Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5861
Revised: June 30, 2010
Accepted: July 7, 2010
Published online: December 14, 2010
AIM: To evaluate the effects of meal size and three segmentations on intragastric distribution of the meal and gastric motility, by scintigraphy.
METHODS: Twelve healthy volunteers were randomly assessed, twice, by scintigraphy. The test meal consisted of 60 or 180 mL of yogurt labeled with 64 MBq 99mTc-tin colloid. Anterior and posterior dynamic frames were simultaneously acquired for 18 min and all data were analyzed in MatLab. Three proximal-distal segmentations using regions of interest were adopted for both meals.
RESULTS: Intragastric distribution of the meal between the proximal and distal compartments was strongly influenced by the way in which the stomach was divided, showing greater proximal retention after the 180 mL. An important finding was that both dominant frequencies (1 and 3 cpm) were simultaneously recorded in the proximal and distal stomach; however, the power ratio of those dominant frequencies varied in agreement with the segmentation adopted and was independent of the meal size.
CONCLUSION: It was possible to simultaneously evaluate the static intragastric distribution and phasic contractility from the same recording using our scintigraphic approach.
- Citation: Américo MF, Ietsugu MV, Romeiro FG, Corá LA, Oliveira RB, Miranda JRA. Effects of meal size and proximal-distal segmentation on gastric activity. World J Gastroenterol 2010; 16(46): 5861-5868
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5861.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5861
Scintigraphy is the gold standard for measuring gastric emptying and offers the advantage of completely characterizing the complex physiology of intragastric distribution of the meal (IDM) between the proximal and the distal regions[1-3]. Quantification of intragastric distribution could help to define abnormal physiology and explain certain functional dyspeptic symptoms[4], especially when global gastric emptying values are normal[3]. Proximal-distal segmentation approaches employed to divide the stomach in proximal and distal regions remain a challenge for studying IDM; therefore, a validation is necessary before incorporating it into clinical practice[3].
At least three segmentation approaches were adopted, based on the proximal stomach defined immediately after a meal, to divide the stomach into two equal areas and using the incisura[1,2,4-7]. No previous studies have compared the results obtained by those three approaches, and several studies have perceived that there may be problems regarding the division of gastric segments into proximal and distal regions[6,8]. In particular, the two gastric compartments might not be easily identifiable, the incisura may not be pronounced, and somewhat arbitrary definitions cannot be always applied[8].
Apart from the well-known phasic motor activity of 3 cpm (cycle per minute) in the distal stomach, a phasic activity of 1 cpm was observed in dogs and humans using different techniques and appears to be concentrated in the proximal region[9-12]. Thus, considering that each frequency of contraction can be associated with one gastric region, the dominant frequencies could facilitate the characterization of the proximal and distal regions of stomach. The functions of the stomach regions can vary according to the nutrient content[7], and there is a clinical recommendation for consumption of smaller and more frequent meals to avoid postprandial symptoms in patients with common gastrointestinal disorders[13,14]. However, there is little information about the effects of meal size on intragastric distribution, especially for semisolid small meals. The aim of this study was to evaluate the effects of meal size and three proximal-distal segmentations on intragastric distribution and gastric motor activity by scintigraphy.
Twelve healthy volunteers (three female and nine male) with a range of body mass indices of 18.5-24.9 kg/m2 and an age of 25-45 years participated in the studies. None had a history of digestive disease or abdominal surgery. Informed written consent was obtained from each participant. The studies were performed in agreement with Declaration of Helsinki and the local Ethics Committee approved the protocol.
Each volunteer was evaluated twice on separate occasions for ingestion of 60 and 180 mL of a semisolid test meal (yogurt containing 1 kcal/mL). The yogurt was chosen to simulate a small meal that is commonly ingested between large meals. In vitro tests were achieved to assure that the Tc-99m tin colloid and the yogurt were adequately blended[12]. The studies were performed in the morning, after an overnight fast, in a randomized order, and were separated by an interval of one wk. Both test meal were labeled with 64 MBq 99mTc-tin colloid as a nonabsorbable carrier and consumed with the volunteers standing upright in front of the gamma camera.
A dual-head gamma camera (Sopha Vision, Model DST, Sophycammera; Medical Sopha Vision America, Twinsburg, OH, USA) equipped with a parallel-hole low-energy and high-resolution collimator was used. The gamma camera was set up to record activity around the 140-keV photopeak of 99mTc. A dynamic set of 1080 frames (1 frame/s) was acquired for 18 min and images were stored in a 64 × 64 matrix for further analysis. A geometric mean of the anterior and posterior gastric counts was determined for each time point and corrected for radionuclide decay[3].
