Published online Sep 28, 2010. doi: 10.3748/wjg.v16.i36.4564
Revised: July 3, 2010
Accepted: July 10, 2010
Published online: September 28, 2010
AIM: To ascertain the role of cardiovascular risk factors, cardiovascular diseases, standard treatments and other diseases in the development of ischemic colitis (IC).
METHODS: A retrospective, case-control study was designed, using matched data and covering 161 incident cases of IC who required admission to our hospital from 1998 through 2003. IC was diagnosed on the basis of endoscopic findings and diagnostic or compatible histology. Controls were randomly chosen from a cohort of patients who were admitted in the same period and required a colonoscopy, excluding those with diagnosis of colitis. Cases were matched with controls (ratio 1:2), by age and sex. A conditional logistic regression was performed.
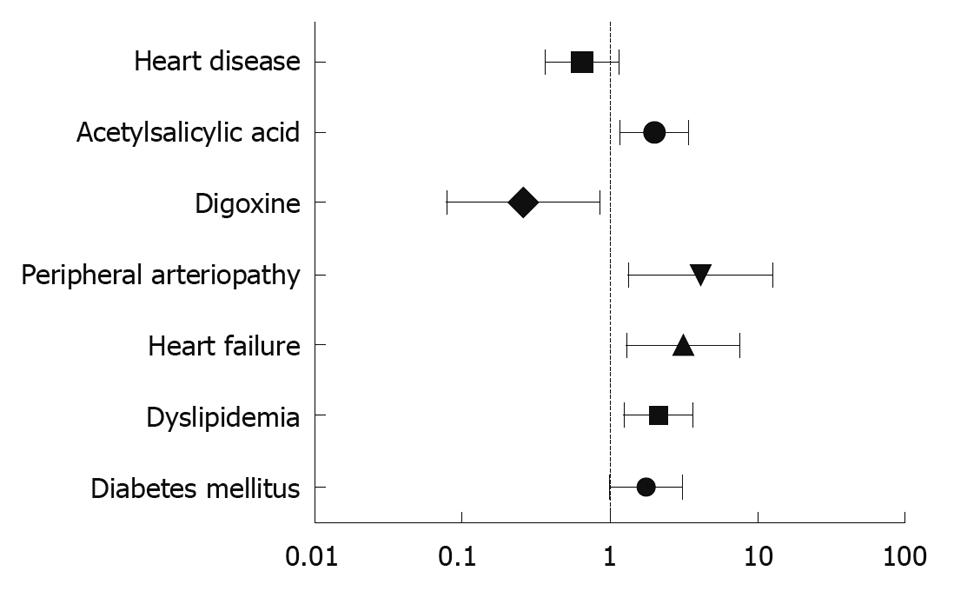
RESULTS: A total of 483 patients (161 cases, 322 controls) were included; mean age 75.67 ± 10.03 years, 55.9% women. The principal indications for colonoscopy in the control group were lower gastrointestinal hemorrhage (35.4%), anemia (33.9%), abdominal pain (19.9%) and diarrhea (9.6%). The endoscopic findings in this group were hemorrhoids (25.5%), diverticular disease (30.4%), polyps (19.9%) and colorectal cancer (10.2%). The following variables were associated with IC in the univariate analysis: arterial hypertension (P = 0.033); dyslipidemia (P < 0.001); diabetes mellitus (P = 0.025); peripheral arterial disease (P = 0.004); heart failure (P = 0.026); treatment with hypotensive drugs (P = 0.023); angiotensin-converting enzyme inhibitors; (P = 0.018); calcium channel antagonists (P = 0.028); and acetylsalicylic acid (ASA) (P < 0.001). Finally, the following variables were independently associated with the development of IC: diabetes mellitus [odds ratio (OR) 1.76, 95% confidence interval (CI): 1.001-3.077, P = 0.046]; dyslipidemia (OR 2.12, 95% CI: 1.26-3.57, P = 0.004); heart failure (OR 3.17, 95% CI: 1.31-7.68, P = 0.01); peripheral arterial disease (OR 4.1, 95% CI: 1.32-12.72, P = 0.015); treatment with digoxin (digitalis) (OR 0.27, 95% CI: 0.084-0.857, P = 0.026); and ASA (OR 1.97, 95% CI: 1.16-3.36, P = 0.012).
