Published online Sep 28, 2010. doi: 10.3748/wjg.v16.i36.4519
Revised: March 3, 2010
Accepted: March 10, 2010
Published online: September 28, 2010
Recent advances in localization techniques, such as the selective arterial secretagogue injection test (SASI test) and somatostatin receptor scintigraphy have promoted curative resection surgery for patients with pancreatic neuroendocrine tumors (PNET). For patients with sporadic functioning PNET, curative resection surgery has been established by localization with the SASI test using secretin or calcium. For curative resection of functioning PNET associated with multiple endocrine neoplasia type 1 (MEN 1) which are usually multiple and sometimes numerous, resection surgery of the pancreas and/or the duodenum has to be performed based on localization by the SASI test. As resection surgery of PNET has increased, several important pathological features of PNET have been revealed. For example, in patients with Zollinger-Ellison syndrome (ZES), duodenal gastrinoma has been detected more frequently than pancreatic gastrinoma, and in patients with MEN 1 and ZES, gastrinomas have been located mostly in the duodenum, and pancreatic gastrinoma has been found to co-exist in 13% of patients. Nonfunctioning PNET in patients with MEN 1 becomes metastatic to the liver when it is more than 1 cm in diameter and should be resected after careful observation. The most important prognostic factor in patients with PNET is the development of hepatic metastases. The treatment strategy for hepatic metastases of PNET has not been established and aggressive resection with chemotherapy and trans-arterial chemoembolization have been performed with significant benefit. The usefulness of octreotide treatment and other molecular targeting agents are currently being assessed.
- Citation: Imamura M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World J Gastroenterol 2010; 16(36): 4519-4525
- URL: https://www.wjgnet.com/1007-9327/full/v16/i36/4519.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i36.4519
As pancreatic neuroendocrine tumors (PNET) are rarely encountered in hospitals, standardization of diagnosis and/or the treatment strategy have not progressed until recently. However, recent advances in localization techniques, such as the selective arterial secretagogue injection test (SASI test) and somatostatin receptor scintigraphy (SRS) have promoted curative resection surgery of PNET[1,2]. As the number of resections has rapidly increased, a few important characteristic pathological features of PNET have been revealed year by year. The World Health Organization pathological classification of PNET was evolutionally simplified in 2003 at the Lion Meeting, and the term carcinoid was declared a misnomer[3] (Table 1). Recently, a study group in the EU published a few guidelines on gastroenteropancreatic neuroendocrine tumors (GEPNET)[5,6]. In this work I will review important progress in the standardization of both surgical and medical treatment strategies for PNET.
| WHO classification | Well-differentiated neuroendocrine tumor | Well-differentiated neuroendocrine carcinoma | Poorly-differentiated neuroendocrine carcinoma |
| Biological behavior | Benign/uncertain behavior | Low malignancy | High malignancy |
| Metastases | - | + | + |
| Ki-67/MIB-1 index (%) | < 2 | 2-20 | > 20 |
| Pathological differentiation | Well-differentiated | Well-differentiated | Poorly-differentiated |
| Vascular invasion | -/+ | + | + |
| Size (diameter) | ≤ 2 cm | > 2 cm | Any size |
In Western countries, PNET is found in about 1 per 100 000 population and represents 1%-2% of all pancreatic neoplasms[5-7]. In the USA, it is suggested that the incidence and prevalence of PNET has substantially increased over the last 30 years probably due to the rapid progress of innovative diagnostic techniques[8]. On the other hand, there have been a few epidemiological studies on NET in Japan[9,10]. In 2006, the Japanese NET study group (NET Work Japan) performed a nationwide survey to examine the epidemiology of GEPNET in Japan, using a stratified random sampling method to select departments of medical facilities where GEPNET were treated in 2005[9,10]. The first survey revealed that the overall prevalence was 2.23 patients per 100 000 population [95% confidence interval (CI): 1.93-2.76] per year. The total number of patients treated for functioning PNET was estimated to be 1627 (95% CI: 1.10-1.57), and the overall prevalence of insulinoma and gastrinoma was 0.84 and 0.23 per 100 000 population per year, respectively. Furthermore, the results in the second survey showed that the incidence of PNET in 2005 was estimated to be 1.01 per 100 000 population per year (95% CI: 0.88-1.25). Accordingly, the incidence of functioning PNET and non-functioning PNET was 0.50 and 0.51 per 100 000 population per year, respectively[9,10]. As the incidence of PNET in the USA has been reported to be about 0.32 per year per 100 000 population by Yao et al[11] PNET seems to develop about three times more frequently in Japan compared to that in the USA.