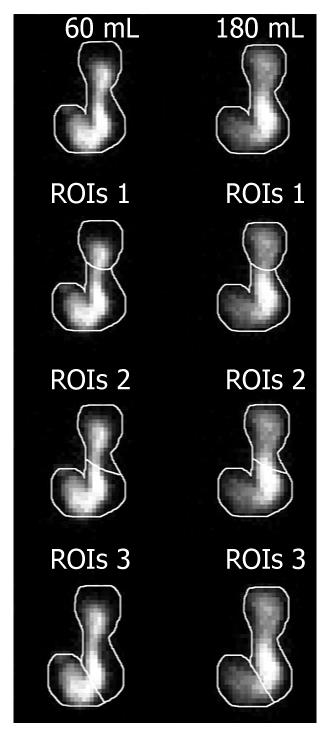
All digital images were analyzed in MatLab (Mathworks Inc., Natick, MA, USA). The total stomach was outlined in the composite image (summation of all images) with a cursor over the largest anterior gastric image obtained by ingestion of 180 mL. The outline for each 180 mL analysis was individually copied and fitted in the 60 mL image of the same subject. This outline was then subdivided into two regions of interest (ROIs) corresponding to the proximal and distal stomach, according to each method used (Figure 1): ROIs 1: The proximal stomach region was the “reservoir” area seen in all subjects in the first postprandial frames and the line used to divide proximal/distal stomach was drawn immediately below this region[2,7,15]; ROIs 2: The image was divided into two equal areas, designated the proximal and distal stomach, by a mid-length separation in the longitudinal axis of the stomach[1,4,16]; and ROIs 3: The proximal and distal regions of the stomach were separated by a fainter band of radioactivity coinciding with the angula; thus the stomach was arbitrarily divided by drawing a line across the incisura angularis[5,6].
The time for meal consumption was measured individually. Thus, a value considering 100% retention of the meal was dependent of the activity at the end of the lag phase (the frame before any activity appeared in the small intestine) and immediately after meal completion. Time zero started when the retention was 100%. For each region (total, proximal and distal stomach) activity time curves, expressed as percentages of activity in the total stomach with 100% of meal retention, were obtained.
The intragastric distribution of 60 and 180 mL were assessed from activity time curves derived from each region and considering the three proximal-distal segmentations by calculating the following parameters: (1) initial retention: the percentage of initial activity (%) contained in the total, proximal and distal stomach at time zero; (2) final retention: the percentage of final activity (%) contained in the total, proximal and distal stomach at 18 min; (3) proximal emptying half-time (T1/2): expressed as the time (min) when the initial retention in the proximal stomach decreased by 50%; (4) maximal distal content: the highest activity value (%) in the distal stomach at any time point in the study; and (5) gastric emptying of the whole stomach (representing% retention over time) was obtained from time zero to 18 min.
Comparisons of the data for three proximal-distal segmentations were made for both meals employing area under curves (AUC) and statistical moment analysis. The AUC derived from the proximal or distal stomach was expressed as percentage of AUC obtained from total stomach. The statistical moment (minutes) was obtained through the temporal average from the proximal or distal distribution curve, normalized by AUC[17]. This quantification allowed determination of a distribution time that could be associated with the midpoint of the proximal and distal distribution curves.
Fast fourier transform (FFT) was employed to analyze phasic contractions in both gastric regions (proximal and distal) and for each type of proximal-distal segmentation. A bi-directional Butterworth band-pass filter with a cutoff frequency at 5-75 mHz (0.3-4.5 cpm) was applied.
Dominant frequencies were expressed as the frequency at which the highest FFT power spectrum was observed in the proximal and distal regions. Values were expressed as power ratios (%), determined by dividing the power of each dominant frequency by the total power (sum of both frequencies), and multiplying the results by 100 for each stomach region in all proximal-distal segmentations[18].
Data were expressed as mean ± SE. The hypothesis of a normal data distribution was confirmed using Shapiro-Wilk’s test. Data obtained by meals of 60 and 180 mL were compared using Student’s t test and P-values less than 0.05 were considered significant. Comparisons among types of proximal-distal segmentation were analyzed by one-way ANOVA and Tukey’s test, with P < 0.05 considered significant.
Our data demonstrated a significant effect of meal size and the three proposed proximal-distal segmentations on intragastric meal distribution and gastric contractility. After ingestion, both meals were rapidly dispersed through the whole stomach with a minimal lag phase. There was no difference between the lag-phase for 60 mL (1.9 ± 0.2 min) and for 180 mL (2.3 ± 0.2 min).