CONCLUSION: The development of an episode of IC was independently associated with diabetes, dyslipidemia, presence of heart failure, peripheral arterial disease and treatment with digoxin or ASA.
- Citation: Fernández JC, Calvo LN, Vázquez EG, García MJG, Pérez MTA, Silva IM, Seara JF. Risk factors associated with the development of ischemic colitis. World J Gastroenterol 2010; 16(36): 4564-4569
- URL: https://www.wjgnet.com/1007-9327/full/v16/i36/4564.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i36.4564
Ischemic colitis (IC) is the most frequent form of intestinal ischemia (70%) and arises in cases where the colon is transitorily deprived of vascular flow. IC may be the result of an occlusive or non-occlusive disease. Clinically, IC is classified as gangrenous or non-gangrenous. Non-gangrenous IC affects the mucosa and submucosa, and is responsible for 80%-85% of cases[1]. Non-gangrenous forms have been subclassified into transitory reversible forms with mild damage, and chronic irreversible forms, which include chronic colitis and stenosis and imply more severe impairment[1]. Development of IC has been associated with a series of diseases and risk factors. In general, any condition or factor that reduces the blood flow to the colon can generate IC. In this respect, a higher incidence of IC has been described in patients who undergo vascular surgery (aortic surgery or coronary bypass in particular)[2,3] or have cardiovascular[4-6] or hematological diseases[7,8]. IC has also been described among patients with microvascular disease, such as systemic lupus erythematosus[9,10], with chronic renal failure that requires dialysis[4] and with use/abuse of certain medications or drugs, such as oral contraceptives, vasoconstrictors, psychotropic drugs, interferon-α, nonsteroidal anti-inflammatory drugs (NSAIDs), 5-HT3 receptor antagonists and cocaine[11,12].
In recent years, different population-based studies have been published which have sought to detect risk factors associated with the development of IC[11,13-15]. Note should be taken of the relationship observed between diagnosis of irritable bowel syndrome and IC, both in case-control and cohort studies[11,13,14]. Similarly, population-based studies of cohorts with chronic obstructive pulmonary disease (COPD) have reported an increased risk of developing an episode of IC among such patients[11].
The prevalence of cardiovascular risk factors, cardiovascular diseases and related medication in cohorts of patients requiring admission due to an episode of IC is high. Thus, in two series published recently in this country, the prevalence of arterial hypertension, dyslipidemia and diabetes was in the order of 57%-66%, 24%-26% and 20%-22%, respectively. In these series, a high prevalence of cardiovascular disease was detected, whether ischemic or hypertensive heart disease (18%-21% and 15.4%, respectively), cerebral vascular disease (12%-20%) or peripheral arterial disease (8%-14%). Lastly, there was a high rate of patients treated with acetylsalicylic acid (ASA) (32%), hypotensive drugs (54%) and diuretics (33.7%-20.7%)[16,17]. Although such prevalence might be linked to the advanced mean age of the cohorts described, there are no population-based studies published that have assessed the possible causal effect of cardiovascular risk factors on the development of IC.
This study sought to: assess whether cardiovascular risk factors, such as hypertension, diabetes, hypercholesterolemia, smoking and cardiovascular diseases, are linked to the development of IC; confirm the relationship between COPD and development of IC in Spain; and finally, assess the effect that standard drug treatments have on the development of IC.
This was a retrospective, observational, case-control study using clinical history data matched in a ratio of 1:2.
