Published online Sep 28, 2010. doi: 10.3748/wjg.v16.i36.4526
Revised: June 2, 2010
Accepted: June 9, 2010
Published online: September 28, 2010
The porphyries are a group of metabolic disorders characterized by deficiencies in the activity of enzymes involved in the biosynthesis of heme. In erythropoietic protoporphyria (EPP), in the majority of cases an autosomal dominant disease, there is a mutation of the gene that encodes ferrochelatase (FECH). FECH deficiency is associated with increased concentrations of protoporphyrin in erythrocytes, plasma, skin and liver. The prevalence of this inherited disorder oscillates between 1:75 000 and 1:200 000. Clinical manifestations of EPP appear in early infancy upon first exposure to the sun. Nevertheless, approximately 5%-20% of patients with EPP develop liver manifestations. Retention of protoporphyrin in the liver is associated with cholestatic phenomena and oxidative stress that predisposes to hepatobiliary disease of varying degrees of severity, such as cholelithiasis, mild parenchymal liver disease, progressive hepatocellular disease with end-stage liver disease and acute liver failure. Liver damage is the major risk in EPP patients, so surveillance and frequent clinical and biochemical liver follow-up is mandatory. The diagnostic approach consists in detecting increased levels of protoporphyrin, decreased activity of FECH and genetic analysis of the FECH gene. A variety of non-surgical therapeutic approaches have been adopted for the management of EPP associated with liver disease, but none of these has been shown to be unequivocally efficacious. Nevertheless, some may have a place in preparing patients for liver transplantation. Liver transplantation does not correct the constitutional deficiency of FECH. Consequently, there is a risk of recurrence of liver disease after liver transplantation as a result of continuing overproduction of protoporphyrin. Some authors recommend that bone marrow transplantation should be considered in liver allograft recipients to prevent recurrence of hepatic disease.
- Citation: Casanova-González MJ, Trapero-Marugán M, Jones EA, Moreno-Otero R. Liver disease and erythropoietic protoporphyria: A concise review. World J Gastroenterol 2010; 16(36): 4526-4531
- URL: https://www.wjgnet.com/1007-9327/full/v16/i36/4526.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i36.4526
The porphyrias are a group of metabolic disorders characterized by deficiencies in the activity of enzymes involved in the biosynthesis of heme, first described by Magnus et al[1,2]. Most are a result of inborn errors of metabolism, but the metabolic defect in some patient may be acquired[3]. Clinical manifestations of porphyrias can be divided in cutaneous or visceral.
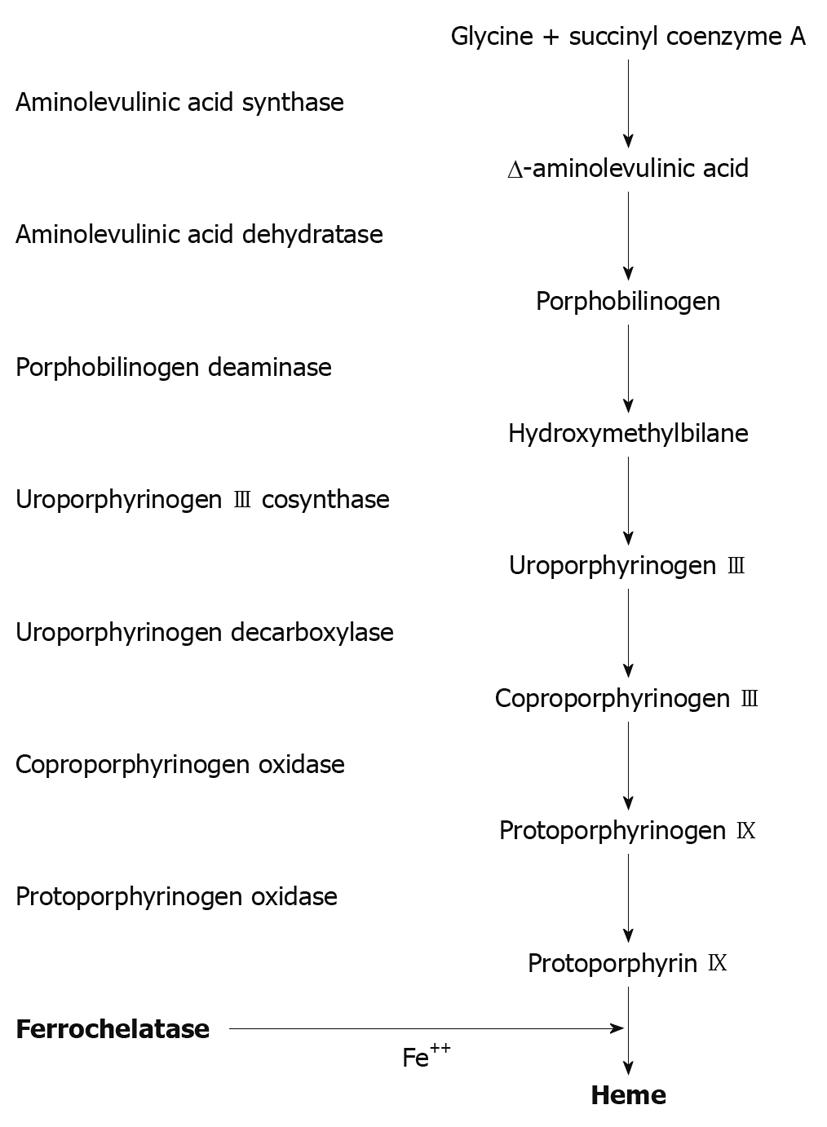