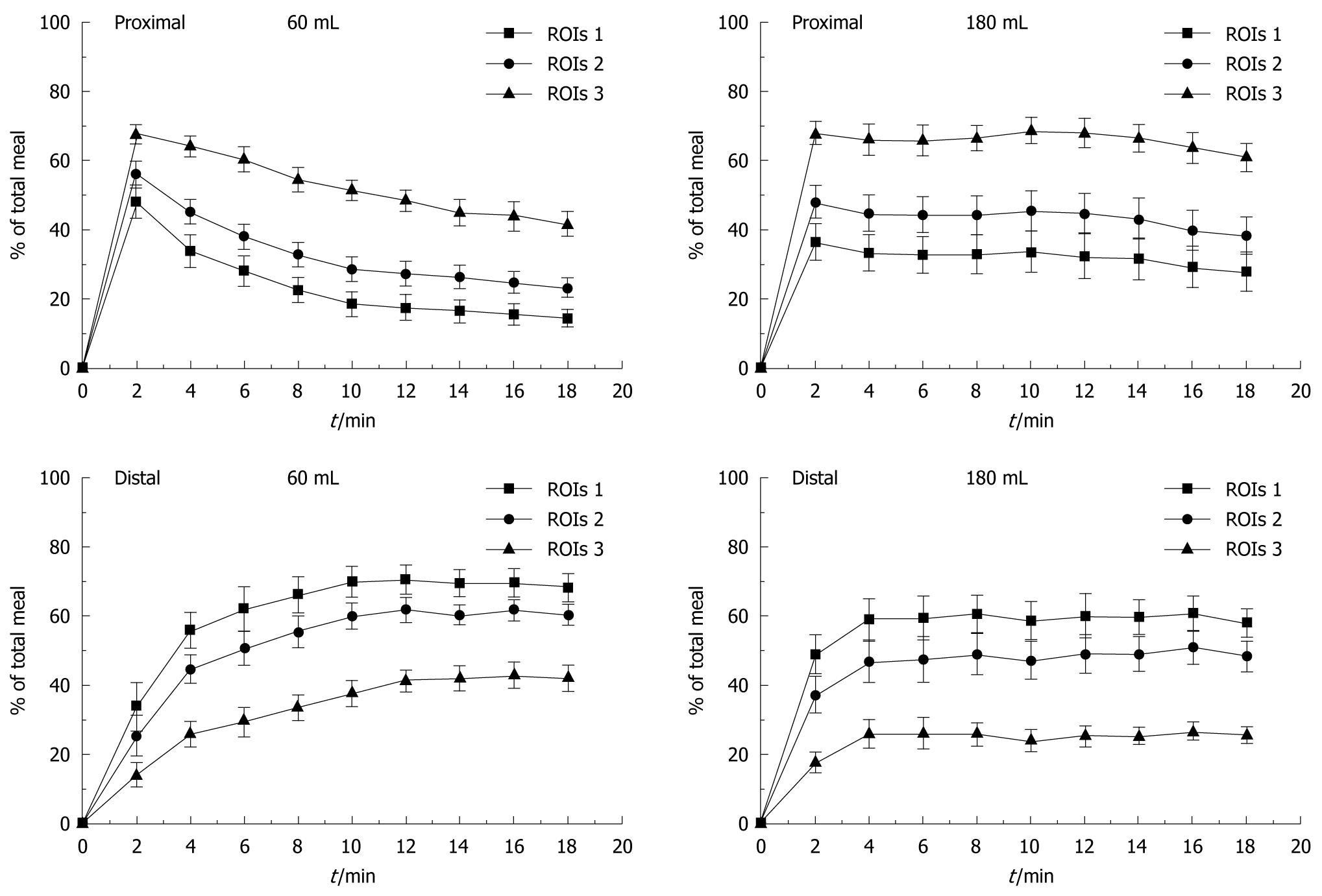
The three proposed proximal-distal segmentations (ROIs) could be applied to all volunteers. Figure 2 shows the profile of IDM of the 60 and 180 mL meal over 18 min for the three types of proximal-distal segmentations. A redistribution of food from the proximal to the distal stomach according to employed segmentation was observed.
There was greater proximal retention after 180 mL ingestion compared to 60 mL, which was proportional to the increase in the area of proximal stomach generated by the type of proximal-distal segmentation. ROIs 1 presented a smaller proximal region and a fast redistribution of the meal for the distal area occurred. By contrast, in ROIs 3, the initial retention in the proximal region was greater than in the other proximal-distal segmentations and presented a slow redistribution of the meal for the distal area. ROIs 2 showed an intermediate pattern. Thus, there was a significant difference among the three ROIs segmentations employed (P < 0.05).