Recurrent peptic ulcers in gastrinoma, necrolytic migratory erythema in glucagonoma, and watery diarrhea in VIPoma are characteristic symptoms due to an excessive increase of the responsible hormone in blood. These symptoms easily lead to the correct diagnosis when the measurement of blood hormone levels is promptly followed. However, the symptoms due to hypoglycemia do not easily lead to the diagnosis of insulinoma[12]. This may sound strange, but it is true. The diagnosis of insulinoma is the most difficult among the functioning PNET. Patients with insulinoma are often misdiagnosed for long periods. The patient eats much food and looks healthy but somewhat strange without any organic illness. We should be very careful in diagnosing insulinoma as there are a number of diseases that cause hypoglycemia, and a variety of special tests are required for insulinoma diagnosis, which will be described below.
Recently, the differential diagnosis of gastrinoma has also become difficult. This is due to both the easy and long-term use of proton pump inhibitors for recurrent peptic ulcers or regurgitation esophagitis without a precise assessment of both serum gastrin levels and gastric hyperacidity status[13,14].
The measurement of serum hormone levels is very useful for the differential diagnosis of PNET other than insulinoma. The normal range of serum gastrin levels in patients with gastrinoma is quite different in patients with and without a history of gastrectomy[1]. When a patient undergoes a distal gastrectomy, normal serum gastrin levels are usually lower than 90 pg/mL[1]. Jensen’s group in NIH performed an aggressive study on both the fasting serum gastrin levels and the gastrin provocative testing of both patients with gastrinoma and normal volunteers[14,15]. They revealed that various physiological conditions were correlated with basal serum gastrin levels, and have recommended that an increase of 120 pg/mL or more as the positive range for the intravenous secretin test[14,15].
C-peptide inhibition test with hog insulin: This test is not 100% reliable for the diagnosis of insulinoma[12], but it can be completed in only 2 h and can serve as a valuable screening tool. Although this test might not be popular currently, we have favored this test for a long time similar to the group at the Mayo Clinic[15].
Intravenous secretin test for insulinoma: When 2 U/kg・body weight of secretin is intravenously administered, plasma insulin level rises more than 200% within 4 min in normal volunteers, but does not rise more than 100% in patients with insulinoma[16,17]. We have developed this test and used it for patients in whom other tests were non-diagnostic in the differential diagnosis of insulinoma.
Intravenous secretin test for gastrinoma: A bolus injection of 2 U/kg・body weight of secretin into the peripheral vein increases the serum level of gastrin by more than 100 pg/mL in patients with gastrinoma, but does not increase the serum level of gastrin in those without gastrinoma. This well known test has been successfully used for the differential diagnosis of gastrinoma since 1972[18]. Although this test has been proved to be useful for years, we have to be careful as this test is also positive in patients with hypergastrinemia due to atrophic gastritis. It has been shown that antral G-cells also have secretin receptors and release gastrin when stimulated with pharmacological doses of secretin[19].