We selected incident cases of IC requiring admission to the Ourense Hospital Complex (Galicia, Spain) during the period January 1998 through March 2003. To detect such cases, endoscopic and pathological anatomy records were searched for diagnoses of IC, segmentary colitis and/or indeterminate colitis, and the pertinent clinical histories were then reviewed. Diagnosis of IC was based on endoscopic findings and/or diagnostic or compatible histology. Individuals with a diagnosis of colitis of any other origin (infectious, inflammatory, diverticulitis, associated with antibiotics or NSAIDs) were excluded from this cohort, as were individuals with IC who did not require admission. IC cases that were prevalent during the study period were likewise excluded. Finally, a total of 161 individuals were included. Their endoscopic location and endoscopic and histological findings are shown in Table 1.
| n (%) | |
| Endoscopic location | |
| Pancolitis | 3 (1.9) |
| Right colon | 10 (6.5) |
| Transverse colon | 34 (21) |
| Splenic angle | 36 (23.2) |
| Descending colon | 69 (44.5) |
| Sigmoid colon | 106 (68.4) |
| Rectum | 38 (24.5) |
| Endoscopic findings | |
| Hyperemia | 98 (65.3) |
| Petechiae | 37 (24.7) |
| Intramucosal bleeding | 30 (20) |
| Fibrin | 43 (26.5) |
| Ulcers | 84 (56) |
| Active bleeding | 9 (6) |
| Necrosis | 12 (8) |
| Estenosis | 12 (8) |
| Histologic findings | |
| Crypt loss | 17 (14.7) |
| Epithelium loss | 13 (8) |
| Edema | 68 (58.6) |
| Inflamatory infiltrate | 87 (75) |
| Capillar thrombosis | 17 (14.7) |
| Necrosis | 40 (34.5) |
| Hemorrhage | 1 (2.5) |
Controls were selected from the cohort of 1555 patients who, during the same time period, required admission to the Ourense Hospital Complex and underwent a colonoscopy. Individuals with diagnosis of colitis of any origin were excluded from this cohort.
Demographic data (age, sex) were collected. Insofar as personal histories were concerned, information was gathered regarding the presence of the following diseases: arterial hypertension; hypercholesterolemia; diabetes mellitus; smoking; COPD; chronic renal failure; heart disease (of hypertensive, ischemic or valvular etiology, heart failure, atrial fibrillation); peripheral arterial disease; abdominal aortic aneurysm surgery; and cerebrovascular disease. With respect to usual medication, we examined whether patients were receiving one or more of the following drugs at date of admission: ASA; NSAIDs; acenocumarol; digoxin (digitalis); diuretics; antidepressants; beta blockers; calcium channel antagonists; angiotensin-converting enzyme inhibitors; angiotensin II receptor antagonists; and antidepressants. Lastly, in the control group we recorded both the reason for a colonoscopy being requested and the endoscopic findings.
Sample size was ascertained on the basis of data on the prevalence of cardiovascular factors known to be present in our cohort of IC patients[17], with the cardiovascular risk factor with the lowest prevalence being used to calculate the sample size. Hence, bearing in mind that smoking registered a prevalence of 10.1%, and accepting an alpha risk of 0.05 and a beta risk of 0.20 in a two-sided test for detection of an odds ratio (OR) of 3160 individuals were required in the case group and 320 in the control group. Cases were matched 1:2 with controls, by age and sex. The EPIDAT 3.1 computer software program was used to calculate sample size and randomization of controls.
This study was formally authorized by the Galician Clinical Research Ethics Committee (code 2008/374) on 15th December 2008.
