Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5822
Revised: September 7, 2010
Accepted: September 14, 2010
Published online: December 14, 2010
AIM: To retrospectively evaluate the magnetic resonance imaging (MRI) features of adult retrorectal tumors and compare with histopathologic findings.
METHODS: MRI features of 21 patients with preoperative suspicion of retrorectal tumors were analyzed based on the histopathological and clinical data.
RESULTS: Fourteen benign cystic lesions appeared hypointense on T1-weighted images, and hyperintense on T2-weighted images with regular peripheral rim. Epidermoid or dermoid cysts were unilocular, and tailgut cysts were multilocular. Presence of intracystic intermediate signal intensity was observed in one case of tailgut cyst with a component of adenocarcinoma. Six solid tumors were malignant lesions and showed heterogeneous intensity on MRI. Mucinous adenocarcinomas showed high signal intensity on T2-weighted and mesh-like enhancing areas on fat-suppressed T2-weighted images. There was a fistula between the mass and anus with an internal opening in mucinous adenocarcinomas arising from anal fistula. Gastrointestinal stromal tumors displayed low signal intensity on T1-weighted images, and intermediate to high signal intensity on T2-weighted images. Central necrosis could be seen as a high signal on T2-weighted images.
CONCLUSION: MRI is a helpful technique to define the extent of the retrorectal tumor and its relationship to the surrounding structures, and also to demonstrate possible complications so as to choose the best surgical approach.
- Citation: Yang BL, Gu YF, Shao WJ, Chen HJ, Sun GD, Jin HY, Zhu X. Retrorectal tumors in adults: Magnetic resonance imaging findings. World J Gastroenterol 2010; 16(46): 5822-5829
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5822
Tumors occurring in the retrorectal space are extremely rare in the adult population, with an estimated incidence of 0.0025-0.014 in the large referral centers[1,2]. The retrorectal space is a potential space that only becomes real when a mass grows within it. The boundaries of the retrorectal region include the posterior wall of the rectum anteriorly and the sacrum posteriorly. This space extends superiorly to peritoneal reflection and inferiorly to the rectosacral fascia and the supralevator space. Laterally, the area is bordered by the ureters and the iliac vessels and the sacral nerve roots.
This region contains the confluence of the embryologic hindgut, neuroectoderm, and the bony pelvis. As such, multiple different tissue types can give rise to retrorectal tumors. Retrorectal tumors may be classified as congenital, neurogenic, osseous or miscellaneous[3,4]. Two-thirds are congenital[2-4], caused by embryological sequestration, abnormalities in midline fusion and incomplete embryological regression. Cystic congenital lesions include epidermoid and dermoid cysts, tailgut cyst (also called mucus-secreting cyst), enterogenic cyst, teratoma, and teratocarcinoma. Neurogenic (including anterior sacral meningoceles), osseous and miscellaneous tumors each account for approximately 10% of retrorectal tumors[3]. Majority of these masses in adults are benign and asymptomatic, however, malignant tumors accounted for 21%[5]. Malignant transformation has also been documented in tailgut cysts and epidermoid cysts[6-8]. Symptoms include pain, change in bowel habit, difficulty with micturition, and neurological signs in the lower limb and perineum[3,4,9].
Retrorectal tumors are often found late and may be managed suboptimally. The current consensus is that the cardinal therapy for patients with retrorectal tumors is surgical[9-11]. Curative resection requires complete excision of the tumor, with an intact capsule for clinically well-circumscribed benign lesions and en bloc resection with clear resection margins for malignant tumors[9,11]. Accurate diagnosis of these conditions before operation is crucial because it can significantly alter clinical management. MRI is a useful technique to evaluate pelvic disorders because of its multiplanar imaging capability and its good soft tissue contrast[2,4,6,8,9,11]. We retrospectively evaluated the MRI features of retrorectal tumors in 21 patients and compared with pathological findings to further characterize the MR imaging findings encountered in retrorectal tumors.
