Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1462
Revised: December 15, 2010
Accepted: December 22, 2010
Published online: March 21, 2011
AIM: To explore the feasibility of dual camera capsule (DCC) small-bowel (SB) imaging and to examine if two cameras complement each other to detect more SB lesions.
METHODS: Forty-one eligible, consecutive patients underwent DCC SB imaging. Two experienced investigators examined the videos and compared the total number of detected lesions to the number of lesions detected by each camera separately. Examination tolerability was assessed using a questionnaire.
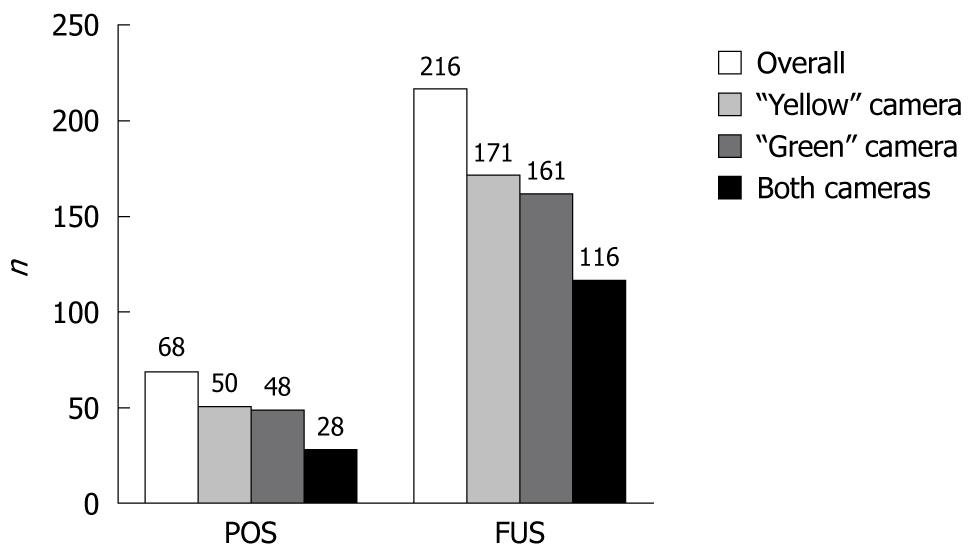
RESULTS: One patient was excluded. DCC cameras detected 68 positive findings (POS) in 20 (50%) cases. Fifty of them were detected by the “yellow” camera, 48 by the “green” and 28 by both cameras; 44% (n = 22) of the “yellow” camera’s POS were not detected by the “green” camera and 42% (n = 20) of the “green” camera’s POS were not detected by the “yellow” camera. In two cases, only one camera detected significant findings. All participants had 216 findings of unknown significance (FUS). The “yellow”, “green” and both cameras detected 171, 161, and 116 FUS, respectively; 32% (n = 55) of the “yellow” camera’s FUS were not detected by the “green” camera and 28% (n = 45) of the “green” camera’s FUS were not detected by the “yellow” camera. There were no complications related to the examination, and 97.6% of the patients would repeat the examination, if necessary.
CONCLUSION: DCC SB examination is feasible and well tolerated. The two cameras complement each other to detect more SB lesions.
- Citation: Triantafyllou K, Papanikolaou IS, Papaxoinis K, Ladas SD. Two cameras detect more lesions in the small-bowel than one. World J Gastroenterol 2011; 17(11): 1462-1467
- URL: https://www.wjgnet.com/1007-9327/full/v17/i11/1462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i11.1462
Small bowel video capsule endoscopy is an established method for examining the small-bowel (SB). However, a recent systematic review of 227 studies showed that the examination’s detection rate of positive findings (POS) (that explain the symptoms for which the test was performed and assist the further management of the patient) varies from 55.3% to 60.5% (overall 59.4%)[1]. To increase the examination’s diagnostic yield, several methods have been proposed, including the use of cathartics and prokinetics, changing body posture, repeating a negative exam, with varying results[2].