No difference was found in the percentage of the meal retained in the total stomach after 60 mL (13.3% ± 3.0%) and 180 mL (13.0% ± 2.4%) ingestion over 18 min. Table 1 compares the effect of meal size and proximal-distal segmentation in IDM parameters, reinforcing the relationship between proximal area and parameters of regional gastric emptying.
| 60 mL | 180 mL | |||||
| ROIs 1 | ROIs 2 | ROIs 3 | ROIs 1 | ROIs 2 | ROIs 3 | |
| Total stomach | ||||||
| Initial retention | 100 | 100 | 100 | 100 | 100 | 100 |
| Final retention | 82.5 ± 3.8 | 83.5 ± 3.2 | 83.8 ± 2.9 | 84.9 ± 3.3 | 85.7 ± 3.1 | 85.5 ± 3.2 |
| Proximal (%) | ||||||
| Initial retention | 39.8 ± 6.8 | 51.7 ± 6.0 | 73.2 ± 3.8a.c | 38.6 ± 6.4 | 52.3 ± 6.4 | 75.2 ± 4.3a.c |
| Final retention | 14.1 ± 2.5 | 22.8 ± 3.0 | 41.6 ± 3.7a.c | 27.7 ± 5.7e | 38.3 ± 5.5g | 60.4 ± 4.2a.c,i |
| T1/2 (min) | 4.2 ± 0.6 | 7.0 ± 0.8 | > 18.0 | 11.0 ± 2.2 | > 18.0 | > 18.0 |
| Distal (%) | ||||||
| Initial retention | 60.1 ± 6.7 | 48.1 ± 6.0 | 26.6 ± 3.8a.c | 61.4 ± 6.4 | 47.6 ± 6.4 | 24.7 ± 4.3a.c |
| Final retention | 68.4 ± 4.0 | 60.7 ± 3.2 | 42.2 ± 4.0a.c | 57.1 ± 4.2 | 47.6 ± 4.5g | 25.1 ± 2.5a.c,i |
| Maximum content | 80.0 ± 4.5 | 69.0 ± 3.7 | 48.0 ± 3.6a.c | 69.0 ± 5.0 | 56.7 ± 5.7 | 32.0 ± 3.8a.c |
Table 2 presents the mean area under the curve and the statistical moment for the proximal and distal regions, for each of the three types of segmentation, after ingestion of the test meals. For the smaller meal, there was a significant difference among ROIs, while the increased meal size generated a difference only between ROIs 1 and 3.
| Proximal | Distal | |||||
| ROIs 1 | ROIs 2 | ROIs 3 | ROIs 1 | ROIs 2 | ROIs 3 | |
| AUC (counts.s) | ||||||
| 60 mL | 27.2 ± 2.9 | 31.4 ± 2.5 | 43.9 ± 1.7ac | 72.8 ± 2.9 | 68.6 ± 2.5 | 56.1 ± 1.7ac |
| 180 mL | 35.3 ± 4.1 | 39.4 ± 3.2 | 49.0 ± 3.8a | 64.7 ± 4.1 | 60.6 ± 3.2 | 51.0 ± 3.8a |
| P | < 0.09 | < 0.031 | < 0.1 | < 0.09 | < 0.031 | < 0.1 |
| Statistical moments (min) | ||||||
| 60 mL | 6.9 ± 0.3 | 7.7 ± 0.2c | 8.5 ± 0.1ac | 10.4 ± 0.3 | 10.5 ± 0.3 | 10.7 ± 0.2 |
| 180 mL | 8.7 ± 0.2 | 9.0 ± 0.1 | 9.2 ± 0.1 | 9.8 ± 0.3 | 10.0 ± 0.3 | 10.1 ± 0.3 |
| P | < 0.000011 | < 0.000031 | < 0.00061 | < 0.2 | < 0.1 | < 0.07 |
Moment calculation demonstrated that the differences between the ROIs were only evident with 60 mL but not with 180 mL meal for the three types of proximal-distal segmentation. Comparison of moments for the distal region for both meals showed no significant differences. The statistical moment obtained in the proximal area was influenced by the type of segmentation adopted (Table 2), representing an option to quantify a time related to intragastric distribution.
