of 6-MP. Azathioprine and 6-mercaptopurine both had a positive effect on maintaining remission. The Peto OR for maintenance of remission with AZA was 2.32 (95% CI: 1.55 to 3.49) with a NNT of 6. The Peto OR for maintenance of remission with 6-MP was 3.32 (95% CI: 1.40 to 7.87) with an NNT of 4. Higher doses of AZA improved response. A steroid-sparing effect with AZA was noted, with a Peto OR of 5.22 (95% CI: 1.06 to 25.68) and NNT of 3 for quiescent disease. Withdrawals due to adverse events were more common in patients treated with AZA (Peto OR 3.74; 95% CI: 1.48 to 9.45, NNT = 20) than with placebo[59].
A steroid-sparing effect has also been confirmed[60]. The following indications for starting thiopurine maintenance therapy are generally accepted: frequent flares (two or more per year), persistent disease activity, and steroid dependence. The thiopurines are slow-acting drugs and an effect is usually observed after 2-3 mo, with approximately 90% of patients who are going to respond doing so within the first 4 mo.
An early AZA maintenance study suggested that the drug might no longer be effective after 3.5 years[61]. However, a subsequent randomized, placebo-controlled withdrawal study showed that AZA remained effective at 3.5 years and beyond[62]. Treton et al[63] showed that even after a long duration of clinical remission under AZA, withdrawal of this drug is associated with a high risk of relapse. Interruption of AZA can be reasonably considered, at least temporarily, in a selected group of patients having no predictive factor of relapse. Debate continues about the potential for a small increase in the risk of lymphoma and this cannot be excluded in long-term treatment with AZA/MP[64,65]. This must be weighed against the improved quality of life from thiopurine therapy for patients with CD and discussed with individual patients.
Methotrexate
In a follow-up to the induction study, patients who had achieved remission after 25 mg intramuscular MTX/week were randomized to 15 mg/wk MTX or placebo. Methotrexate was found to be significantly better than placebo at maintaining remission[66]. However, side effects were significantly higher than with placebo. Methotrexate has not been studied after remission has been induced surgically or by other drugs (such as corticosteroids). In general, MTX is considered an alternative to AZA/MP for the maintenance of remission. It also has steroid-sparing properties and the mean time to respond is about 2 mo.
With regard to the ECCO consensus on CD management, patients receiving azathioprine or mercaptopurine who relapse should be evaluated for adherence to therapy and have their dose optimized. Change of their maintenance therapy to methotrexate or anti-TNF therapy should be considered[1].
Budesonide
Low doses of budesonide (3 or 6 mg) have been studied for their potential to maintain remission. The maintenance of remission with budesonide has been studied in several controlled trials. These have been reviewed by Benchimol et al[38] in a Cochrane Systematic Review, based on 10 controlled trials. Eight studies used a controlled ileal release form of budesonide, while three used a pH-modified release formulation. Budesonide is not more effective than placebo or weaning prednisolone for maintenance of remission in CD. Some modest benefits are noted in patients receiving budesonide compared with placebo in terms of lower CDAI scores and longer time to relapse of disease. However, these benefits are offset by higher treatment-related adverse event rates and more frequent adrenocorticoid suppression in patients receiving budesonide.
The ECCO Consensus on CD management states that corticosteroids are not effective for maintenance of medically-induced remission in CD. Budesonide may delay relapse after medically-induced remission, but is not effective at maintaining remission for 12 mo. Budesonide can replace prednisolone in steroid-dependent patients to improve tolerability[1].
POSTOPERATIVE CD (SURGICALLY-INDUCED REMISSION)
About 75% of CD patients with ileal or ileocolonic disease will require surgery within the first 20 years of diagnosis[66,67]. Recurrence rates after surgical resection are high, but are influenced by the definition of recurrence[68]. After the first resection, up to 80% of patients show an endoscopic recurrence within the first year, although most patients are not symptomatic[66,67,69]. Up to 20% have clinical symptoms and 5% require further surgical intervention within the first year. After 5 years, about half have had a clinical relapse. Neither systemic corticosteroids nor budesonide are effective at preventing postoperative relapse[69-73]. Risk factors for postoperative recurrence have rarely been studied in a prospective manner. Continued smoking is the most consistently described risk factor for postoperative relapse[57,74]. Rutgeerts has shown that preoperative disease activity and endoscopic lesions in the neoterminal ileum within the first year after surgery are also associated with a higher risk of postoperative recurrence[69]. A more recent study has suggested that CD patients with CARD15 mutations have a higher risk of postoperative relapse compared to patients without mutated CARD15[74].
5-ASA
Despite the controversies about 5-ASA in the medical treatment of active or quiescent CD, data on the prevention of postoperative recurrence are relatively solid. A meta-analysis by Cammà described a risk reduction of 13.1% in clinical relapse during mesalazine treatment compared to placebo[58]. A subsequent placebo-controlled trial reported no effect of mesalazine after surgical resection for CD, except in patients with isolated small bowel resection[75]. 5-ASA is well tolerated and generally recommended after resection[74]. The ECCO Consensus on CD management suggested that high dose mesalazine is an option for patients with an isolated ileal resection[1].