In erythropoietic protoporphyria (EPP) there is a mutation of the gene that encodes ferrochelatase (FECH) in the long arm of chromosome 18. This enzyme catalyzes the insertion of ferrous iron into the protoporphyrin IX ring to form heme (Figure 1)[4,5]. FECH expression has a heme-dependent negative feedback regulation at a post-transcriptional level in such a way that FECH is decreased by increasing the level of intracellular heme[6]. EPP exhibits both recessive and dominant patterns of inheritance and a high degree of allelic heterogeneity with incomplete penetrance. Most heterozygotes are asymptomatic. Symptoms do not occur unless FECH activity is less than 30% of normal, but such low levels are not present in a majority of patients[7]. Recently a new pattern of EPP has been described related to gain-of-function mutations in the aminolevulinic acid synthase 2 gene[8].
Cells which synthesize heme are predominantly erythroblasts/reticulocytes in the bone marrow (80%) and hepatocytes (20%). Deficiency of FECH results in increased release of protoporphyrin, which binds to albumin in plasma and subsequently undergoes hepatic extraction. Normally, most protoporphyrin in hepatocytes is secreted into bile; the remainder undergoes transformation into heme. Some protoporphyrin in bile is returned to the liver as a consequence of the enterohepatic circulation; the remaining protoporphyrin in the intestine undergoes fecal excretion. Protoporphyrin is insoluble and hence unavailable for renal excretion. In EPP, subnormal biotransformation of protoporphyrin into heme results in accumulation of protoporphyrin in hepatocytes[9].
FECH deficiency is associated with increased concentrations of protoporphyrin in erythrocytes, plasma, skin and liver. Retention of protoporphyrin in skin predisposes to acute photosensitivity. As a result of absorption of ultraviolet light (400 nm) by protoporphyrin in plasma and erythrocytes when blood circulates through the dermal vessels, free radicals are formed, erythrocytes become unstable and injury to the skin is induced[10]. A significant increase in the hepatobiliary excretion on protoporphyrin can damage the liver through both cholestatic phenomena and oxidative stress[9] predisposing to hepatobiliary disease of varying degrees of severity[11-13].
EPP is generally suspected by the presence of acute photosensitivity of the skin and can be confirmed by detection of a plasmatic fluorescence peak at 634 nm. It is also useful finding increased levels of protoporphyrin in feces and the demonstration of an excess of free protoporphyrin in erythrocytes[12]. Screening for FECH mutation on one allele or aminolevulinic acid synthase 2 gain-of-function mutation in selected family members may be useful, especially in genetic counseling. If one parent is affected with EPP with a FECH allele mutation, the risk of the offspring developing EPP is less than 2.5%, therefore, screening for the presence of the FECH IVS3-48C allele in the other parent may be helpful to estimate the probability for the offspring[12,14,15].
Liver biopsy confirms hepatic disease in EPP by the presence of protoporphyrin deposits in the hepatocytes that can be observed as a brown pigment within the biliary canaliculi and the portal macrophages. Macroscopically, the cirrhotic liver can have a black color due to protoporphyrin deposits. Using polarized light the characteristic Maltese cross shape of birefringent crystalline pigment deposits is found. The examination of liver tissue under a Wood’s lamp reveals a red fluorescence due to protoporphyrin. Liver biopsy is not helpful for estimation of prognosis of liver disease[11,13].