Imaging techniques such as computed tomography, ultrasonography (US), endoscopic US (EUS), or intraoperative US (IOUS) have been useful for the localization of most PNET greater than 2 cm in diameter[20-23]. However, imaging techniques have difficulty in visualizing PNET less than 5 mm, and cannot identify a functioning PNET among various types of PNET including nonfunctioning PNET[20-23]. As the functioning PNET shows characteristic symptoms even when less than 5 mm, the SASI test is useful for preoperative localization of functioning PNET leading to curative resection surgery[1,20-22].
SRS is indispensable for localization of ectopic NET and the distribution of NET throughout the body[24].
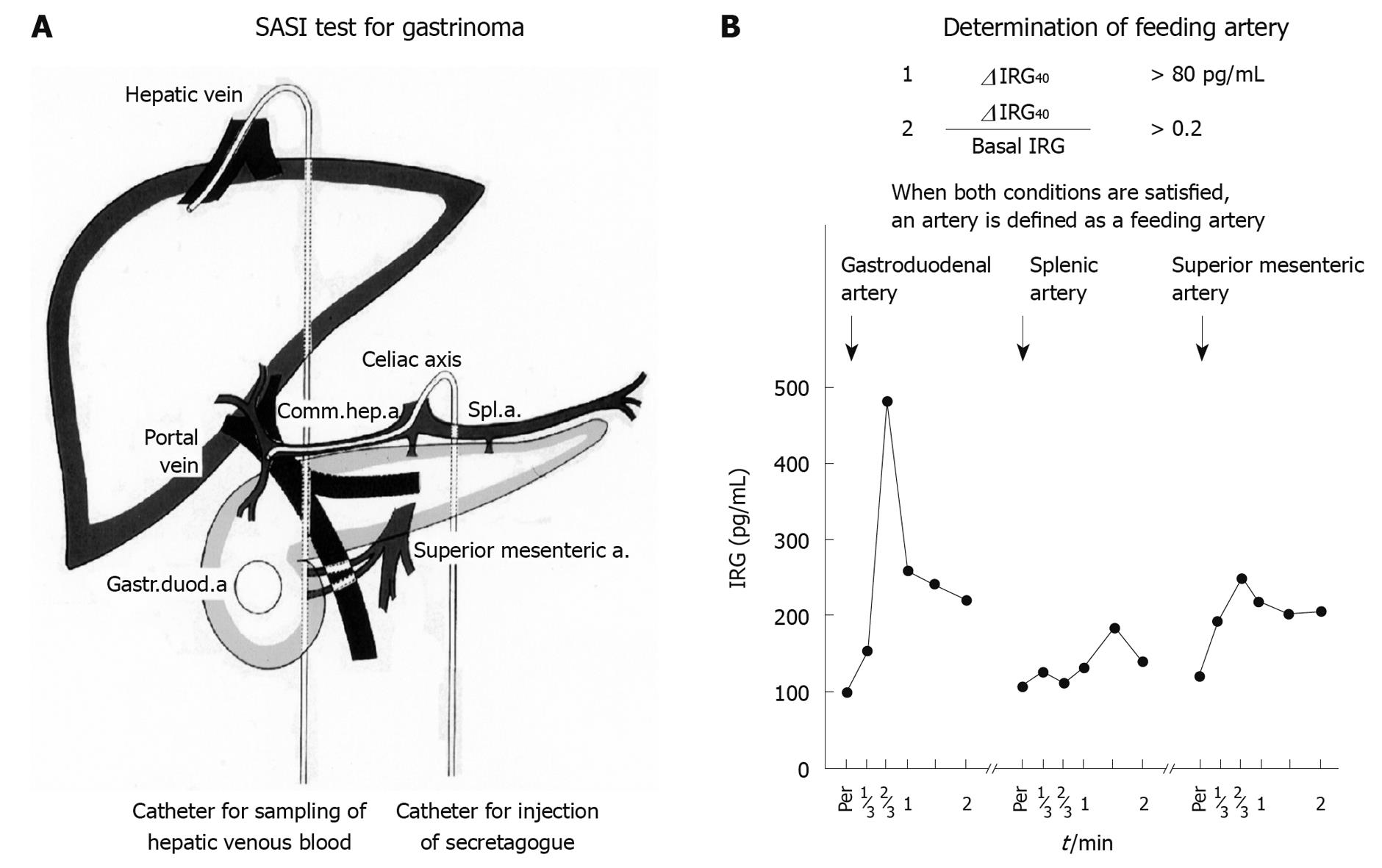
The SASI test was first described for localization of gastrinoma, and has gradually proved useful for the localization of other symptomatic PNET[1,20-22,25]. At the time of abdominal arteriography, secretagogue (30 U of secretin for gastrinoma and 1 mL of 8.5% calcium gluconate for insulinoma and glucagonoma) is injected into the splenic artery, the gastroduodenal artery and the superior mesenteric artery. Then, 2 mL blood samples are drawn from the hepatic vein through a catheter inserted via the femoral vein, before and 20, 40 and 60 s after the injection of secretagogue to detect the change in hormone levels in hepatic venous blood. When the rise in hormone levels 40 s after injection is significantly higher than measurement errors, the artery is diagnosed as a feeding artery of PNET. Functioning PNET is then located in the feeding area of the identified feeding artery. More precise localization is possible by injecting secretagogue into a branch of the identified artery. When the splenic artery is identified as a feeding artery of insulinoma, more precise localization is possible by injecting calcium solution into the distal, middle and proximal portion of the splenic artery[20]. Both the sensitivity and specificity of the SASI test for both gastrinoma and insulinoma has been shown to be more than 90%, respectively[20,21,25] (Figure 1).