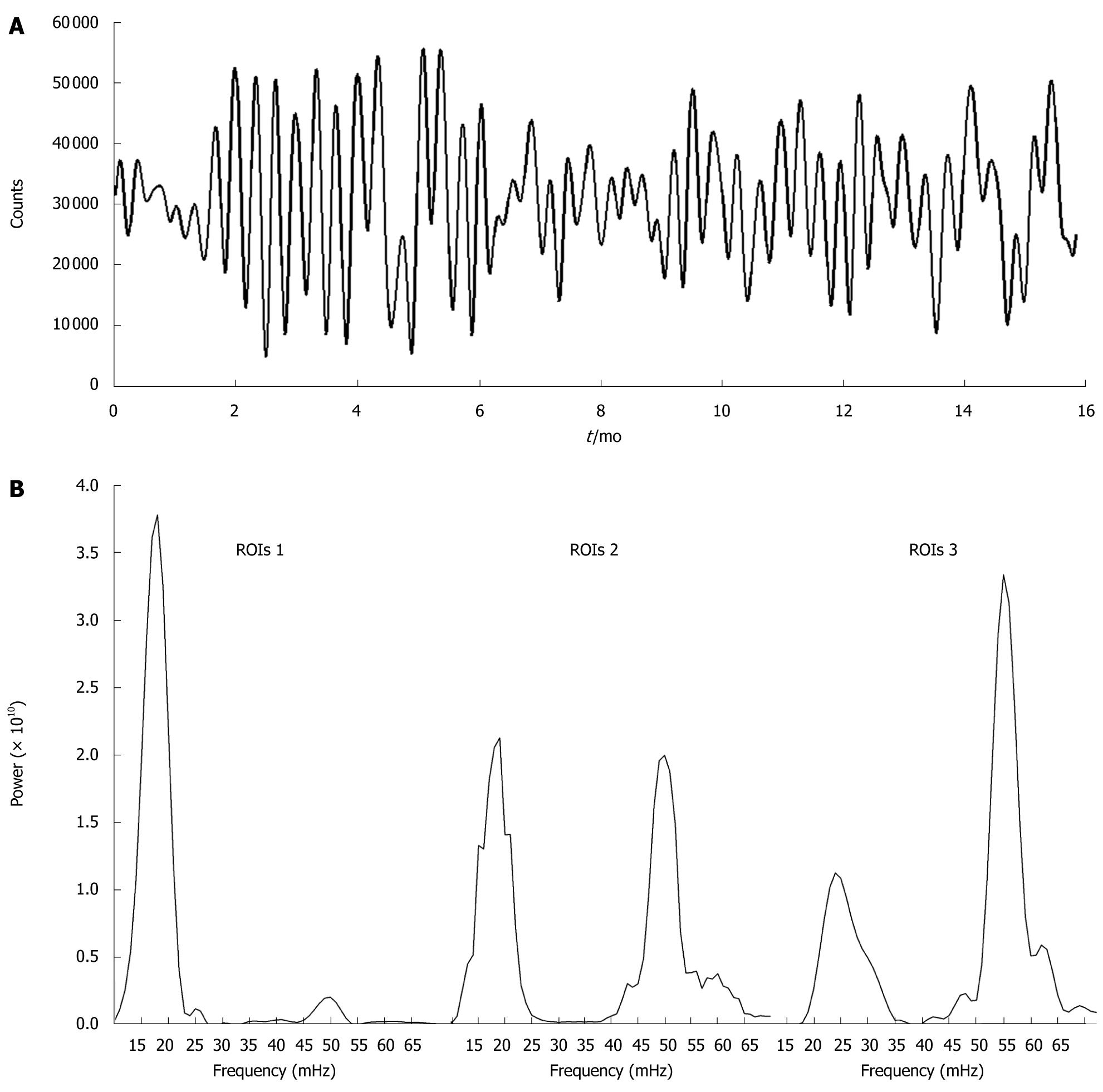
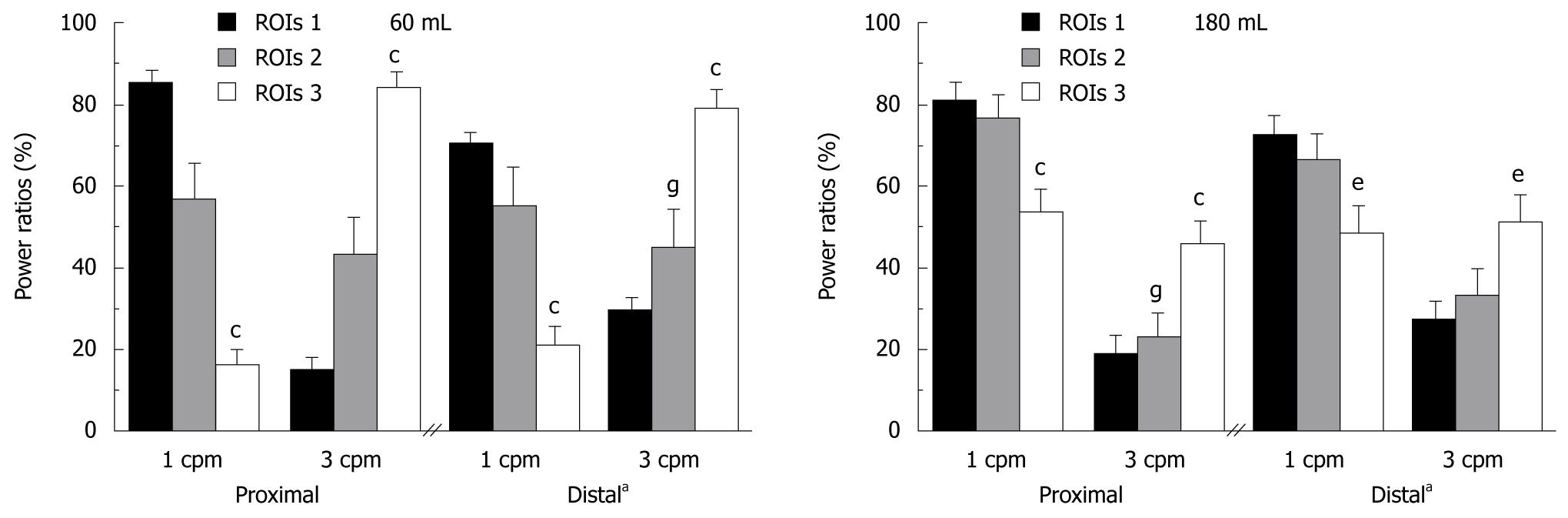
An important finding was both dominant frequencies were recorded in the proximal stomach (16.0 ± 1.0 mHz or 1 cpm and 50.0 ± 2.0 mHz or 3 cpm) and in the distal stomach (16.0 ± 1.0 and 50.0 ± 1.0 mHz), independently of gastric segmentation. The dominant frequencies were observed to overlap in the signal of proximal stomach at different time points (Figure 3). Power ratio calculations indicated that there was a rearrangement of the maximum power of each frequency (1 and 3 cpm) according to the segmentation type.