EPP usually presents in childhood, but there are a few reports of cases presenting in adults[16,17]. The commonest mode of presentation is acute photosensitivity of the skin. It affects areas exposed to the sun and tends to be intractable. A few minutes of exposure to the sun induces pruritus, erythema, swelling and pain. Longer periods of exposure may induce second degree burns. After repetitive exposure, patients may present with lichenification, hypopigmentation, hyperpigmentation and scarring of the skin[10,16,18].
Clinical findings suggestive of liver disease appear in approximately 5%-20% of patients[11-13,19]. The susceptibility of individual patients with EPP to protoporphyrin-induced liver damage is highly variable. The host factors responsible for this variable susceptibility are unknown. The spectrum of hepatobiliary disease associated with EPP is wide. It includes cholelithiasis, mild parenchymal liver disease, progressive hepatocellular disease and end-stage liver disease[12].
Liver damage in EPP has been attributed to precipitation of insoluble protoporphyrin in bile canaliculi and to protoporphyrin-induced oxidative stress. The latter arises as a consequence of excess unmetabolized protoporphyrin interacting with the hepatocellular membrane and inducing impaired function of the Na+/K+-ATPase pump within the membrane. The accumulation of excess protoporphyrin, that does not undergo biliary excretion, exacerbates cholestasis and further reduces the excretion of protoporphyrin[20]. These pathophysiological phenomena may result in hepatic inflammation, progressive hepatocellular disease, hepatic fibrosis, and, eventually, cirrhosis[11-13].
Cholelithiasis: Insoluble protoporphyrin in bile may act as nuclei for stone formation. Cholelithiasis is frequent in EPP (10%-20%), due to the accumulation of free protoporphyrin and increased biliary protoporphyrin concentration. Clinical manifestations of cholelithiasis and choledocolithiasis are similar to lithiasis by cholesterol or bilirubin[5]. Many patients with EPP and gallstones undergo cholecystectomy. Subsequent analysis of the stones reveals that they are birefringent and contain high concentrations of protoporphyrin. Some authors believe that EPP should be suspected when cholelithiasis presents in childhood[21].
Mild parenchymal liver disease: Most patients (20%) present with a mild liver disease, characterized by increased levels of aminotransferases and/or cholestatic enzymes. Typically, in patients with mild disease, there are no symptoms. Patients can also present with splenomegaly and hepatomegaly. Liver biopsy in such patients may reveal features of appreciable hepatocellular injury[5,13,22-24].
Progressive hepatocellular disease: Symptoms include upper abdominal pain and jaundice. There may be associated rapid deterioration of photosensitivity because of decreased secretion of protoporphyrin into bile, secondary to cholestasis and hemolysis[12].
It is rare for the initial presentation of EPP to be manifestations of progressive hepatocellular disease[25]. When jaundice is clinically evident, hepatocellular disease is advanced and hepatic clearance function is appreciably reduced. Blood protoporphyrin levels increase further, but fecal protoporphyrin excretion decreases[14].
End-stage liver disease: Only 5% of liver damage presents as an acute liver insufficiency. Progressive hepatocellular disease ultimately leads to cholestatic hepatocellular failure, which often has an acute onset, and a rapidly progressive, irreversible course. Progressive hepatocellular disease in EPP is usually fatal within months if liver transplantation is not undertaken (see below)[25-42].
There is no consensus regarding optimal surveillance for patients with EPP. Liver biopsy is the gold standard to assess the degree of hepatic damage. Results of non-invasive methods, such as serum biochemical liver tests, do not correlate closely with the degree of hepatic injury. In a review, Anstey and Hift[11] proposed the following indications for liver biopsy in patients with EPP: (1) Presence of null mutations or autosomal recessive disease; (2) Family history of EPP-related liver disease; (3) Presence of risk factors for the development of liver disease, such as markers of viral hepatitis, factors suggestive of non-alcoholic fatty liver disease, and alcohol abuse; (4) Abnormal results of serum biochemical liver tests; (5) Evidence of hepatocellular decompensation; and (6) To relieve a patient’s anxiety or to comply with a patient’s preference.
The optimal frequency of blood tests to monitor patients with EPP has not been established. Some authors advocate serum biochemical liver tests every 6 mo; others prefer to have these tests done annually up to the age of 20 years and then biennially[43,44].