SRS is clearly able to visualize PNET more than 2 cm in diameter in the body, at a glance, and has contributed to the staging of PNET[26-28]. SRS can visualize 100% of gastrinomas larger than 3 cm in diameter, but only 20% of gastrinomas less than 5 mm, and 30% of gastrinomas less than 1 cm[27]. Thus, SRS visualized 73% of gastrinomas, 100% of glucagonomas, 88% of VIPomas, 73% of non-symptomatic GEPET, and only 46% of insulinomas, depending on both the extent of the presence and the differences in subtypes of somatostatin receptors, and the size of the tumor[27,28]. For the localization of ectopic PNET, SRS is an indispensable test[24].
IOUS is useful in estimating the character of a tumor and to measure the distance between a PNET and the main pancreatic duct. In addition, the form and size of a PNET can be measured more correctly with IOUS than any other preoperative imaging technique[29]
Rapid immunoassay of insulin (IRI) and radioimmunoassay of gastrin (IRG) are useful for estimating the extent of the curability of surgery. Intraoperative measurement of both blood glucose levels and insulin using the same rate of drip infusion of glucose solution is helpful for estimating the curability of insulinoma resection[12]. The intraoperative secretin test with rapid radioimmunoassay of serum gastrin are useful for confirming the curability of gastrinoma resection surgery[30].
The best treatment for PNET is curative surgical resection[5-8,31,32]. This needs to be performed before liver metastasis develops. Most PNET grow without invading the adjacent pancreatic parenchyma, and can reach a size of 1 cm[1,31].
For a benign small PNET such as a benign sporadic insulinoma, enucleation is indicated wherever it is located in the pancreas, as long as it is 5 mm from the main pancreatic duct (MPD)[12,31]. Other sporadic functioning PNET such as gastrinomas, glucagonomas and VIPomas are thought to be potentially malignant and often multiple. Therefore, for these tumors pancreatic resection with lymph node dissection is indicated[31-33]. When the tumors are less than 5 mm in diameter, enucleation might also be indicated. R0 resection surgery for sporadic PNET has brought about complete relief of the characteristic difficult symptoms without recurrence[20,30-32].
In the case of multiple PNET, we must consider whether or not the patient has MEN 1. Serum calcium level and parathyroid hormone level require to be measured first, because the penetration rate of hyperparathyroidism is more than 90% in MEN 1. Genetic analysis is then performed. In patients with MEN 1, both PNET and duodenal NET are often multiple and microscopically numerous, and most are nonfunctioning[34-38].