We reviewed the clinical and radiological findings in 21 patients (13 women, 8 men; age range, 16-74 years; mean age, 39.3 years) with preoperative suspicion of retrorectal tumor who were treated at our hospital between January 2006 and December 2008. Institutional review board permission was obtained for retrospective review without informed consent. MRI was performed in all patients with a standardized protocol. Data from clinic charts, hospital medical records, radiological and pathological reports of these patients were collected retrospectively. The clinical findings at presentation included rectal fullness (n = 7), low back pain (n = 2), constipation (n = 6), symptoms due to recurrent perirectal abscess and/or fistula (n = 5) and no apparent symptom (n = 1). Only two patients had postanal dimples. Digital examination of the rectum revealed a mass located posteriorly or laterally to the rectum in 20 patients. Laboratory tests demonstrated elevated carcino-embryonic antige (CEA) in two patients (2/21).
All 21 patients underwent preoperative MRI. MR examinations were achieved on a 1.5-T unit using an eight-channel phased-array pelvic coil (Siemens, MR Magnetom Sonata, Germany). Neither bowel preparation nor IV contrast enhancement was performed. Before performing MR, the rectum was distended by a balloon filled with 60-80 mL saline. The MR examination was performed with the patient in a supine position. MR examination protocol included sagittal, axial, coronal T2-weighted images, and axial (n = 21) T1-weighted images. T2-weighted imaging (TR/TE, 4000/97; echo-train length 13, field of view 20 cm, 4 mm slice thickness, no interslice gap, 256 × 256 matrix) were performed in the sagittal plane (n = 21), axial plane (n = 21) and coronal plane (n = 9), and followed with fat-suppression in sagittal and axial plane. Imaging parameters for T1-weighted sequences were: TR/TE, 620/12; echo-train length 1, field of view 22 cm, 4 mm slice thickness, no interslice gap, 320 × 240 matrix. Overall acquisition time varied from 20 to 30 min.
Magnetic resonance images were reported by two experienced radiologists. Four features of the retrorectal tumor were assessed. (1) the tumor location and extent were defined by the most cephalad sacral vertebra involved; (2) tumor size was measured in the largest two dimensions; (3) tumor morphology was assessed by examining the internal signal characteristics and the tumor margin. A cystic tumor was diagnosed when the lesion displayed over 80% cystic elements and a solid tumor when the lesion showed more than 80% solid elements; the remainders were classified as heterogeneous tumors; and (4) the tumor margin was assessed as well-defined (a smooth or lobular contour without surface projections), irregular (with surface projections) or clearly invasive (the tumor breached an adjacent structure).
Eighteen patients underwent surgery for retrorectal tumor. One patient with a gastrointestinal stromal tumor (GIST) arising from the rectum received abdominal perineal resection (APR). Two patients, diagnosed as having mucinous adenocarcinoma arising from fistulae-in-ano, received radiochemotherapy because the patients refused surgery. Histopathological studies demonstrated 7 malignant tumors (2 mucinous adenocarcinomas arising from fistula, 2 gastrointestinal stromal tumors, 1 tailgut cyst with component of mucinous adenocarcinoma, 1 adenocarcinoma of the anal duct, and 1 primary retrorectal adenocarcinoma) and 14 benign cysts (6 epidermoid cysts, 7 tailgut cysts and 1dermoid cyst). Patients with malignant tumors were significantly older than those with benign tumors (52.5 years vs 34.6 years). There was no gender difference in malignant tumors (male/female ratio, 4/3), and women comprised a larger proportion of the patients with benign cysts (male/female ratio, 3/11).