The difference is shown in the power spectrum of these frequencies according to the type gastric segmentation (Figure 4). A difference could be observed in the distal region between 60 and 180 mL: for 180 mL meal there was an increase of the 1 cpm and a decrease of the 3 cpm in ROIs 2 and 3. There were no significant changes for ROIs 1.
The results showed that meal distribution in the human stomach differs according to the volume used (60 or 180 mL) and noticeably according to proximal-distal image segmentation used. Dynamic gastric scintigraphy was effective for determining two dominant frequencies (1 and 3 cpm), and the magnitude of these contractions in both the proximal and distal stomach.
It has been previously shown that volume and meal size influenced gastric emptying[19,20]; thus, it seems logical to advise patients to decrease the size and increase the frequency of meals during certain conditions, such as pregnancy, gastroesophageal reflux disease, and functional dyspepsia[14]. Our meals were chosen to investigate IDM and motility patterns using semisolid small meals in order to establish a pattern in healthy volunteers. Thereafter, our approach can be applied in patients to verify IDM and gastric motor activity after the recommended consumption of a smaller meal.
The emphasis of most previous studies was on total gastric emptying[19], despite the fact that regional gastric emptying is more frequently abnormal than total gastric emptying[2]. The partitioning of ingested meals between the proximal and distal stomach is related to the genesis of dyspeptic symptoms, such as early satiety, fullness, and nausea[1,4,21]. The meal distribution within the area of the stomach might strongly influence the way in which the stomach is subdivided[4]. Unfortunately, the methods used for defining these two areas are still controversial and poorly defined[15]. Our data demonstrated a direct relationship between gastric compartment size and IDM. The choice of the segmentation technique should consider variations in the stomach shape[16] and the objective of the study. For example, using ROIs 1 it is possible analyze details of the fundic accommodation process’ whereas employing ROIs 2 both dominant frequencies can be equally observed, and ROIs 3 is useful to evaluate antral contractility.
Generally, twenty minutes after the ingestion of a larger meal, a gradual decrease in proximal stomach activity begins, with a corresponding increase in the distal stomach, indicating a redistribution of food from the proximal to the distal stomach[1,4,5]. Hence, our IDM data were obtained during this initial stage, showing that the 60 mL meal quickly began to be redistributed to the distal region; whereas, using 180 mL, there was a slower redistribution from proximal to distal stomach in all segmentations adopted (Figure 2). In the curves obtained from ingestion of 180 mL was impossible calculate T1/2 from the proximal stomach during the 18 minutes of recording, except for ROIs 1, reinforcing the retention in the proximal region. In the final retention values, there was a significant difference between three ROIs segmentation and both meals ingested (Table 1).
To quantify the IDM, we used the statistical moments, which have previously been utilized only in pharmaceutical approaches[17], and the traditional AUC. The statistical moments and AUC provide complementary information about the data observed. Both quantification methods showed that proximal-distal segmentations have more effect for the 60 mL meal than for 180 mL. However, there was no difference in the statistical moment between ROIs in the distal curve profile, whereas there was a significant difference in AUC. Distal accumulation time was defined previously by our group as the time elapsing from the meal ingestion until the activity reached 99% of the maximum value in the sigmoidal tracing over the distal stomach curves[12]. The distribution time obtained by the statistical moment was very close to the distal accumulation time, but can be employed for any kind of curve, including proximal stomach curves.