Recently, a dual camera capsule (DCC) endoscope (PillCam Colon, Given Imaging, Yoqneam, Israel) designed for colon examination has been introduced to the market. This DCC is 6 mm longer than the conventional SB capsule (PillCam Small Bowel 2, Given Imaging, Yoqneam, Israel). Its specific technological properties include: two cameras - the “yellow” and the “green”, enabling the device to acquire video images from both ends, optics with more than twice the coverage area of the small bowel capsule, automatic light control, a frame acquisition rate of four frames per second (double the rate of the SB capsule), and a total operating duration of approximately 10 h. After initial capsule activation and several minutes of image transmission, the capsule enters a delay mode of approximately 2 h, after which it spontaneously restarts the transmission of images. Similarly to the SB capsule, the system includes a sensor array and data recorder connected to the patient during the procedure. The recorded data are downloaded into the Rapid Viewer (Given Imaging, Yoqneam, Israel) workstation for review of the video. The reviewer can review images captured from each individual camera and from both cameras simultaneously[3].
Given these properties, this capsule has the theoretical potential to detect more lesions than the conventional SB capsule during SB capsule examination. Whether this advantage is relevant clinically remains to be proven. The aim of our study was to explore the feasibility and tolerability of DCC SB imaging and to examine if the capsule’s two cameras can complement each other to detect more SB lesions.
The study was approved by the Ethics Committees of both Hospitals and each participant gave informed consent.
This prospective, non-randomized, feasibility study on consecutive outpatients was conducted in two Academic Departments in Athens, Greece, over a 5-mo period from September 2008 to January 2009. We included 41 patients with indications for SB capsule endoscopy. Exclusion criteria were: known or suspected gastrointestinal obstruction, stricture or fistula, conditions associated with delayed capsule endoscopy gastric/small bowel transit time (i.e. diabetes mellitus), gastrectomy, pregnancy, paralysis or impaired mobility, medication use that could affect gastrointestinal motility (i.e. prokinetics, antidepressants, anticholinergics, and narcotics) or mucosa visibility (iron, sucralfate), and renal impairment in which sodium phosphate is contraindicated.
All patients received detailed written instructions on their bowel preparation. They ingested only clear liquids the day before the procedure and they fasted for 8 h overnight, prior to testing.
Capsule examinations started at between 8 and 10 o’clock the next morning. After its initial activation, the DCC remained beside the patients during the delay mode period. Patients ingested the DCC, after its definite re-activation, approximately 2 h later. Thereafter, patients were checked every 10 min with the Real Time Viewer (Given Imaging, Yoqneam, Israel) until the entrance of the capsule into the duodenum. At this time, they drank 45 mL of sodium phosphate (Phospholaxat, Pharma Line, Athens, Greece) and were advised to drink two liters of water during the following 2 h[4]. This type of SB preparation has been shown to improve mucosa visibility during SB capsule endoscopy in a prospective, randomized, double blind study[4] and it is the preferred preparation strategy in our Departments. Patients ate a light snack 4 h after DCC ingestion and they dined when recording ceased. Before discharge, patients were asked to fill a questionnaire, answering the following questions: (1) “Will you repeat the examination, if needed?” (yes - no); (2) “How comfortable were you during the examination” (very comfortable - comfortable - neither comfortable nor uncomfortable - uncomfortable - very uncomfortable); and (3) “Did you experience any unwanted symptoms during the examination?” (yes - no, if yes, please describe). In cases where the DCC had not been expelled before discharge, we advised patients to search for the capsule in their feces during the following days. In cases of capsule retention, patients were followed clinically and radiologically until capsule excretion.
Downloaded videos were first read locally for patients’ clinical management and thereafter, they were coded and submitted to two investigators for video review and data analysis.