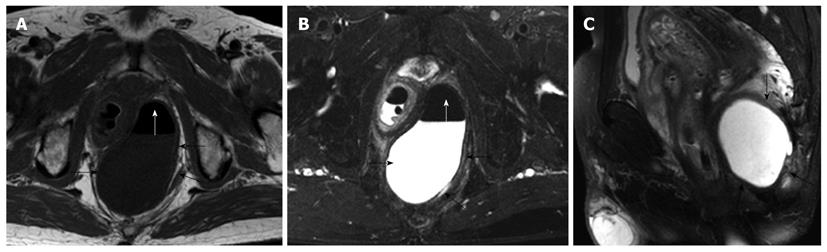
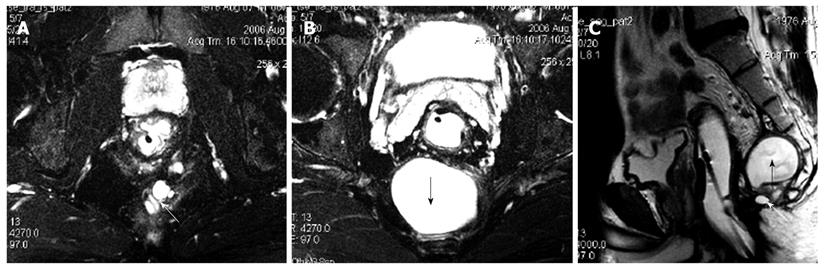
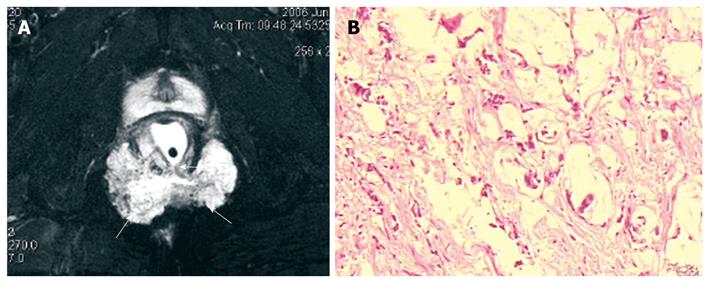
MRI demonstrated that benign cystic lesions were primarily located in the retrorectal space in 12 cases, with downward and lateral extension to ischiorectal fossa in two cases (Figure 1). The rectum appeared anterior or lateral in five patients. The mean largest diameter of the lesions was 4.8 cm (range 0.7-10 cm). Both epidermoid cysts and dermoid cysts were unilocular (Figure 1). There were numerous cysts in all tailgut cysts, shown as a large main cyst and associated with other smaller cysts in the same location (Figure 2). All the cystic lesions appeared hypointense on T1-weighted images, and hyperintense on T2-weighted images. As far as non-complicated cysts (10/14) were concerned, the cystic mass was well-circumscribed by a regular peripheral rim. The rim was hypointense on all sequences. The borders of the cystic lesions were irregular in 4 patients, who had an operative history with a misdiagnosis as having abscess or fistulae.
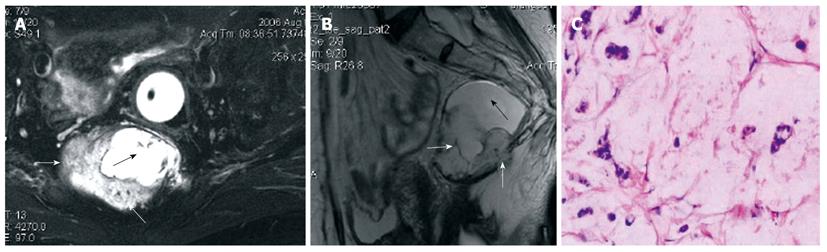
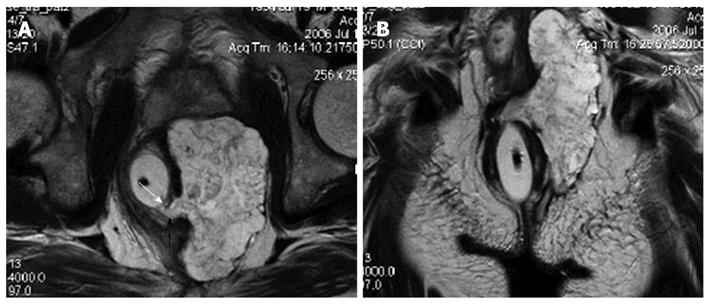
Heterogeneous tumor was found in one case of tailgut cyst with a component of adenocarcinoma (Figure 3). The rectum was compressed anteriorly but without evidence of invasion. The cystic portion appeared hypointense on T1-weighted images and hyperintense on T2-weighted images with irregular borders. The malignant portion presented as intermediate signal intensity on T2-weighted and fat-suppression with irregular margin.