A variety of non-surgical therapeutic approaches have been adopted for the management of EPP associated with progressive hepatocellular disease. However, none of these has been shown to be unequivocally efficacious. Nevertheless, some may have a place in preparing patients for liver transplantation[45]. The pathophysiology of this disease suggests several potential therapeutic targets (Table 1). Such targets include attempts to induce bile flow, to render bile less toxic, to reduce protoporphyrin production in the bone marrow, to reduce the circulating pool of protoporphyrin, to promote hepatocellular metabolism and transport of protoporphyrin, to protect hepatocytes from toxic damage, and to interrupt the enterohepatic circulation[11-13,46].
| Pathogenic mechanism | Treatment |
| Induce bile flow | Ursodeoxycholic acid |
| Reduce protoporphyrin production | Parenteral iron |
| Transfusion of erythrocytes | |
| Infusions of hematin | |
| Reduce protoporphyrin levels | Plasmapheresis |
| Extracorporeal albumin dialysis | |
| Interrupt enterohepatic circulation | Cholestyramine |
| Activated charcoal | |
| Protect hepatocytes from toxic damage | N-acetyl cysteine |
| Remove the principal source of protoporphyrin | Bone marrow transplant |
| Erythropoietic protoporphyria-related liver failure | Liver transplant |
Ursodeoxycholic acid: This bile acid is administered to promote biliary secretion of protoporphyrin. Results of its use in EPP are controversial. However, it is known to alter the composition of bile, to protect hepatocytes from the cytotoxic effect of hydrophobic bile acids, and to stimulate biliary secretion by several distinct mechanisms[46,47].
Parenteral iron and transfusion of erythrocytes: The objective of administering iron and/or erythrocytes is to suppress erythropoiesis and, hence, reduce the protoporphyrin level. In theory, iron therapy should not work since it stimulates heme synthesis via 5-aminolevulinate synthase. However, results of this approach in patients with EPP are contradictory. It has been reported that iron therapy may exacerbate hepatic dysfunction[48,49] whereas in some case reports the correction of iron deficiency has improved EPP[50,51]. Nevertheless, the mechanism of this favorable response to iron therapy remains unknown, so more studies with a significant number of patients are necessary to clarify the role of iron therapy in the medical treatment of EPP. Because of the lack of definitive clinical data, the contradictory reports and the theoretical possibility of exacerbating hepatic dysfunction, the decision to use this therapy should be individualized.
Infusions of hematin: Hematin appears to reduce excess protoporphyrin production in the bone marrow. It has been administered to patients with EPP (3-4 mg/kg iv) who develop a crisis after liver transplantation (see below)[52].
Plasmapheresis: Circulating levels of protoporphyrin can be decreased by plasma exchange[53].
Extracorporeal albumin dialysis: This type of dialysis is used to decrease circulating levels of albumin-bound toxins. Accordingly, in patients with EPP, damage to the hepatobiliary system may be reduced by using this approach to reduce plasma concentrations of protoporphyrin. It may also reduce levels of protoporphyrin in erythrocytes, when protoporphyrin subsequently diffuses out of these cells into the plasma. A greater reduction in erythrocyte protoporphyrin levels following treatment with a molecular adsorbent recirculating system (9.1%) than following plasmapheresis (0.8%) or treatment with the Prometheus system (5.9%) has been documented in a case study of a patient with EPP and liver disease[25,54].
Cholestyramine: This orally administered resin reduces circulating levels of protoporphyrin by binding to protoporphyrin in the intestine and, hence, interrupting the enterohepatic circulation. It is usually used in combination with other treatment approaches[55].
Activated charcoal: Like cholestyramine, activated charcoal also binds to protoporphyrin in the intestine and prevents its absorption. It is cheap and readily available. It seems to be effective in reducing circulating protoporphyrin levels[56,57].
N-acetyl cysteine: In liver diseases free radicals are increased, thereby damaging the hepatic tissue[58]. In addition, nitric oxide has deleterious effects in the presence of reactive oxygen species, participating in the pathophysiology of different liver diseases[59,60].
N-acetyl cysteine (NAC) modulates the expression of inducible oxide synthase in hepatocytes[61], and this action could be effective in the attenuation of oxidative and nitrosative stress in liver injury. Based on these findings, it is suggested that antioxidant therapy might be beneficial in the treatment of liver damage of different etiologies[62]. There is little experience with NAC in EPP, only clinical reports such a 32-year-old man with EPP who developed progressive hepatocellular disease and was treated with NAC 300 mg/kg body weight per day IV infusion for 3 wk. This treatment was associated with an improvement in hepatocellular function, in particular, serum levels of hepatic enzymes normalized[63].