Azathioprine/Mercaptopurine
A recent meta-analysis evaluated 4 controlled trials comparing azathioprine (n = 3) or 6-MP (n = 1) with control arms (placebo with or without antibiotic induction therapy or mesalamine). In the overall analysis, purine analogs were more effective than control arms in preventing clinical recurrence at 1 year (mean difference, 95% CI: 8, 1%-15%, P = 0.021, NNT = 13) and at 2 years (mean difference, 95% CI: 13, 2%-24%, P = 0.018, NNT = 8). In sensitivity analyses, the efficacy of purine analogs was superior to that of placebo for the prevention of clinical and endoscopic recurrence at 1 year (mean differences, 95% CI: 13, 1.8%-25%, P = 0.025, NNT = 7, and 23, 9%-37%, P = 0.0016, NNT = 4, respectively). At 1 year, in the overall analysis, purine analogs were more effective than control arms were in preventing severe (Rutgeerts score i2-4) endoscopic recurrence (mean difference, CI 95%: 15, 1.8%-29%, P = 0.026, NNT = 7), but they were not effective in the prevention of very severe (i3-4) recurrence. The rate of adverse events leading to drug withdrawal was higher in thiopurine-treated patients than in control arms (17.2% vs 9.8%, respectively, P = 0.021)[76].
Although there are no robust data to support the use of AZA/MP for preventing postoperative relapse, many clinicians use these drugs for this indication[77].
The ECCO Consensus on CD management recommends prophylactic treatment after small intestinal resection. Thiopurines are more effective than mesalazine or imidazole antibiotics alone for preventing both clinical and endoscopic recurrences. Azathioprine/6-mercaptopurine are the drug of choice in patients with a risk factor for early postoperative recurrence[1].
Antibiotics
In a randomized, placebo-controlled trial of metronidazole, a significant decrease in the incidence of severe endoscopic recurrence was observed after ileal resection[78]. Metronidazole therapy significantly reduced clinical recurrence rates at 1 year, but it is rarely used for this indication because of poor tolerability. Another nitroimidazole antibiotic, ornidazole, has been studied by the same group. Ornidazole significantly reduced the clinical and endoscopic recurrence rate at 1 year compared to placebo, but still more patients in the ornidazole group dropped out because of side effects[79].
Imidazole antibiotics, as suggested by the ECCO Consensus on CD management, may be a therapeutic option after ileocolic resection but are poorly tolerated[1].
CONCLUSION
Based on currently available data from randomized, placebo-controlled trials and meta-analyses we have described the conventional therapy of CD. Biological therapy has opened new therapeutic horizons and novel treatment goals; however, current guidelines advocate a step-up approach to treatment, with the addition of more powerful therapies as the severity of disease or refractoriness to therapy increases. In contrast to the cautious, conventional step-up approach, a proactive top-down approach to treatment has been proposed. This regimen advocates biological and immunomodulator therapy at an early stage, shortly after diagnosis of CD.
However, it is important to keep in mind that the CD course in the majority of patients is relatively mild. About three-quarters of patients suffering from CD present at diagnosis with inflammatory disease and one-quarter with either stricturing and/or penetrating disease[80,81]. After the first year of diagnosis, 55% of patients are in remission and 15% have only mild disease, leaving around 30% suffering from frequently active disease. Generally, 20% display active disease during each of the first 7 years[82]. In a Markov model, a representative patient with CD spends 65% of lifetime disease course in medical or surgical remission, 27% with mild disease, 7% with severe disease, and 1% in surgery[55]. Disease behavior tends to alter over time towards a more aggressive phenotype characterized by the development of disabling complications such as abscesses, internal fistulae and strictures[2]. Most of these complications need surgical interventions that lead to more disabling disease. In a Norwegian cohort, the probability of surgery was 14%, 27% and 38% at 1, 5 and 10 years, respectively[83].