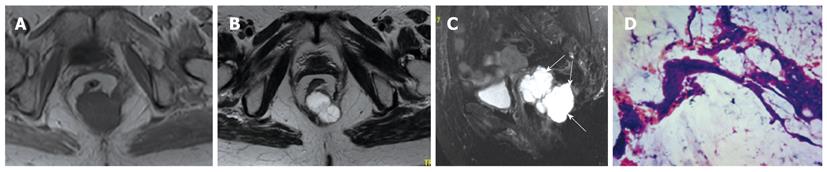
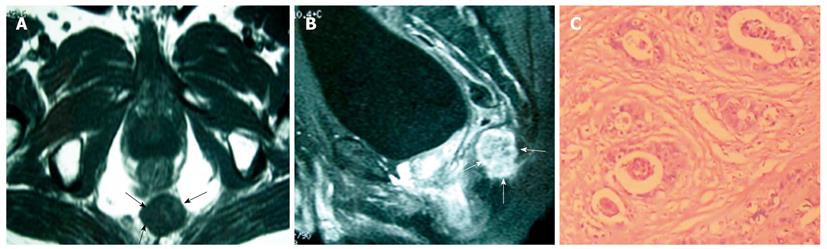
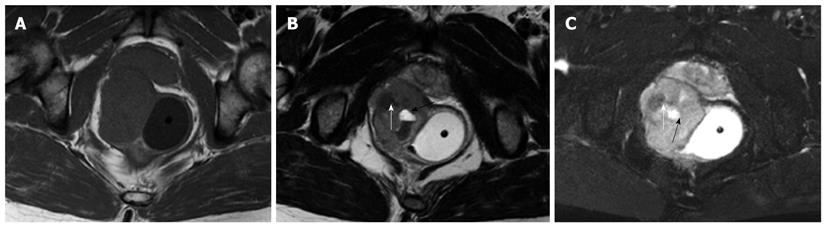
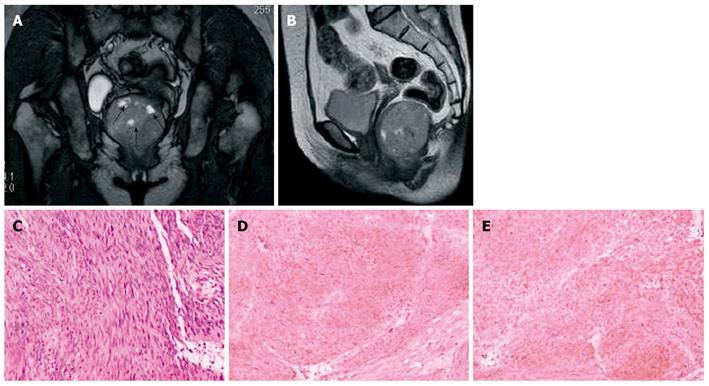
The six solid tumors were malignant lesions confirmed by histopathology. Three patients were diagnosed as having mucinous adenocarcinoma, two as having mucinous adenocarcinomas arising from chronic perianal fistulae, and 1 as having adenocarcinoma of the anal duct (Figure 4). MRI showed a larger mass in the pelvis, extending to the ischiorectal fossa. The mucin, which forms the major tissue component of mucinous tumor, showed high signal intensity on T2-weighted fast SE images and mesh-like enhancing areas on fat-suppressed T2-weighted images (Figure 5). As for mucinous adenocarcinoma arising from fistula-in-ano, there was a fistula between the mass and the anus with an internal opening in anorectum (Figure 6). One was diagnosed as primary presacral adenocarcinoma after operation, and MRI identified a well-circumscribed 3.5 cm × 4 cm solid lesion with irregular margin in the presacral space, not involving sacrum or rectum (Figure 7). The other two were diagnosed as gastrointestinal stromal tumors (GISTs), one appeared to arise from the rectum (Figure 8), and the other appeared in presacral space (Figure 9A and B). Tumors were larger than 5 cm in at least one transverse dimension. MRI showed low signal intensity on T1-weighted image, and heterogeneous signal intensity on T2-weighted image. We suspected a central necrosis based on the T2-weighted imaging with a high signal. In immunohistochemical studies, diffuse and strong immunoreactivity for CD34 and CD117 were seen throughout the tumors (Figure 9C and D). Conversely, the tumor cells were negative for both muscle markers (smooth muscle actin, desmin) and neural markers (S-100 protein, neuron specific enolase).