There has been controversy regarding resection surgery for nonfunctioning PNET in MEN 1[31-33]. Recently, Goudet et al[36] revealed in a cohort study of 758 patients with MEN 1, that gastrinoma, nonfunctioning PNET and glucagonomas-vipomas-somatostatinomas had a high risk of death after adjustment for age, gender and diagnosis period. These PNET should be resected as early as possible before the development of hepatic metastases[31,35,36].
So far, extended distal pancreatectomy and enucleation of PNET more than 1 cm in diameter in the pancreatic head has been recommended for the prevention of liver metastases[35]. Total pancreatectomy is, so far, not indicated, because of a significant decrease in the quality of life of patients[37,38]. However, we know that some patients with PNET in MEN 1 rapidly develop liver metastases and die within a few years, therefore we will, in future, perform total pancreatectomy for selected patients based on advanced genetic analysis[38].
Pancreatic hypoglycemia in MEN 1 is often caused by multiple insulinomas which are located mostly in the body or tail of the pancreas[39]. Distal pancreatectomy is indicated for these types of insulinomas guided by the SASI test with calcium[12,39].
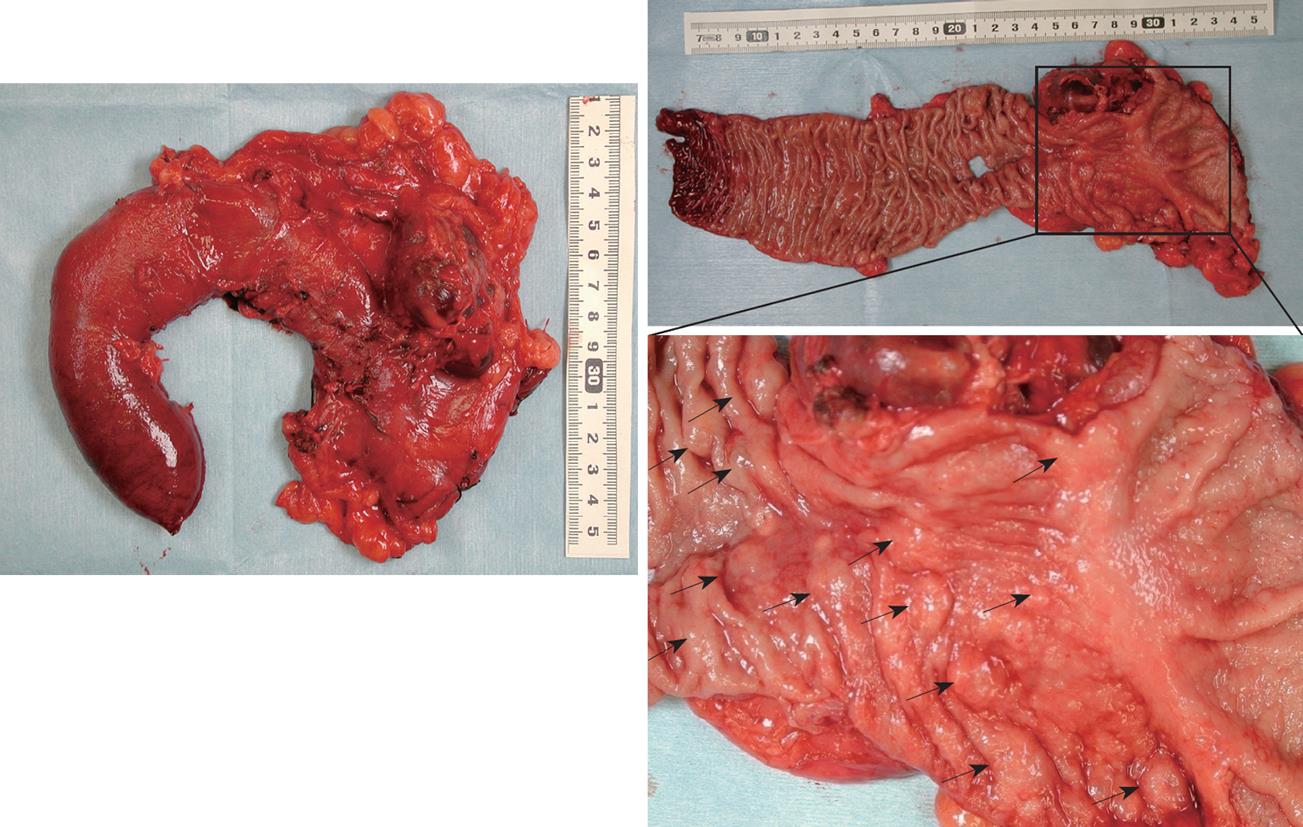
Recently, increased resection surgery for gastrinoma in patients with MEN 1 revealed that gastrinomas in MEN 1 were located mostly in the duodenum and rarely in the pancreas[40-43]. We have performed curative resection of gastrinomas in 16 patients with MEN 1 using pancreaticoduodenectomy (PD) or partial duodenal resection or pancreas preserving total duodenectomy (PPTD)[44]. In all patients, duodenal gastrinomas existed; as a single tumor in 42%, multiple tumors in 50% and numerous tumors in 13% (Figure 2). In addition, it was revealed that in two of 16 patients, pancreatic gastrinomas co-existed with multiple duodenal gastrinomas. These were resected guided by localization with the SASI test. When the patient with MEN 1 has more than five duodenal gastrinomas, we would recommend PPTD instead of PD for curative surgery[20,43,44]. The purpose of PPTD is to prevent recurrence of duodenal gastrinoma by total resection of the entire duodenum and to preserve full pancreatic function without resection of the pancreatic head. PPTD can be performed without any complications and seems less invasive than PD.