Both investigators had experience of at least 200 capsule endoscopy studies (SB and colon), they were aware of the study indication, but they were blinded to the results of the local review. They examined the videos independently, in random order, using the Rapid Reader, version 5.1 (Given Imaging Ltd, Yoqneam, Israel) software, as outlined below: (1) they rated the overall quality of SB mucosa visibility using a four steps scale: bad - fair - good - excellent[5]; (2) they counted the POS and the findings of unknown significance (FUS)[6] detected by either the “yellow” or the “green” camera only, or by both cameras. POS are those that explain the symptoms for which the test was performed, assist the further management of the patient, or are subsequently confirmed by other diagnostic modalities. Findings of uncertain significance (red spots, small erosions, lymphangiectasias, etc.) are those that fail to completely explain the symptoms, thus necessitating further investigation[6]; and (3) they provided a diagnosis for each examination according to the “yellow” and “green” camera’s findings. In cases of disagreement, a decision was made by a third independent experienced investigator.
The tolerability of the examinations was assessed by the completed questionnaires. Any serious adverse events were also recorded.
The main results of the study are reported in descriptive manner. Qualitative data are presented as absolute and value percent and quantitative data are presented as median value (IQR).
Agreement between investigators for the quality of SB mucosa visibility and between the two cameras regarding diagnosis was assessed by κ statistics. Correlations were assessed by regression analysis. For these comparisons, a P value of < 0.05 indicated statistical significance.
We prospectively enrolled 41 consecutive patients who met the study’s criteria. In one patient, the capsule remained in the stomach for 9 h, for no apparent reason. The capsule was excreted 2 d later but the patient did not consent to undergo another examination. One capsule failed to re-activate after entering the delay mode, but the patient received another capsule that worked properly. Therefore, we report the results of 40 DCC SB examinations. The patients’ baseline characteristics are shown in Table 1.
| Male sex | 29 (72.5) |
| Age (yr) | 58 ± 16 |
| Height (cm) | 168.1 ± 9.3 |
| Weight (kg) | 73.6 ± 12.8 |
| Indication | |
| Iron deficiency anemia | 13 (32.5) |
| Obscure gastrointestinal bleeding | 15 (37.5) |
| Crohn’s disease | 1 (2.5) |
| Chronic diarrhea | 7 (17.5) |
| Other | 4 (10) |
DCC reached the cecum in 36/40 (85%) patients. DCC’s gastric and SB transit times were 45.5 (IQR: 17.7-78.5) min and 200 (IQR: 117-277) min, respectively. There was moderate - good agreement between the two investigators regarding SB mucosa visibility (κ = 0.66, P < 0.001). Thirty two (80%) and 27 (67.5%) cases were rated with good-excellent mucosa visibility by investigators 1 and 2, respectively.
There was excellent correlation (r > 0.9, P < 0.001) between the two investigators regarding the number of findings detected by DCC. Therefore, we analyzed the mean value of their measurements. Overall, DCC cameras detected 68 POS in 50% of the cases. The “yellow” and the “green” camera detected 50 and 48 POS, respectively, while both cameras detected 28 POS findings simultaneously. Figure 1 shows that the two DCC cameras detected different POS: 44% (n = 22) of the “yellow” camera’s POS were not detected by the “green” camera and 42% (n = 20) of the “green” camera’s POS were not detected by the “yellow” camera. More precisely, Figure 2 shows that the POS detected by the two capsule’s cameras were identical only in 6 of the 20 cases. Moreover, there were two (0.05%) cases (case 18 and 20 in Figure 2) where only one camera -the “green” one- detected significant findings (three and one angiodysplasias, respectively).
All participants had FUS and the capsule’s cameras detected 216 FUS overall; 171 detected by the “yellow” camera, 161 detected by the “green” one, and 116 by both of them simultaneously (Figure 1). Moreover, 32% (n = 55) of the “yellow” camera’s FUS were not detected by the “green” camera and 28% (n = 45) of the “green” camera’s FUS were not detected by the “yellow” camera.