The boundaries of the presacral region include the posterior wall of the rectum and the sacrum. This space extends superiorly to peritoneal reflection and inferiorly to rectosacral fascia and the supralevator space. Laterally, the area is bordered by the ureters, the iliac vessels, and the sacral nerve roots. Because this area contains totipotential cells that differentiate into three germ cell layers, a multitude of tumor types may be encountered. Traditionally, these lesions are divided into congenital, inflammatory, neurogenic, osseous and miscellaneous types[3,4]. Clinical diagnosis may be delayed because of non-specific symptoms. Symptomatic patients typically complain of vague, longstanding pain in the perineum or low back, and change in bowel habit[1,5,8]. Because detection is often difficult and delayed, patients frequently present with tumors that have reached a considerable size and involve multiple organ systems, thus complicating their treatment. Singer et al[12] reported 7 patients who underwent an average of 4.7 invasive procedures or operations before the correct diagnosis of a retrorectal lesion was made.
The diagnosis and management of these tumors has evolved in recent years because of improved imaging modalities, especially the MRI. MRI is often used in diagnosing and managing the patients with presacral tumors, as it can provide excellent anatomic detail and soft-tissue contrast. Kim et al[13] asserted that for the evaluation of a presacral mass, MRI has the advantage over CT of being able to offer multiplanar capabilities and good tissue contrast. MRI is a valuable tool for preoperative evaluation, imaging and characterizing lesions, estimating their extent and the risk of malignancy, distinguishing organ-confined disease from tumor spread into adjacent structures, and deciding upon the most appropriate intervention strategies and imaging follow-up requirements.
About half of the presacral tumors are congenital lesions, and most of them are developmental cysts (epidermoid, dermoid, tailgut cysts, and teratomas). On MRI, presacral cyst usually has low signal intensity on T1-weighted images and high signal intensity on T2-weighted images[8]. However, it may have high signal intensity on T1-weighted images due to presence of mucinous materials, high protein content, or hemorrhage in the cyst[6,8]. All of the presacral cysts in our cases were hypointense on T1-weighted images and hyperintense on T2-weighted images. Among the presacral cystic masses, epidermoid cyst, dermoid cyst, rectal duplication cyst, and meningocele are usually unilocular[14]. The presence of fat content on fat-saturated images is suggestive of a dermoid cyst[8]. Rectal duplication cysts often communicate with the rectal lumen and are anterior to the rectum. Anterior meningocele is a well-defined unilocular thin-walled, fluid-filled lesion of the retrorectal space with a stalk that may be seen communicating with the thecal sac. In contrast, tailgut cyst is usually multicystic[6]. In our study, we observed that epidermoid cysts and dermoid cysts appeared unilocular on MRI, and tailgut cysts appeared as a large cyst accompanied by small peripheral cysts. We believe that the unilocular or multilocular characteristics are very important because of the malignant potential of a tailgut cyst. A few cases of degenerated tailgut cysts have been documented in the literature[6,15-17]. The possibility of malignant transformation must be considered in the presence of heterogeneous tumor. Our findings, that a cystic lesion displayed heterogeneous signal intensity on T2-weighed MR images with markedly irregular wall, and the malignant portion presented as irregular margin with intermediate signal intensity on T2-weighted and fat-suppression, are consistent with what has been described in the literature[6,17].