Bone marrow transplantation: The purpose of this approach is to remove the tissue primarily responsible for the overproduction of protoporphyrin. It is a frequently discussed option, but the incidence of associated adverse events has limited its use as a treatment for EPP. However, some authors advocate bone marrow transplantation as a complementary treatment in an attempt to avoid liver re-transplantation in patients who have undergone liver transplantation for EPP-associated liver disease (see below)[26].
Liver transplantation: More than 40 patients with EPP and hepatocellular disease, who have undergone liver transplantation, have been reported in the world literature[12]. Liver transplantation does not correct the constitutional deficiency of FECH. Consequently, there is a risk of recurrence of liver disease after liver transplantation as a result of the continuing overproduction of protoporphyrin. A review of 20 transplanted cases of EPP in the USA led the authors to recommend that bone marrow transplantation should be considered in liver allograft recipients to prevent recurrence of hepatic disease[45]. Patients with EPP are prone to certain perioperative complications of liver transplantation. Management involves adopting appropriate precautions or treatment options. Phototoxic abdominal burns may be induced; the mechanism is analogous to that responsible for skin photosensitivity. Such burns can be avoided by fitting filters to lamps in the operating theater[64]. Acute neuropathy is a major complication. It is associated with severe abdominal pain, an acute deterioration in hepatocellular function, and an increase in erythrocyte protoporphyrin levels. Treatment options include hematin infusions and/or plasmapheresis[25]. Acute protoporphyrin-mediated damage to the liver allograft may occur secondary to high circulating levels of protoporphyrin at the time of transplantation. This complication can be prevented by taking short-term measures to reduce levels of protoporphyrin at the time of surgery[54,65]: (1) Cholecystectomy; (2) Vaccines against viral hepatitis; and (3)Avoid hepatotoxic drugs. Avoid drugs that might develop a drug induced liver injury.
In EPP there is a mutation of the gene that encodes FECH, the enzyme that catalyzes the insertion of ferrous iron into the protoporphyrin IX ring to form heme. EPP presents both recessive and dominant patterns of inheritance. Recently, a new pattern of EPP has been described related to gain-of-function mutations in the aminolevulinic acid synthase 2 gene. FECH deficiency is associated with increased concentrations of protoporphyrin in erythrocytes, plasma, skin and liver. A significant increase in the hepatobiliary excretion of protoporphyrin can damage the liver through both cholestatic phenomena and oxidative stress predisposing to a wide spectrum of hepatobiliary disease of varying degrees of severity that includes cholelithiasis, mild parenchymal liver disease, progressive hepatocellular disease and end-stage liver disease. The susceptibility of individual patients with EPP to protoporphyrin-induced liver damage is highly variable. There is no consensus regarding optimal surveillance for patients with EPP. Liver biopsy is the gold standard to assess the degree of hepatic damage. A variety of non-surgical therapeutic approaches have been adopted for the management of EPP associated with progressive liver disease, but none of these has been shown to be unequivocally efficacious. Liver transplantation does not correct the constitutional deficiency of FECH and, as a result of the continuing overproduction of protoporphyrin, there is a risk of recurrence of liver disease after liver transplantation.