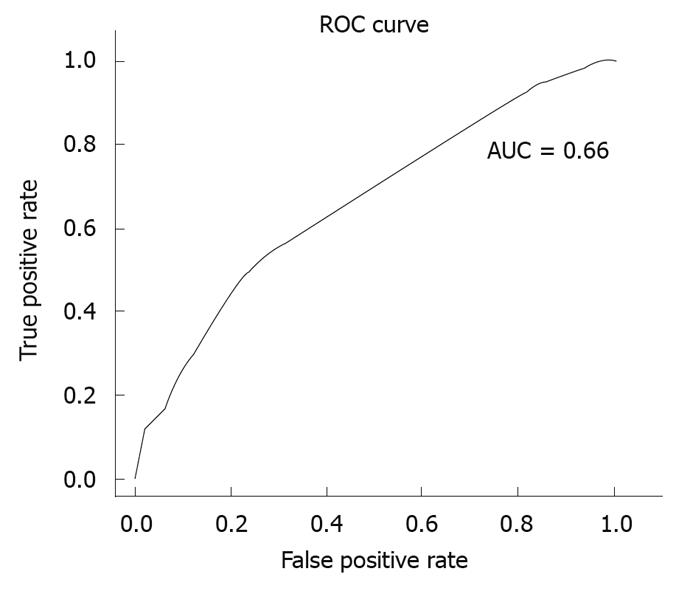
As a first step, we conducted a descriptive analysis of the variables covered by the study. Univariate and multivariate analyses were then performed using conditional logistic regression. In the multivariate analysis, account was taken of those variables that had proved statistically significant in the univariate analysis and those that were deemed to be clinically relevant. The goodness-of-fit of the models generated was ascertained using the pseudo R2 coefficient of determination and the area under the curve (AUC), with an alpha error = 0.05 and beta error = 0.2 being considered in all tests. The statistical analysis was performed using the free R software (http://www.r-project.org). Odds ratios and their confidence intervals (CIs) were graphically depicted using the Graph Pad Prism 5.0 program.
A total of 483 individuals, 161 cases and 322 controls, were included in the study; mean age 75.67 ± 10.03 years (cases: 75.42 ± 10.58, controls: 75.79 ± 9.75, P = 0.56), 55.9% women (cases: 55.9%, controls: 55.9%, P = 0.5). The reasons given for performing a colonoscopy in controls were: lower gastrointestinal hemorrhage, 114 (35.4%); ferropenic anemia, 109 (33.9%); abdominal pain, 64 (19.9%); diarrhea syndrome, 31 (9.6%); intestinal rhythm disorder, 22 (6.8%); and melena, 13 (4%). The endoscopic findings detected in the controls were: diverticular disease, 98 (30.4%); hemorrhoids, 82 (25.5%); polyps, 64 (19.9%); colorectal cancer, 33 (10.2%); and angiodysplasias, 24 (7.5%).
The clinical history and standard pharmacologic treatments of cases and controls are shown in Tables 2 and 3, respectively, along with the pertinent ORs, CIs and significance levels in the univariate conditional logistic regression.
| Case (%) | Control (%) | P value | OR | 95% CI | |
| Arterial hypertension | 56.5 | 46.6 | 0.034 | 1.543 | 1.035-2.301 |
| Dyslipidemia | 19.8 | 12.1 | 0.025 | 1.793 | 1.075-2.992 |
| Diabetes mellitus | 26.7 | 12.7 | < 0.001 | 2.427 | 1.506-3.911 |
| Smoking habit | 10.5 | 9.9 | 0.825 | 1.075 | 0.564-2.049 |
| Chronic renal failure | 4.3 | 4.6 | 0.877 | 0.93 | 0.372-2.33 |
| Valve heart disease | 7.4 | 6.8 | 0.806 | 1.094 | 0.535-2.234 |
| Ischemic heart disease | 18 | 14.9 | 0.381 | 1.254 | 0.756-2.08 |
| Hypertensive heart disease | 15.5 | 13.4 | 0.516 | 1.195 | 0.698-2.044 |
| Atrial fibrillation | 14.3 | 14 | 0.923 | 1.028 | 0.585-1.808 |
| Heart failure | 10.5 | 5 | 0.026 | 2.253 | 1.103-4.604 |
| Peripheral arteriopathy | 8.1 | 2.2 | 0.004 | 4.658 | 1.643-13.21 |
| Abdominal aorta surgery | 1.2 | 0 | 0.096 | ||
| Cerebrovascular disease | 12.4 | 9.6 | 0.339 | 1.348 | 0.731-2.483 |
| COPD | 18 | 18.6 | 0.864 | 0.956 | 0.576-1.587 |
| Case (%) | Control (%) | P value | OR | 95% CI | |
| Antihypertensives | 51.5 | 41 | 0.023 | 1.595 | 1.067-2.383 |
| Beta-blockers | 5.6 | 5.6 | 1 | 1 | 0.443-2.26 |
| Ca2+ channel antagonists | 22.3 | 14.6 | 0.027 | 1.771 | 1.065-2.946 |
| ACE inhibitors | 27.3 | 18.3 | 0.018 | 1.776 | 1.102-2.861 |