There was agreement between the investigators in 39/40 cases regarding the diagnosis. Disagreement occurred for one chronic diarrhea case with erosions detected by DCC and the case was finally assigned to a diagnosis of Crohn’s disease by the third investigator. Overall, the diagnostic yield of DCC SB examination was 50%. The diagnostic yield of each DCC camera is shown in Table 2: 45% and 50% for the “yellow” and the “green” camera, respectively (P = 0.987). The agreement between the DCC cameras diagnosis was high (κ = 0.92, P < 0.001). However, there was numerical difference because of the two cases with angiodysplasia(s) missed by the “yellow” camera.
| Diagnosis | “yellow” camera | “green” camera | P |
| 0.987 | |||
| Normal examination | 22 (55) | 20 (50) | |
| Angiodysplasia(s) | 7 (17.5) | 9 (22.5) | |
| Active bleeding (unidentified source) | 2 (5) | 2 (5) | |
| Crohn’s disease | 7 (17.5) | 7 (17.5) | |
| Polyp(s) | 2 (5) | 2 (5) |
There were no serious adverse events related to the examination. There was no capsule retention. Forty (97.6%) of the 41 initially included participants stated that they would repeat DCC SB examination -if necessary. 90% of patients were not uncomfortable during the examination, while 20% of them reported mild adverse reactions related to bowel preparation (Table 3).
| n (%) | |
| “How comfortable were you during the examination?” | |
| Very comfortable | 3 (7.5) |
| Comfortable | 7 (17.5) |
| Neither comfortable nor uncomfortable | 26 (65) |
| Uncomfortable | 3 (7.5) |
| Very uncomfortable | 1 (2.5) |
| “Did you experience any unwanted symptom(s) during the examination?” | |
| Yes1 | 8 (20) |
| Abdominal cramps | 4 |
| Abdominal bloating | 7 |
| Urgency | 2 |
| Nausea | 1 |
| No | 32 (80) |
The diagnostic yield of SB capsule endoscopy varies across the indications of the examination[1]. Capsule endoscopy is expensive and time consuming; therefore efforts have been made to improve its performance[2]. Meta analysis has shown that only purgative small bowel preparation increases the diagnostic yield of the examination[7], while other modalities have shown conflicting results[2]. The properties of the new DCC designed for colon examination (mainly the two cameras and the rate of capturing images) provide an opportunity to test its utility in SB imaging. Our study showed, for the first time, that SB capsule endoscopy with DCC is feasible, well tolerated, and that the two cameras have the potential to detect more SB lesions by complementing each other. While our study was underway, a study was published in abstract form examining the same hypothesis. Investigators from Portugal using DCC SB endoscopy detected 44 SB findings overall - 24 exclusively from the “yellow” camera, 13 from the “green”, and only one from both - in 10 patients, concluding that using a device with two cameras would increase the diagnostic acuity of capsule endoscopy SB examination[8]. Our study revealed that each DCC camera misses 40% of the significant and 30% of the FUS detected by the other one. It is not clear why this happens. Although the SB lumen is small enough in diameter to allow lesion detection by one camera, our study indicates that because the capsule endoscope is trembling and rotating in SB lumen, there might be blind spots along its passage that can be detected on a second camera recording. More importantly, there were two cases with significant SB examination findings detected by only one camera, raising the possibility that the correct diagnosis might have been missed if the small bowel had been explored with a single camera video capsule. This finding might extrapolate to one additional positive DCC SB examination for every 20 false negative conventional SB capsule explorations, in which case a formal comparison between the two capsules would confirm our study results. However, this assumption has to be proven in a controlled trial.
Two previous studies highlighted the results of sequential SB capsule endoscopy in patients with obscure gastrointestinal bleeding. In each study, 51[9] and 40[10] patients, respectively, underwent sequential SB capsule endoscopy with two different capsule endoscopy systems (PillCam SB and Olympus EndoCapsule). Patients ingested the capsules in a randomized order and data showed that the DY of the two systems was similar. However in the German study[10], the second capsule detected angiodysplasias in two (5%) more patients. The results of these studies differ from the results of studies that investigated the value of a second capsule endoscopy, using the same endoscopy system, in patients with obscure gastrointestinal bleeding or iron deficiency anemia with one previous non-diagnostic capsule investigation. Bar-Meir et al[11] showed significant lesions in seven of the 20 included severe iron deficiency patients, Jones et al[12] revealed positive finding in 18 of 24 obscure gastrointestinal bleeders, while Viazis et al[13] established a diagnosis in 37 of 76 obscure gastrointestinal bleeders who underwent second look capsule endoscopy. Whether dual camera SB capsule endoscopy can reduce repeat SB examination (including capsule endoscopy) until a confirmed diagnosis remains to be investigated.