MRI is useful in predicting whether a tumor is benign or malignant. A cystic tumor with a smooth, well-circumscribed margin and no features of invasion or enhancement with gadolinium is benign, and a heterogeneous tumor, or a solid tumor with an irregular margin, is usually malignant[9]. The six solid tumors were malignant confirmed by histopathology in this series. Two patients with adenocarcinoma arising from fistula-in-ano displayed presacral tumor. Several characteristic MRI findings may help diagnose mucinous adenocarcinoma arising from fistula-in-ano. Histopathologically, mucinous colorectal carcinomas comprise large pools of extracellular mucin lined by columns of malignant cells, cords and vessels, which give rise to a typical mesh-like internal structure[18]. The mucin, which forms the major tissue component of mucinous tumors, has high signal intensity on T2-weighted fast SE images. Two authors reported that a fistula between the mass and the anus is a characteristic finding of mucinous adenocarcinoma arising from fistula-in-ano on MRI[19,20]. Two patients showed a fistula tract between the mass and the anus in agreement with the previous reports.
GIST is a non-epithelial neoplasm arising from the wall of the gastrointestinal tract. GIST is thought to originate from the interstitial cell of Cajal, an intestinal pacemaker cell[21]. GISTs are most often located in the stomach (39%-70%) and small intestine (20%-35%), whereas the colon and rectum (5%-12%) are less common locations[22-25]. Some GISTs primarily arise in the omentum, mesentery, or retroperitoneum and are unrelated to the tubular gastrointestinal tract. It is even rarer that GIST originates from presacral space. On MRI, solid portions of tumor typically show low signal intensity on T1-weighted images, intermediate to high signal intensity on T2-weighted images, and enhancement after administration of gadolinium[26]. The markedly high signal seen on T2-weighted MRI should be considered as a feature strongly indicating a diagnosis of GIST[27]. GISTs usually involve the muscularis propria of the gastrointestinal wall, so the characteristic image is that of a well-circumscribed, smooth, intramural mass with exophytic growth. The case with rectal GIST in this series presented as well-defined, eccentric mural mass that expanded the rectal wall and extended into the right ischiorectal fossa. As in GISTs at other locations, central areas of necrosis could be seen in our cases.
Adenocarcinomas of the presacral region are distinctly unusual. Most of the cases represent direct extension or metastatic spread from rectal cancer. Although malignant replacement of these cysts is possible, complete replacement of the cyst epithelium and other elements by adenocarcinoma has not been reported[5]. However, potential sources such as gastrointestinal, pancreatic and prostatic tissues were eliminated and no evidence of a developmental cyst was found histopathologically in this case. The tumor described in the present report should be regarded as a primary presacral adenocarcinoma, as reported by Zamir et al[5] and Puccio et al[28]. Pelvic MRI demonstrated a retrorectal heterogeneous solid lesion on both T1-weighted and T2-weighted images and a markedly high signal was seen on T2-weighted images after fat-saturation.
A further benefit of MRI being confirmed is whether routine preoperative biopsy is necessary[29]. Historically, the role of preoperative biopsy of retrorectal tumors has been a controversial topic in the general surgery. When preoperative MRI is available, the indications for biopsy can be limited to patients whose mass may represent metastatic disease or lymphoma[9]. The risk of a routine biopsy can, therefore, be avoided.
Retrorectal tumors can be best managed by surgery. Careful surgical planning is important by selecting appropriate approaches, such as an anterior approach (abdominal), posterior approach (perineal), or a combined abdominoperineal approach. MRI will help define the margins of resection and the relationship between the tumor and the sacral level. If the tumor is positioned below the mid-body of S3, a perineal approach can be considered. All tumors that extend above S3 often require an abdominal or combined approach.
In conclusion, retrorectal tumor is a rare entity that is difficult to diagnose. Our series supports that MRI is a useful examination when a retrorectal tumor is suspected. Cystic lesions with a smooth wall on MRI are typically benign, whereas heterogeneous or solid tumors are usually malignant. However, final diagnosis should be based on the pathological examination after surgical resection. MRI is a helpful technique to define the extent of the tumor, its relationship to the surrounding structures and also to demonstrate possible complications in order to choose the best surgical approaches.