| Nitrates | 10.5 | 9.3 | 0.67 | 1.143 | 0.617-2.117 |
| ARB | 1.2 | 4 | 0.109 | 0.358 | 0.102-1.259 |
| NSAIDs | 10.5 | 9.9 | 0.823 | 1.077 | 0.561-2.07 |
| Digoxin | 3.1 | 7.4 | 0.060 | 0.383 | 0.141-1.042 |
| Antidepressants | 3.7 | 6.2 | 0.222 | 0.540 | 0.201-1.453 |
| Diuretics | 26.1 | 26.1 | 1 | 1 | 0.656-1.525 |
| ASA | 31.6 | 18 | < 0.001 | 2.153 | 1.371-3.382 |
| Acenocumarol | 5.6 | 5.6 | 1 | 1 | 0.443-2.26 |
In the multivariate analysis, a statistically significant relationship was found between diagnosis of IC and the following variables: diabetes mellitus (P = 0.029); hypercholesterolemia (P = 0.007); heart failure (P = 0.034); peripheral arterial disease (P = 0.015); and continued treatment with digoxin (P = 0.018) or ASA (P = 0.012). To control for the effect of heart disease, a model was then fitted including the significant variables adjusted for diagnosis of heart disease of any origin. The results yielded by this model can be seen in Table 4 and Figure 1. The pseudo R2 coefficient of determination was 0.086. Figure 2 depicts the receiver operator characteristic curve of the model and its AUC.
| Variables | P value | Coefficient | OR | 95% CI |
| Diabetes mellitus | 0.04600 | 0.5671 | 1.7632 | 1.0101-3.0776 |
| Dyslipidemia | 0.00437 | 0.7550 | 2.1277 | 1.2658-3.5762 |
| Heart failure | 0.01064 | 1.1539 | 3.1705 | 1.3079-7.6853 |
| Peripheral arteriopathy | 0.01461 | 1.4109 | 4.0997 | 1.3211-12.7219 |
| Digoxin | 0.02651 | -1.3173 | 0.2679 | 0.0836-0.8576 |
| Acetylsalicylic acid | 0.01219 | 0.6808 | 1.9755 | 1.1600-3.3640 |
| Heart disease | 0.14541 | -0.4224 | 0.6554 | 0.3711-1.1575 |
Although development of IC has been associated with diverse phenomena that reduce mesenteric perfusion, there are no studies that have assessed the relationship between IC and cardiovascular risk factors and cardiovascular diseases. In one case-control study of autopsies, a statistically significant relationship was found between diagnosis of fatal IC and presence of heart failure, valvular heart disease, acute myocardial infarction and previous surgery[18]. The results of this study are limited, however, in that it covered a series of autopsies in which serious forms of IC were diagnosed. In the majority of patients, IC develops as a transitory or self-limited form[16,17,19,20]. In our study, both mild or transitory and severe forms were included. We detected an independent relationship between development of IC and previous diagnoses of cardiovascular risk factors (diabetes mellitus, hypercholesterolemia) and cardiovascular diseases (peripheral arterial disease, heart failure). However, no relation was found with smoking habit. In fact, the prevalence of smoking habit was low. The most recent data available in Spain show that the proportion of the population over 65 years who are actively smoking is low: 8.5%[21]. Our findings thus support the hypothesis of IC as a disease associated with a reduction in mesenteric blood flow.