Apart from establishing a diagnosis, SB capsule endoscopy also estimates the extent of the disease in cases of multiple angiodysplasias and Crohn’s disease, in which the involvement of different parts of the small bowel might dictate different therapeutic approaches. Therefore, missing lesions may underestimate disease extent. For example in case 3 with multiple small bowel ulcers/erosions shown in Figure 2, the “yellow” camera detected erosions throughout the recording, while all “green” camera’s POS were detected by the end of the recording (terminal ileum).
In our study, mucosa visibility was good-excellent in 68% and 80% of the cases, as rated by each investigator, respectively, with a half dose colonoscopy preparation regimen given after the insertion of the capsule at the duodenum. While this purge is given as a boost in colon capsule endoscopy studies, in our departments we use it as the standard preparation scheme for SB capsule endoscopy to both avoid prolonging the capsule’s gastric transit time and to improve mucosa visibility, specifically in the ileum[4]. This preparation was both effective and well tolerated, causing mild adverse reactions in only 20% of the participants, which is similar to the complication rate published in the literature[2,7].
Finally, we addressed patient’s acceptance, which is one of the major issues for a successful diagnostic modality[14]. The acceptance rate of DCC SB examination in our study was high: all participants (apart from one who was excluded initially) agreed to repeat the study if necessary. More importantly, there was no capsule retention and 90% of the patients did not feel uncomfortable during the examination.
Our study was a prospective feasibility trial aiming to find grounds for further formal investigation of DCC SB imaging. Therefore, the results should be interpreted with caution in clinical practice and some reservations should be noted. Firstly, the study design is not optimal for detecting firm conclusions and the sample size is small. The ideal trial to explore whether DCC endoscopy of the SB results in higher DY compared to the conventional capsule, should include patients with one specific indication (e.g. obscure gastrointestinal bleeding) who would ingest a single camera capsule operating on a different frequency than the DCC initially and the DCC later, or vice versa. Secondly, the capsule is 6 mm longer and this might result in swallowing difficulties, in a higher retention rate of the capsule in the stomach, and in a higher rate of incomplete SB examinations[3]. All our patients swallowed the capsule easily. However, in one case, the capsule remained in the stomach during the life span of the capsule’s battery, without any predisposing factor. We did not detect increased DCC gastric and small bowel transit time, compared to those published with the conventional SB capsule[7], and the rate of complete SB examinations with cecal visualization was 85%, similar to that reported in the literature with the conventional SB capsule[7]. Furthermore, complete colon examination was observed in 42.5% of the cases without any further purgative boost; however, bad-moderate bowel cleansing in 79%-85% of the cases prevented any thorough colon examination (data not shown in the results). Thirdly, the longer time required to review the videos from each individual camera and from both cameras simultaneously might be an issue. However, we have not addressed this parameter. Lastly, it might be not acceptable by many patients to wait 2 h until the definite DCC activation. However for the purpose of our study, we have not tried the capsule’s initial activation in advance.
In conclusion, we showed that DCC SB endoscopy is feasible and well tolerated. Moreover, the two capsule’s cameras complement each other to detect more SB lesions. It remains to be determined whether DCC SB endoscopy can increase the diagnostic yield of capsule endoscopy SB examination and if it can improve the clinical outcome of patients undergoing the examination.
Wireless video-capsule endoscopy is a non-invasive and patient friendly examination, which has revolutionized small-bowel (SB) exploration. It has been proved to be superior to any other radiographic or endoscopic modality for SB mucosa examination. However, it is expensive and its diagnostic yield varies across the indications. Until recently several methods have been introduced to improve the method’s diagnostic yield, with conflicting results.