It is important to emphasize that partitioning of ingested meals between the proximal and distal stomach is related to gastric accommodation. Abnormal IDM might be a consequence of disturbed proximal stomach accommodation[4] in a considerable subset of patients with functional dyspepsia and might have a role in symptom production, such as early satiety and weight loss[6]. Studies of dyspepsia have shown a preferential accumulation in the distal stomach, suggesting defective postprandial relation of the proximal stomach; however, it is difficult to draw general conclusions because each study employed a different proximal-distal partition. It would be interesting to provide data on the relationship between meal size, proximal-distal segmentation, IDM, and accommodation in this patient group. Information on the accommodation process will have clinical value, especially for studying patients with dyspepsia and normal gastric emptying, and it may contribute directly to improved medical therapy[22].
Fast Fourier transformation of our scintigraphic recordings defined two dominant frequencies in the distal as well as the proximal stomach, in all volunteers (Figure 3). Meal size did not affect the dominant frequencies of the contractions or their power, but there was a large difference in the power spectra of these frequencies, based on image segmentation. The power ratio of the proximal signal was rearranged for the maximum power of each frequency (1 and 3 cpm), according to segmentation type. Hence, there was a power gradient from ROIs 1 to ROIs 3, where 1 cpm decreases and 3 cpm increases in the human proximal stomach (Figures 3 and 4). This motor activity around 1 cpm on the proximal stomach[9,10,23] has not been extensively documented in humans, although it has been correlated with functional dyspepsia[9], mainly due to methodological issues, such as differences in barostat systems[24] and/or filter parameters employed in data analysis[12].
In summary, the results of research and/or diagnosis can be deeply influenced by the proximal-distal segmentation method adopted. Two dominant frequencies (1 and 3 cpm) can be simultaneously registered in the proximal and distal stomach, but the proximal-distal segmentation should be considered carefully to analyze their power spectra. The protocol developed in this study can be applied in patients with several disorders, with the advantages of simultaneous evaluation of IDM and gastric contractions.
The stomach is composed of two distinct functional regions. The distal stomach is capable of generating 3 vigorous contractions per minute (cpm), which cause reduction in size of ingested particles and subsequent emptying. The proximal stomach is primarily concerned with storage of the ingested food, with a slight contraction activity around 1 cpm. During a meal, the stomach continuously adapts its size to the content by gradually relaxing its musculature, performing the so-called accommodation to distension. Intragastric distribution of the meal (IDM) between the proximal and distal stomach is related to the accommodation process, and is useful for defining abnormal physiology and for explaining certain functional disease symptoms.
Proximal-distal segmentation approaches employed to divide the stomach into proximal and distal regions remain a challenge in the study of IDM. Unfortunately, the methods used for defining these two areas are still controversial and poorly defined. Validation is necessary before incorporating these methods into clinical practice. Two dominant frequencies (1 and 3 cpm) can be registered simultaneously in the proximal and distal stomach, but the proximal-distal segmentation should be considered carefully to analyze their power spectra. The motor activity around 1 cpm on the proximal stomach has been correlated with functional dyspepsia. In this context, the frequency of contraction can be explored to elucidate certain disease patterns.
Scintigraphy already is the gold standard for measuring gastric emptying and offers the advantage of completely characterizing the complex physiology of IDM between stomach regions. New studies can be exploited to refine and extend its use in clinical practice. The functions of the stomach regions can vary according to the nutrient content and there is a clinical recommendation for consumption of smaller and more frequent meals to avoid postprandial symptoms in patients with common gastrointestinal disorders. However, there is little information about the effects of meal size on intragastric distribution, especially for semisolid small meals. In the area of functional disorders research, considerable effort is being expended on how to convert basic knowledge into benefits for patients’ treatment. Therefore, in the present study we compared three kinds of segmentation in normal volunteers and showed that segmentation is remarkably important in the evaluation of IDM and gastric motility. This observation is particularly relevant when assessing patients.
Abnormal IDM might be a consequence of disturbed proximal stomach accommodation in a considerable subset of patients with functional dyspepsia and might have a role in symptom production, such as early satiety and weight loss. Studies of dyspepsia have shown a preferential accumulation in the distal stomach, suggesting defective postprandial accommodation in the proximal stomach; however, it is difficult to draw general conclusions, because each study employed a different proximal-distal partition. Thus, providing data on the relationship between meal size, proximal-distal segmentation, IDM, and accommodation in this patient group is very important. Information about the accommodation process will have clinical value, especially in the study of patients with dyspepsia and normal gastric emptying, and might directly influence medical therapy.
IDM represents the distribution of gastric contents between the proximal and distal stomach during gastric emptying. Dyspepsia is a medical condition characterized by chronic or recurrent pain in the upper abdomen, bloating, and fullness.
The authors evaluated the effects of meal size and three segmentations on intragastric distribution of meal and gastric motility by scintigraphy. This paper is an interesting report.