We have indicated PPTD for multiple duodenal gastrinomas (more than 5 or numerous gastrinomas)[43]. In 7 patients with MEN 1, more than 5 multiple duodenal gastrinomas were suspected during surgery and PPTD was performed. However, postoperative pathological diagnosis revealed that in 3 patients, only one or two duodenal gastrinomas existed, and other submucosal tumors which were thought to be gastrinomas during surgery were diagnosed as hyperplasia of Brunner’s glands in the postoperatively fixed paraffin specimen.
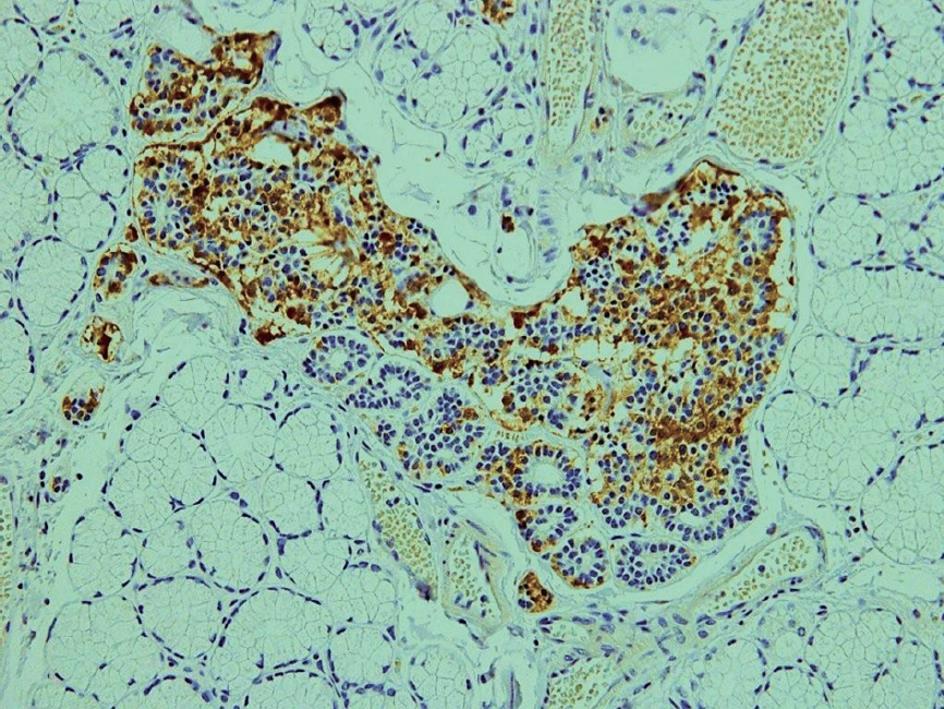
We performed immunohistochemical staining of the duodenal Brunner’s glands with anti-gastrin serum, and found that there were clusters of gastrin-producing cells in the hyperplasia of duodenal Brunner’s glands in all duodenal specimens after PPTD (Figure 3). Recently, Klöppel et al[45] reported that in patients with MEN 1, mutations in the menin gene can cause the development of clusters of gastrin-producing cells in the duodenal Brunner’s glands, which are thought to be precursor lesions of gastrinoma in patients with MEN 1. This may explain the high rate of postoperative recurrence of duodenal gastrinomas in patients with MEN 1, and may theoretically support the usefulness of PPTD as a curative surgery for these patients[43,44].
A few guidelines on the treatment of GEPNET have been published, such as the NCCN (National Comprehensive Cancer Network) guideline and Consensus guidelines by the European NET Study Group (ENETS)[5,6,8,44]. In both of these guidelines, resection surgery is first recommended for resectable hepatic metastases of GEPNET when the metastases are limited to the liver[5,6,46-49]. Now, the use of various types of cytotoxic chemotherapy for rapidly growing GEPNET and octreotide for slow growing well-differentiated GEPNET have been standardized[5,6]. These guidelines are also available for PNET.