Wireless capsule endoscopy is an evolving technology. Recently, a new capsule endoscope designed for colon examination has been introduced to the market. It is a dual camera capsule (DCC) that compared to the single camera conventional SB capsule has a theoretical advantage to detect more SB lesions.
This theoretical advantage has been tested in 41 consecutive patients with an indication for SB mucosa examination and DCC endoscopy was performed to examine whether the two cameras complement each other to detect more lesions than each of them alone. The results showed that each camera missed 40% of the findings that explain patient’s symptoms detected by the other one. More importantly, there were two cases with significant SB examination findings detected by only one camera, raising the possibility that the correct diagnosis might have been missed if the small bowel had been explored with a single camera video-capsule. In conclusion, this study revealed that DCC SB endoscopy is feasible, well tolerated, and that the two capsule’s cameras complement each other to detect more SB lesions.
Given that a feasibility trial can not reach firm conclusions, the hypothesis that DCC may increase the diagnostic yield of wireless capsule SB endoscopy must be tested in a formal way. If proven, DCC SB endoscopy will be the standard for SB mucosa exploration.
Wireless video-capsule endoscopy is an endoscopy system equipped with a capsule endoscope that acquires images from the gut lumen and transmits them to a data recorder using wireless emission technology. Images are processed and reviewed in a video format using a commercially available computer.
The trial of a colon capsule to view the small intestine is an innovative idea and the results indicate that it has considerable clinical importance. This is a well designed and clinically relevant study.
Peer reviewers: Zvi Fireman, MD, Associate Professor of Medicine, Head, Gastroenterology Department, Hillel Yaffe Med Ctr, POB 169, 38100, Hadera, Israel; Richard Hu, MD, MSc, Division of Gastroenterology, Department of Medicine, Olive view-UCLA Medical Center, 14445 Olive View Drive, Los Angeles, CA 91342, United States; William Dickey, Altnagelvin Hospital, Londonderry, BT47 6SB, Northern Ireland, United Kingdom
S- Editor Wang JL L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. |
| 2. | Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-227. |
| 3. | Adler DG, Chand B, Conway JD, Diehl DL, Kantsevoy SV, Kwon RS, Mamula P, Rodriguez SA, Shah RJ, Song LM. Capsule endoscopy of the colon. Gastrointest Endosc. 2008;68:621-623. |
| 4. | Triantafyllou K, Kalli T, Ladas SD. Small bowel purge after the entrance of the capsule in the duodenum results to better quality of bowel preparation for video -capsule endoscopy. Prospective, randomized, double-blind, placebo-controlled, real time viewer assisted study. Gastrointest Endosc. 2008;134:A-339. |
| 6. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. |
| 7. | Rokkas T, Papaxoinis K, Triantafyllou K, Pistiolas D, Ladas SD. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol. 2009;104:219-227. |
| 8. | Almeida N, Figueiredo P, Lopes S, Freire P, Gouveia H, Leitão MC. Is it the end of the single camera small bowel capsule as we know it? Endoscopy. 2008;40:A30. |
| 9. | Cave DR, Fleischer DE, Leighton JA, Faigel DO, Heigh RI, Sharma VK, Gostout CJ, Rajan E, Mergener K, Foley A. A multicenter randomized comparison of the Endocapsule and the Pillcam SB. Gastrointest Endosc. 2008;68:487-494. |
| 10. | Hartmann D, Eickhoff A, Damian U, Riemann JF. Diagnosis of small-bowel pathology using paired capsule endoscopy with two different devices: a randomized study. Endoscopy. 2007;39:1041-1045. |
| 11. | Bar-Meir S, Eliakim R, Nadler M, Barkay O, Fireman Z, Scapa E, Chowers Y, Bardan E. Second capsule endoscopy for patients with severe iron deficiency anemia. Gastrointest Endosc. 2004;60:711-713. |
| 12. | Jones BH, Fleischer DE, Sharma VK, Heigh RI, Shiff AD, Hernandez JL, Leighton JA. Yield of repeat wireless video capsule endoscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:1058-1064. |
| 13. | Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69:850-856. |