Moreover, we detected an independent relationship between IC and treatment with ASA or digoxin. NSAIDs in general, and ASA in particular, are known to have a harmful effect on the gastrointestinal tract. NSAIDs-related colitis has been described in connection with both short-[12,22] and long-term treatments[23]. Different clinical observations and case-control studies have linked the use of NSAIDs or salicylates to diagnosis of acute diarrhea syndrome[24] and exacerbation of inflammatory colitis (ulcerative colitis or Crohn’s disease)[23]. Insofar as the relationship with the development of IC is concerned, there is little evidence. There is one clinical report in which the use of meloxicam is associated with the development of IC[12]. In the case-control study published by Collin et al[25] a statistically significant relationship was observed between use of NSAIDs and development of segmentary non-gangrenous colitis. In our study, an independent relationship was detected between use of ASA and development of IC. This did not apply to continued use of NSAIDs, once ASA had been excluded. The mechanism associated with tissue damage has not been fully elucidated. Studies based on a model of colitis associated with trinitrobenzene sulfonic acid have confirmed the ability of NSAIDs to exacerbate colitis[26]. This effect is brought about by inhibition of cycloxygenase-2 (COX-2)[26]. COX-2 is also the principal agent responsible for the process that leads to resolution of the inflammation. Prostaglandin D2 acts as a termination signal, by reducing granulocyte infiltration[27]. Similarly, inhibition of COX-2 and prostaglandins with their vasodilatory effect could increase vascular resistance in the splanchnic circulation[22]. As regards the protective effect of treatment with digoxin observed in our study, little has been published. Indeed, only one case-control study has been reported, in which use of digoxin was found to have a harmful effect[25]. Nevertheless, this study failed to analyze confounding variables, such as diagnoses of heart failure and heart disease, which we introduced into our analysis. The protective effect could be linked to the positive inotropic effect associated with digoxin treatment, which would generate an increase in splanchnic blood flow.
Our patients with IC had an advanced age; the mean age was 75.42 ± 10.58 years. This finding is similar to the data published recently in series of patients with IC in our country[16,19,20]. It is noteworthy that, despite the advanced age of the patients, the prognosis was fairly good: only 5.9% required surgery and 4.7% died during hospitalization[17].
Our study has a number of limitations, the first of which is that it was a retrospective study. Although the collection of clinical history data was based on a protocol drawn up at our hospital, conditions related to development of IC, such as constipation and irritable bowel syndrome, could not be evaluated[11,13,14,20]. The control group might suffer from selection bias as a result of it being a group of patients who required admission. However, the selection criterion - performance of a colonoscopy - was not related to the variables analyzed. At all events, in view of the fact that these were patients who required admission, the control group might have had a frequency of cardiovascular risk factors, cardiovascular diseases and drug use higher than that of the general population. In this respect, the conclusions of this study must be validated in the context of a population-based cohort study. Since incidence of IC in the general population is low, ranging from 4.5 to 44 cases per 100 000 population[11], designing a cohort study to assess the effect of these variables may prove complicated.
Another of this study’s limitations resides in the lack of some clearly defined criteria for diagnosis of IC. At our health center, diagnosis of IC, after excluding other etiologies, was based on typical endoscopic findings of IC associated with a definitive or compatible histology. Endoscopic findings are known to have a high degree of accuracy for diagnosis of IC[28]. We used widely accepted histological criteria for diagnosis of IC[1,29,30], with definitive histology being defined as detection of mucosal infarction, clots or fibrin in the capillaries or submucosal hemorrhage. In addition, compatible histology was defined as detection of a loss of mucin and superficial epithelial cells, mild or moderate inflammatory infiltrate, edema or vascular congestion. Currently, there are no diagnostic criteria that enable NSAIDs-related colitis to be specifically diagnosed. The doubt remains, therefore, as to whether two different entities are involved or whether NSAIDs in general, and ASA in particular, have a role in the pathogeny of IC. In a previously published study, we reported a statistically significant relationship between recurrent IC and continued treatment with ASA[17].