Tumors occurring in the retrorectal space are extremely rare in the adult population. Retrorectal tumors may be classified as congenital, neurogenic, osseous or miscellaneous types. Majority of these masses are benign and asymptomatic, however, malignant tumors accounted for 21%. Curative resection requires a complete excision of the tumor, with an intact capsule for clinically well-circumscribed benign lesions and en bloc resection with clear resection margins for malignant tumors. Accurate diagnosis of these conditions before operation is crucial because it can significantly alter clinical management.
The diagnosis and management of retrorectal tumors have evolved in recent years because of improved imaging modalities, especial magnetic resonance imaging (MRI). MRI is a valuable tool for preoperative evaluation, imaging and characterizing lesions, estimating their extent and the risk of malignancy, distinguishing organ-confined disease from tumor spread into adjacent structures, and deciding upon the most appropriate intervention strategies and imaging follow-up requirements.
This series supports that MRI is a useful examination when a retrorectal tumor is suspected. Cystic lesions with a smooth wall on MRI are typically benign, whereas heterogeneous or solid tumors are usually malignant. MRI is a helpful technique to define the extent of the tumor, its relationship to the surrounding structures and also to demonstrate possible complications in order to choose the best surgical approach.
MRI is a useful technique to evaluate retrorectal tumors. It can provide optimal information about tumor location, size, extent, the risk of malignancy and strategy for therapeutic intervention in the patients with retrorectal tumor.
Retrorectal space, also called presacral space, is a potential space limited anteriorly by the fascia propria of the rectum and posteriorly by Waldeyer’s rectosacral fascia. Laterally, it is limited by the iliac vessels and the ureters and extends along the lateral ligaments of the rectum, communicating superiorly at the level of the peritoneal reflection with the retroperitoneal space.
This article well documented the MRI features of retrorectal lesions, and this will interest our readers.
Peer reviewers: Philip H Gordon, Professor, Department of Surgery, McGill University, 3755 Cote Ste. Catherine Road, Suite G-314, Montreal, Quebec, H3T 1E2, Canada; Francis Seow-Choen, MBBS, FRCSEd, FAMS, Professor, Seow-Choen Colorectal Centre, Mt Elizabeth Medical Centre, Singapore, 3 Mt Elizabeth Medical Centre #09-10, 228510, Singapore
S- Editor Shi ZF L- Editor Ma JY E- Editor Ma WH
| 2. | Wolpert A, Beer-Gabel M, Lifschitz O, Zbar AP. The management of presacral masses in the adult. Tech Coloproctol. 2002;6:43-49. |
| 3. | Jao SW, Beart RW Jr, Spencer RJ, Reiman HM, Ilstrup DM. Retrorectal tumors. Mayo Clinic experience, 1960-1979. Dis Colon Rectum. 1985;28:644-652. |
| 4. | Glasgow SC, Birnbaum EH, Lowney JK, Fleshman JW, Kodner IJ, Mutch DG, Lewin S, Mutch MG, Dietz DW. Retrorectal tumors: a diagnostic and therapeutic challenge. Dis Colon Rectum. 2005;48:1581-1587. |
| 5. | Zamir G, Wexner SD, Pizov G, Reissman P. Primary presacral adenocarcinoma. Report of a case. Dis Colon Rectum. 1998;41:1056-1058. |
| 6. | Aflalo-Hazan V, Rousset P, Mourra N, Lewin M, Azizi L, Hoeffel C. Tailgut cysts: MRI findings. Eur Radiol. 2008;18:2586-2593. |