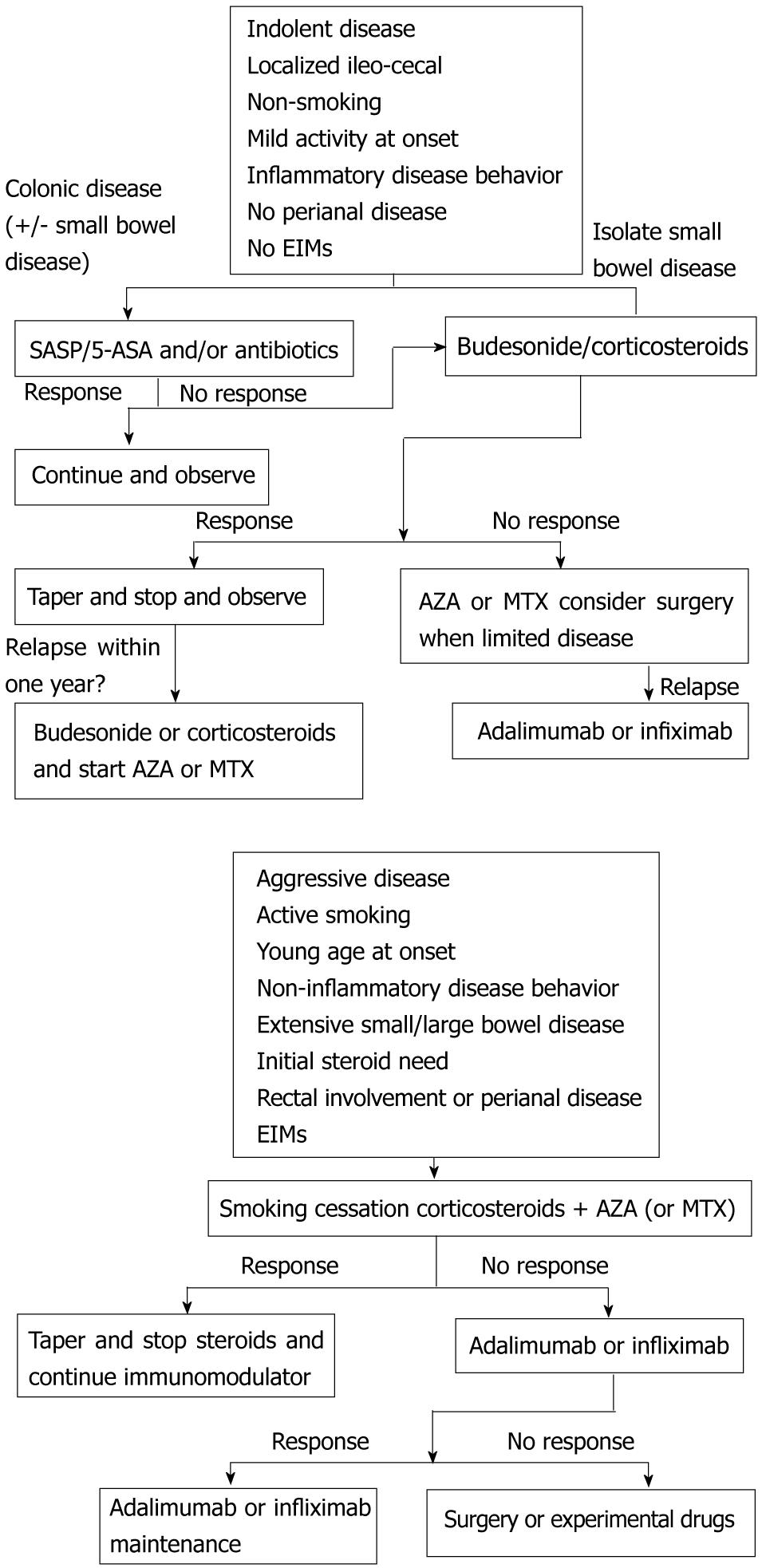
Specific clinical, serological and/or genetic predictors are needed to help identify patients with the highest risk of developing a disabling disease. At present, no predictors which have been fully validated and replicated in adequately powered studies are available. Active smoking, age less than 40 years, extensive length of affected digestive tract, perianal lesions and steroid therapy during the first flare have been proposed as predictors of a worse prognosis in medically-treated CD patients[29]. Furthermore, in an Olmsted County cohort, patients with ileal or ileocolonic extent at baseline were five to seven times more likely to experience an evolution in disease behavior from non-penetrating, non-fistulizing to fistula, abscesses or strictures than those with isolated colonic extent[84]. The only biological index that has been identified as a predictor of more severe clinical course of adult CD is C-reactive protein (CRP)[85]. Obviously, we don’t need predictors at diagnosis when the disease is already considered as severe (stricturing or perforating lesions, multifocal or extensive lesions, severe systemic damage not reversible with treatment). Initiation of immunosuppressives or biologicals early in the disease course in patients at risk of, or already with, complicated disease seems reasonable since this may induce long-term deep remission. The goal should be the induction of mucosal healing and the achievement of symptom-free everyday life, both with minimal use of steroids. Clearly, the potential of early immunosuppressive or biological initiation must be weighed against the possibility of increased risk of treatment side effects, such as more frequent infections or a higher rate of malignancies. Overtreating patients who would have a benign course of the disease is the wrong choice, because of the risk of drug-induced complications. To obtain a sustained remission in CD it is important to optimize conventional therapy, to strictly monitor patients, to identify patients for biological therapy with full consideration of the individual risk/benefit profile, and to introduce biologics in a timely manner, identifying patients who would most benefit from early use (Figure 1).
Figure 1 Treatment Algorithms.
EIMs: Extraintestinal manifestations; SASP: Sulfasalazine; 5- ASA: Five-aminosalicylic acid; AZA: Azathioprine; MTX: Methotrexate.
In conclusion, conventional treatments still remain an important option for management of patients with CD.