In conclusion, we detected a statistically independent relationship between some cardiovascular risk factors and cardiovascular diseases, and development of IC. This relationship must, however, be confirmed in cohort studies. Similarly, the role of ASA in the development of IC must be evaluated.
Ischemic colitis (IC) is the most frequent form of intestinal ischemia (70%) and arises in cases where the colon is transitorily deprived of vascular flow. Development of IC has been associated with a series of diseases and risk factors. In general, any condition or factor that reduces the blood flow to the colon could generate IC. In recent years, different population-based studies have been published which have sought to detect risk factors associated with the development of IC, especially irritable bowel syndrome.
Although IC has been related to may conditions, the relationship with cardiovascular diseases and associated treatments is based on low evidence studies.
In this case-control study based on clinical data recordings we have performed a logistic regression analysis. We found an independent association between cardiovascular diseases (presence of heart failure, peripheral arterial disease) and cardiovascular risk factors (diabetes, dyslipidemia) and IC. Furthermore, we have detected that acetylsalicylic acid is independently associated with the development of IC. As long as there are no definite criteria that differentiate nonsteroidal anti-inflammatory drugs-related colitis from IC, the doubt remains as to whether two different entities are involved or whether acetylsalicylic acid has a role in the pathogeny of IC.
IC should be considered as a cardiovascular disease. This relationship must, however, be confirmed in cohort studies. Finally, the role of acetylsalicylic acid in the pathogeny of IC should be prospectively evaluated.
This paper is well-organized and well-investigated about the risk factors associated with the development of IC.
Peer reviewers: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan; Rene Lambert, Professor, International Agency for Research on Cancer, 150 Cours Albert Thomas, Lyon 69372 cedex 8, France
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum. 1996;39:88-100. |
| 2. | Järvinen O, Laurikka J, Salenius JP, Lepäntalo M. Mesenteric infarction after aortoiliac surgery on the basis of 1752 operations from the National Vascular Registry. World J Surg. 1999;23:243-247. |
| 3. | Guttormson NL, Bubrick MP. Mortality from ischemic colitis. Dis Colon Rectum. 1989;32:469-472. |
| 4. | Björck M, Bergqvist D, Troëng T. Incidence and clinical presentation of bowel ischaemia after aortoiliac surgery--2930 operations from a population-based registry in Sweden. Eur J Vasc Endovasc Surg. 1996;12:139-144. |
| 5. | Collet T, Even C, Bouin M, Lecluse E, Piquet MA, Crampon D, Grollier G, Dao T, Verwaerde JC. Prevalence of electrocardiographic and echocardiographic abnormalities in ambulatory ischemic colitis. Dig Dis Sci. 2000;45:23-25. |
| 6. | Hourmand-Ollivier I, Bouin M, Saloux E, Morello R, Rousselot P, Piquet MA, Dao T, Verwaerde JC. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol. 2003;98:1573-1577. |
| 7. | Yee NS, Guerry D 4th, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med. 2000;132:595-596. |
| 8. | Green BT, Branch MS. Ischemic colitis in a young adult during sickle cell crisis: case report and review. Gastrointest Endosc. 2003;57:605-607. |