| 7. | Schwarz RE, Lyda M, Lew M, Paz IB. A carcinoembryonic antigen-secreting adenocarcinoma arising within a retrorectal tailgut cyst: clinicopathological considerations. Am J Gastroenterol. 2000;95:1344-1347. |
| 8. | Yang DM, Kim HC, Lee HL, Lee SH, Kim GY. Squamous cell carcinoma arising from a presacral epidermoid cyst: CT and MR findings. Abdom Imaging. 2008;33:498-500. |
| 9. | Woodfield JC, Chalmers AG, Phillips N, Sagar PM. Algorithms for the surgical management of retrorectal tumours. Br J Surg. 2008;95:214-221. |
| 10. | Buchs N, Taylor S, Roche B. The posterior approach for low retrorectal tumors in adults. Int J Colorectal Dis. 2007;22:381-385. |
| 11. | Pappalardo G, Frattaroli FM, Casciani E, Moles N, Mascagni D, Spoletini D, Fanello G, Gualdi G. Retrorectal tumors: the choice of surgical approach based on a new classification. Am Surg. 2009;75:240-248. |
| 12. | Singer MA, Cintron JR, Martz JE, Schoetz DJ, Abcarian H. Retrorectal cyst: a rare tumor frequently misdiagnosed. J Am Coll Surg. 2003;196:880-886. |
| 13. | Kim MJ, Kim WH, Kim NK, Yun MJ, Park YN, Lee JT, Yoo HS. Tailgut cyst: multilocular cystic appearance on MRI. J Comput Assist Tomogr. 1997;21:731-732. |
| 14. | Dahan H, Arrivé L, Wendum D, Docou le Pointe H, Djouhri H, Tubiana JM. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics. 2001;21:575-584. |
| 15. | Jarboui S, Jarraya H, Mihoub MB, Abdesselem MM, Zaouche A. Retrorectal cystic hamartoma associated with malignant disease. Can J Surg. 2008;51:E115-E116. |
| 16. | Cho BC, Kim NK, Lim BJ, Kang SO, Sohn JH, Roh JK, Choi ST, Kim SA, Park SE. A carcinoembryonic antigen-secreting adenocarcinoma arising in tailgut cyst: clinical implications of carcinoembryonic antigen. Yonsei Med J. 2005;46:555-561. |
| 17. | Lim KE, Hsu WC, Wang CR. Tailgut cyst with malignancy: MR imaging findings. AJR Am J Roentgenol. 1998;170:1488-1490. |
| 18. | Teixeira CR, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. The clinical significance of the histologic subclassification of colorectal carcinoma. Oncology. 1993;50:495-499. |
| 19. | Fujimoto H, Ikeda M, Shimofusa R, Terauchi M, Eguchi M. Mucinous adenocarcinoma arising from fistula-in-ano: findings on MRI. Eur Radiol. 2003;13:2053-2054. |
| 20. | Hama Y, Makita K, Yamana T, Dodanuki K. Mucinous adenocarcinoma arising from fistula in ano: MRI findings. AJR Am J Roentgenol. 2006;187:517-521. |
| 21. | O’leary T, Berman JJ. Gastrointestinal stromal tumors: answers and questions. Hum Pathol. 2002;33:456-458. |
| 22. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. |
| 23. | Sturgeon C, Chejfec G, Espat NJ. Gastrointestinal stromal tumors: a spectrum of disease. Surg Oncol. 2003;12:21-26. |
| 24. | Trupiano JK, Stewart RE, Misick C, Appelman HD, Goldblum JR. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol. 2002;26:705-714. |
| 25. | Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655-664. |
| 26. | Takao H, Yamahira K, Doi I, Watanabe T. Gastrointestinal stromal tumor of the retroperitoneum: CT and MR findings. Eur Radiol. 2004;14:1926-1929. |
| 27. | Caramella T, Schmidt S, Chevallier P, Saint Paul M, Bernard JL, Bidoli R, Bruneton JN. MR features of gastrointestinal stromal tumors. Clin Imaging. 2005;29:251-254. |
| 28. | Puccio F, Solazzo M, Marcianò P, Fadani R, Regina P, Benzi F. Primary retrorectal adenocarcinoma: report of a case. Tech Coloproctol. 2003;7:55-57. |
| 29. | Abel ME, Nelson R, Prasad ML, Pearl RK, Orsay CP, Abcarian H. Parasacrococcygeal approach for the resection of retrorectal developmental cysts. Dis Colon Rectum. 1985;28:855-858. |