Peer reviewers: Matilde Bustos, MD, PhD, Hepatology and Gene Therapy Area, Center for Applied Medical Research, Avda Pio XII, 55, 31008 Pamplona, Spain; Sebastian Mueller, MD, PhD, Professor of Medicine, Department of Internal Medicine, Salem Medical Center, and Center for Alcohol Research, University of Heidelberg, Zeppelinstraße 11 - 33, Heidelberg, 69121, Germany
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
| 1. | Magnus IA, Jarrett A, Prankerd TA, Rimington C. Erythropoietic protoporphyria. A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet. 1961;2:448-451. |
| 2. | Moore MR. Biochemistry of porphyria. Int J Biochem. 1993;25:1353-1368. |
| 3. | Ventura P, Cappellini MD, Rocchi E. The acute porphyrias: a diagnostic and therapeutic challenge in internal and emergency medicine. Intern Emerg Med. 2009;4:297-308. |
| 4. | Meerman L. Erythropoietic protoporphyria. An overview with emphasis on the liver. Scand J Gastroenterol Suppl. 2000;79-85. |
| 5. | Richard E, Robert-Richard E, Ged C, Moreau-Gaudry F, de Verneuil H. Erythropoietic porphyrias: animal models and update in gene-based therapies. Curr Gene Ther. 2008;8:176-186. |
| 6. | Sakaino M, Kataoka T, Taketani S. Post-transcriptional regulation of the expression of ferrochelatase by its variant mRNA. J Biochem. 2009;145:733-738. |
| 7. | Kong XF, Ye J, Gao DY, Gong QM, Zhang DH, Lu ZM, Lu YM, Zhang XX. Identification of a ferrochelatase mutation in a Chinese family with erythropoietic protoporphyria. J Hepatol. 2008;48:375-379. |
| 8. | Whatley SD, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton MN, Elder GH, Holme SA, Anstey AV, Parker M. C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am J Hum Genet. 2008;83:408-414. |
| 9. | Holme SA, Worwood M, Anstey AV, Elder GH, Badminton MN. Erythropoiesis and iron metabolism in dominant erythropoietic protoporphyria. Blood. 2007;110:4108-4110. |
| 10. | Tsuboi H, Yonemoto K, Katsuoka K. Erythropoietic protoporphyria with eye complications. J Dermatol. 2007;34:790-794. |
| 11. | Lecha M, Puy H, Deybach JC. Erythropoietic protoporphyria. Orphanet J Rare Dis. 2009;4:19. |
| 12. | Anstey AV, Hift RJ. Liver disease in erythropoietic protoporphyria: insights and implications for management. Gut. 2007;56:1009-1018. |
| 13. | Bruguera M, Herrero C. [Liver disease in erythropoietic protoporphyria]. Gastroenterol Hepatol. 2005;28:632-636. |
| 14. | Sassa S. Modern diagnosis and management of the porphyrias. Br J Haematol. 2006;135:281-292. |
| 15. | Thunell S, Harper P, Brun A. Porphyrins, porphyrin metabolism and porphyrias. IV. Pathophysiology of erythyropoietic protoporphyria--diagnosis, care and monitoring of the patient. Scand J Clin Lab Invest. 2000;60:581-604. |
| 16. | Berroeta L, Man I, Goudie DR, Whatley SD, Elder GH, Ibbotson SH. Late presentation of erythropoietic protoporphyria: case report and genetic analysis of family members. Br J Dermatol. 2007;157:1030-1031. |
| 17. | Henderson CA, Jones S, Elder G, Ilchyshyn A. Erythropoietic protoporphyria presenting in an adult. J R Soc Med. 1995;88:476P-477P. |
| 18. | Poh-Fitzpatrick MB. Molecular and cellular mechanisms of porphyrin photosensitization. Photodermatol. 1986;3:148-157. |
| 19. | Holme SA, Anstey AV, Finlay AY, Elder GH, Badminton MN. Erythropoietic protoporphyria in the U.K.: clinical features and effect on quality of life. Br J Dermatol. 2006;155:574-581. |
| 20. | Knobler E, Poh-Fitzpatrick MB, Kravetz D, Vincent WR, Muller-Eberhard U, Vincent SH. Interaction of hemopexin, albumin and liver fatty acid-binding protein with protoporphyrin. Hepatology. 1989;10:995-997. |
| 21. | Todd DJ. Gallstones in children. Am J Dis Child. 1991;145:971-972. |
| 22. | Bonkovsky HL, Schned AR. Fatal liver failure in protoporphyria. Synergism between ethanol excess and the genetic defect. Gastroenterology. 1986;90:191-201. |
| 23. | Frank M, Doss MO. Severe liver disease in protoporphyria. Curr Probl Dermatol. 1991;20:160-167. |