Peer reviewer: Atsushi Nakajima, Professor, Division of Gastroenterology, Yokohama City University Graduate School of Medicine, 3-9 Fuku-ura, Kanazawa-ku, Yokohama 236-0004, Japan
S- Editor Wang JL L- Editor Stewart GJ E- Editor Ma WH
| 1. | Troncon LE, Bennett RJ, Ahluwalia NK, Thompson DG. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut. 1994;35:327-332. |
| 2. | Gonlachanvit S, Maurer AH, Fisher RS, Parkman HP. Regional gastric emptying abnormalities in functional dyspepsia and gastro-oesophageal reflux disease. Neurogastroenterol Motil. 2006;18:894-904. |
| 3. | Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753-763. |
| 4. | Piessevaux H, Tack J, Walrand S, Pauwels S, Geubel A. Intragastric distribution of a standardized meal in health and functional dyspepsia: correlation with specific symptoms. Neurogastroenterol Motil. 2003;15:447-455. |
| 5. | Kuiken SD, Samsom M, Camilleri M, Mullan BP, Burton DD, Kost LJ, Hardyman TJ, Brinkmann BH, O'Connor MK. Development of a test to measure gastric accommodation in humans. Am J Physiol. 1999;277:G1217-G1221. |
| 6. | Feinle C, Kunz P, Boesiger P, Fried M, Schwizer W. Scintigraphic validation of a magnetic resonance imaging method to study gastric emptying of a solid meal in humans. Gut. 1999;44:106-111. |
| 7. | Collins PJ, Houghton LA, Read NW, Horowitz M, Chatterton BE, Heddle R, Dent J. Role of the proximal and distal stomach in mixed solid and liquid meal emptying. Gut. 1991;32:615-619. |
| 8. | Blat S, Guérin S, Chauvin A, Bobillier E, Le Cloirec J, Bourguet P, Malbert CH. Role of vagal innervation on intragastric distribution and emptying of liquid and semisolid meals in conscious pigs. Neurogastroenterol Motil. 2001;13:73-80. |
| 9. | Simrén M, Vos R, Janssens J, Tack J. Unsuppressed postprandial phasic contractility in the proximal stomach in functional dyspepsia: relevance to symptoms. Am J Gastroenterol. 2003;98:2169-2175. |
| 10. | Quigley EM. Gastric and small intestinal motility in health and disease. Gastroenterol Clin North Am. 1996;25:113-145. |
| 11. | de Zwart IM, Mearadji B, Lamb HJ, Eilers PH, Masclee AA, de Roos A, Kunz P. Gastric motility: comparison of assessment with real-time MR imaging or barostat measurement initial experience. Radiology. 2002;224:592-597. |
| 12. | Américo MF, Oliveira RB, Romeiro FG, Baffa O, Corá LA, Miranda JR. Scintigraphic validation of AC Biosusceptometry to study the gastric motor activity and the intragastric distribution of food in humans. Neurogastroenterol Motil. 2007;19:804-811. |
| 13. | Kahrilas PJ. Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700-1707. |
| 14. | Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut. 2006;55:1685-1691. |
| 15. | Collins PJ, Horowitz M, Chatterton BE. Proximal, distal and total stomach emptying of a digestible solid meal in normal subjects. Br J Radiol. 1988;61:12-18. |
| 16. | Troncon LE, Herculano JR Jr, Savoldelli RD, Moraes ER, Secaf M, Oliveira RB. Relationships between intragastric food maldistribution, disturbances of antral contractility, and symptoms in functional dyspepsia. Dig Dis Sci. 2006;51:517-526. |
| 17. | Podczeck F, Newton JM, Yuen KH. The description of the gastrointestinal transit of pellets assessed by gamma scintigraphy using statistical moments. Pharm Res. 1995;12:376-379. |
| 18. | Usami A, Mizukami Y, Onji M. Abnormal gastric motility in liver cirrhosis: roles of secretin. Dig Dis Sci. 1998;43:2392-2397. |
| 19. | Doran S, Jones KL, Andrews JM, Horowitz M. Effects of meal volume and posture on gastric emptying of solids and appetite. Am J Physiol. 1998;275:R1712-R1718. |
| 20. | Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685-1694. |
| 21. | Boeckxstaens GE, Hirsch DP, Kuiken SD, Heisterkamp SH, Tytgat GN. The proximal stomach and postprandial symptoms in functional dyspeptics. Am J Gastroenterol. 2002;97:40-48. |
| 22. | Maurer AH, Parkman HP. Update on gastrointestinal scintigraphy. Semin Nucl Med. 2006;36:110-118. |
| 23. | Azpiroz F, Malagelada JR. Physiological variations in canine gastric tone measured by an electronic barostat. Am J Physiol. 1985;248:G229-G237. |