| 9. | Kistin MG, Kaplan MM, Harrington JT. Diffuse ischemic colitis associated with systemic lupus erythematosus--response to subtotal colectomy. Gastroenterology. 1978;75:1147-1151. |
| 10. | Richardson SC, Willis J, Wong RC. Ischemic colitis, systemic lupus erythematosus, and the lupus anticoagulant: case report and review. Gastrointest Endosc. 2003;57:257-260. |
| 11. | Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther. 2004;19:729-738. |
| 12. | Garcia B, Ramaholimihaso F, Diebold MD, Cadiot G, Thiéfin G. Ischaemic colitis in a patient taking meloxicam. Lancet. 2001;357:690. |
| 13. | Walker AM, Bohn RL, Cali C, Cook SF, Ajene AN, Sands BE. Risk factors for colon ischemia. Am J Gastroenterol. 2004;99:1333-1337. |
| 14. | Suh DC, Kahler KH, Choi IS, Shin H, Kralstein J, Shetzline M. Patients with irritable bowel syndrome or constipation have an increased risk for ischaemic colitis. Aliment Pharmacol Ther. 2007;25:681-692. |
| 15. | Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol. 2006;101:1069-1079. |
| 16. | Montoro MA, Santolaria SB, Sánchez-Puértolas B, Vera F, GTECIE Group. Clinical characteristics and outcome of ischemic colitis in Spain. A multicentre and prospective study (ICS study). Gut. 2006;55:A208. |
| 17. | Fernandez Seara J, Vega Garcia-Luengos M, Gonzalez Vazquez E, Nunez Calvo L, Cubiella Fernandez J. Characteristics and Predictive Factors of Severity, in Hospital Mortality and Relapse. Open Gastroenterol J. 2009;3:1-7. |
| 18. | Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Fatal colonic ischemia: A population-based study. Scand J Gastroenterol. 2006;41:1312-1319. |
| 19. | Medina C, Vilaseca J, Videla S, Fabra R, Armengol-Miro JR, Malagelada JR. Outcome of patients with ischemic colitis: review of fifty-three cases. Dis Colon Rectum. 2004;47:180-184. |
| 20. | Añón R, Boscá MM, Sanchiz V, Tosca J, Almela P, Amorós C, Benages A. Factors predicting poor prognosis in ischemic colitis. World J Gastroenterol. 2006;12:4875-4878. |
| 21. | Available from: http://www.ine.es. |
| 22. | d'Halluin PN, Turlin B, Polard E, Dinasquet M, Pagenault M, Rioux N, Gosselin M, Bretagne JF, Heresbach D. [Selective COX-2 inhibitor-associated colitis: two case reports]. Gastroenterol Clin Biol. 2003;27:932-935. |
| 23. | Gleeson MH, Davis AJ. Non-steroidal anti-inflammatory drugs, aspirin and newly diagnosed colitis: a case-control study. Aliment Pharmacol Ther. 2003;17:817-825. |
| 24. | Etienney I, Beaugerie L, Viboud C, Flahault A. Non-steroidal anti-inflammatory drugs as a risk factor for acute diarrhoea: a case crossover study. Gut. 2003;52:260-263. |
| 25. | Collin R, Hochain P, Czernichow P, Petit A, Manchon ND, Berkelmans I. Non-steroidal anti-inflammatory drugs and segmental non-gangrenous colitis: a case-control study. Eur J Gastroenterol Hepatol. 1993;5:715-719. |
| 26. | Ajuebor MN, Singh A, Wallace JL. Cyclooxygenase-2-derived prostaglandin D(2) is an early anti-inflammatory signal in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G238-G244. |
| 27. | Wallace JL. COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. ScientificWorldJournal. 2006;6:577-588. |
| 28. | Assadian A, Senekowitsch C, Assadian O, Hartleb H, Hagmüller GW. Diagnostic accuracy of sigmoidoscopy compared with histology for ischemic colitis after aortic aneurysm repair. Vascular. 2008;16:243-247. |
| 29. | Bower TC. Ischemic colitis. Surg Clin North Am. 1993;73:1037-1053. |
| 30. | Price AB. Ischaemic colitis. Curr Top Pathol. 1990;81:229-246. |