| 24. | Anstey AV, Hift RJ. Liver disease in erythropoietic protoporphyria: insights and implications for management. Postgrad Med J. 2007;83:739-748. |
| 25. | McGuire BM, Bloomer JR. Use of extracorporeal albumin dialysis for erythropoietic protoporphyria. Liver Transpl. 2007;13:639-640. |
| 26. | Zhang F, Lu L, Qian X, Pu LY, Li GQ, Wang XH. Liver transplantation for erythropoietic protoporphyria with hepatic failure: a case report. Transplant Proc. 2008;40:1774-1776. |
| 27. | Samuel D, Boboc B, Bernuau J, Bismuth H, Benhamou JP. Liver transplantation for protoporphyria. Evidence for the predominant role of the erythropoietic tissue in protoporphyrin overproduction. Gastroenterology. 1988;95:816-819. |
| 28. | Polson RJ, Lim CK, Rolles K, Calne RY, Williams R. The effect of liver transplantation in a 13-year-old boy with erythropoietic protoporphyria. Transplantation. 1988;46:386-389. |
| 29. | Bloomer JR, Weimer MK, Bossenmaier IC, Snover DC, Payne WD, Ascher NL. Liver transplantation in a patient with protoporphyria. Gastroenterology. 1989;97:188-194. |
| 30. | Wagner S, Doss MO, Wittekind C, Bäcker U, Meessen D, Schmidt FW. [Erythrohepatic protoporphyria with rapidly progressing liver cirrhosis]. Dtsch Med Wochenschr. 1989;114:1837-1841. |
| 31. | Mion FB, Faure JL, Berger F, McGregor B, Perrot H, Paliard P. Liver transplantation for erythropoietic protoporphyria. Report of a new case with subsequent medium-term follow-up. J Hepatol. 1992;16:203-207. |
| 32. | Sarkany RP, Cox TM. Autosomal recessive erythropoietic protoporphyria: a syndrome of severe photosensitivity and liver failure. QJM. 1995;88:541-549. |
| 33. | Frank M, Doss MO. [Liver cirrhosis in protoporphyria: bile acid therapy and liver transplantation]. Z Gastroenterol. 1995;33:399-403. |
| 34. | Dellon ES, Szczepiorkowski ZM, Dzik WH, Graeme-Cook F, Ades A, Bloomer JR, Cosimi AB, Chung RT. Treatment of recurrent allograft dysfunction with intravenous hematin after liver transplantation for erythropoietic protoporphyria. Transplantation. 2002;73:911-915. |
| 35. | Do KD, Banner BF, Katz E, Szymanski IO, Bonkovsky HL. Benefits of chronic plasmapheresis and intravenous heme-albumin in erythropoietic protoporphyria after orthotopic liver transplantation. Transplantation. 2002;73:469-472. |
| 36. | Steinmüller T, Doss MO, Steffen R, Blumhardt G, Bechstein WO, Frank M, Sieg I, Kretschmar R, Neuhaus P. [Liver transplantation in erythrohepatic protoporphyria]. Dtsch Med Wochenschr. 1992;117:1097-1102. |
| 37. | Lock G, Holstege A, Mueller AR, Christe W, Doss MO, Schölmerich J, Neuhaus P. Liver failure in erythropoietic protoporphyria associated with choledocholithiasis and severe post-transplantation polyneuropathy. Liver. 1996;16:211-217. |
| 38. | de Torres I, Demetris AJ, Randhawa PS. Recurrent hepatic allograft injury in erythropoietic protoporphyria. Transplantation. 1996;61:1412-1413. |
| 39. | Reichheld JH, Katz E, Banner BF, Szymanski IO, Saltzman JR, Bonkovsky HL. The value of intravenous heme-albumin and plasmapheresis in reducing postoperative complications of orthotopic liver transplantation for erythropoietic protoporphyria. Transplantation. 1999;67:922-928. |
| 40. | Meerman L, Haagsma EB, Gouw AS, Slooff MJ, Jansen PL. Long-term follow-up after liver transplantation for erythropoietic protoporphyria. Eur J Gastroenterol Hepatol. 1999;11:431-438. |
| 41. | Nguyen L, Blust M, Bailin M, Melendez L, Raines DE. Photosensitivity and perioperative polyneuropathy complicating orthotopic liver transplantation in a patient with erythropoietic protoporphyria. Anesthesiology. 1999;91:1173-1175. |
| 42. | Jiménez-Sáenz M, Caunedo-Alvarez A, Rojas M, Mata M, Villar JL, Piñar A, Herrerías-Gutiérrez J. [Severe liver failure in erythropoietic protoporphyria. A report of a case treated by liver transplantation]. Med Clin (Barc). 1999;113:176-179. |
| 43. | Sarkany RP, Norris PG. Hepatic complications of erythropoietic protoporphyria. Br J Dermatol. 1994;130:258-259. |
| 44. | Mooyaart BR, de Jong GM, van der Veen S, Driessen LH, Beukeveld GJ, Grond J, Gips CH. Hepatic disease in erythropoietic protoporphyria. Dermatologica. 1986;173:120-130. |
| 45. | McGuire BM, Bonkovsky HL, Carithers RL Jr, Chung RT, Goldstein LI, Lake JR, Lok AS, Potter CJ, Rand E, Voigt MD. Liver transplantation for erythropoietic protoporphyria liver disease. Liver Transpl. 2005;11:1590-1596. |
| 46. | Pirlich M, Lochs H, Schmidt HH. Liver cirrhosis in erythropoietic protoporphyria: improvement of liver function with ursodeoxycholic acid. Am J Gastroenterol. 2001;96:3468-3469. |
| 47. | Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525-531. |
| 48. | Milligan A, Graham-Brown RA, Sarkany I, Baker H. Erythropoietic protoporphyria exacerbated by oral iron therapy. Br J Dermatol. 1988;119:63-66. |
| 49. | Dobozy A, Csató M, Siklósi C, Simon N. Transfusion therapy for erythropoietic protoporphyria. Br J Dermatol. 1983;109:571-576. |
| 50. | Gordeuk VR, Brittenham GM, Hawkins CW, Mukhtar H, Bickers DR. Iron therapy for hepatic dysfunction in erythropoietic protoporphyria. Ann Intern Med. 1986;105:27-31. |
| 51. | Holme SA, Thomas CL, Whatley SD, Bentley DP, Anstey AV, Badminton MN. Symptomatic response of erythropoietic protoporphyria to iron supplementation. J Am Acad Dermatol. 2007;56:1070-1072. |
| 52. | Potter C, Tolaymat N, Bobo R, Sharp H, Rank J, Bloomer J. Hematin therapy in children with protoporphyric liver disease. J Pediatr Gastroenterol Nutr. 1996;23:402-407. |
| 53. | Tung BY, Farrell FJ, McCashland TM, Gish RG, Bacon BR, Keeffe EB, Kowdley KV. Long-term follow-up after liver transplantation in patients with hepatic iron overload. Liver Transpl Surg. 1999;5:369-374. |
| 54. | Eefsen M, Rasmussen A, Wulf HC, Brock A, Hansen BA. Erythropoietic protoporphyria and pretransplantation treatment with nonbiological liver assist devices. Liver Transpl. 2007;13:655-657. |
| 55. | McCullough AJ, Barron D, Mullen KD, Petrelli M, Park MC, Mukhtar H, Bickers DR. Fecal protoporphyrin excretion in erythropoietic protoporphyria: effect of cholestyramine and bile acid feeding. Gastroenterology. 1988;94:177-181. |
| 56. | Gorchein A, Chong SK, Mowat AP. Oral activated charcoal in protoporphyria with liver damage. Br J Clin Pharmacol. 1989;27:703P. |
| 57. | Gorchein A, Foster GR. Liver failure in protoporphyria: long-term treatment with oral charcoal. Hepatology. 1999;29:995-996. |
| 58. | Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3:526-536. |
| 59. | García-Monzón C, Majano PL, Zubia I, Sanz P, Apolinario A, Moreno-Otero R. Intrahepatic accumulation of nitrotyrosine in chronic viral hepatitis is associated with histological severity of liver disease. J Hepatol. 2000;32:331-338. |
| 60. | Sanz-Cameno P, Medina J, García-Buey L, García-Sánchez A, Borque MJ, Martín-Vílchez S, Gamallo C, Jones EA, Moreno-Otero R. Enhanced intrahepatic inducible nitric oxide synthase expression and nitrotyrosine accumulation in primary biliary cirrhosis and autoimmune hepatitis. J Hepatol. 2002;37:723-729. |
| 61. | Majano PL, Medina J, Zubía I, Sunyer L, Lara-Pezzi E, Maldonado-Rodríguez A, López-Cabrera M, Moreno-Otero R. N-Acetyl-cysteine modulates inducible nitric oxide synthase gene expression in human hepatocytes. J Hepatol. 2004;40:632-637. |
| 62. | Medina J, Moreno-Otero R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs. 2005;65:2445-2461. |
| 63. | Sperl J, Procházková J, Martásek P, Subhanová I, Franková S, Trunecka P, Jirsa M. N-acetyl cysteine averted liver transplantation in a patient with liver failure caused by erythropoietic protoporphyria. Liver Transpl. 2009;15:352-354. |
| 64. | Wahlin S, Srikanthan N, Hamre B, Harper P, Brun A. Protection from phototoxic injury during surgery and endoscopy in erythropoietic protoporphyria. Liver Transpl. 2008;14:1340-1346. |
| 65. | Meerman L, Verwer R, Slooff MJ, van Hattum J, Beukeveld GJ, Kleibeuker JH, Haagsma EB. Perioperative measures during liver transplantation for erythropoietic protoporphyria. Transplantation. 1994;57:155-158. |