It has been proved that resection of hepatic metastases improves the outcome of patients with PNET. Various types of resection surgery have been performed to achieve a macroscopic curative resection of hepatic metastases. Bettini et al[49] in Verona have reported on the usefulness of resection surgery combined with cytotoxic chemotherapy for prolongation of survival. They performed hepatic resection surgery whenever more than 90% of the hepatic metastases could be dissected, and used cytotoxic chemotherapy with CDDP, etoposide and 5-fluorouracil (5-FU) with streptozotocin as well as octreotide[49].
Radiofrequency ablation (RF) has been performed in addition to surgical resection of the liver for multiple hepatic metastases, for example, for metastases located deep in the hepatic parenchyma[50]. However, a number of complications after RF have been reported, especially following percutaneous RF. Therefore RF should be performed very carefully[51].
As the few guidelines on GEPNET describe, cytotoxic chemotherapy with CDDP and etoposide, streptozotocin with or without 5-FU, etc., has been recommended for rapidly growing or poorly differentiated GEPNET[5,6]. For slow growing NET, octreotide with or without interferon α has been recommended[5,6].
In addition, prospective studies of mTor inhibitors with or without octreotide and tyrosine kinase inhibitors are currently underway[52,53]. New cytotoxic chemotherapy with temozolomide and capecitabine have also been reported to be effective in a small series of patients with malignant NET.[54]. These drugs are also expected to be one of the new agents for PNET.
Curative resection surgery for sporadic PNET has almost been standardized using the SASI test for localization of PNET. The treatment strategy for PNET with MEN 1 has not been established, but resection surgery has been proved to contribute to the prolongation of survival in patients with MEN 1. Advances both in new chemotherapy including molecular targeting therapy and genetic analysis of PNET in patients with MEN 1 will lead us to a new treatment strategy for hereditary PNET.
Peer reviewers: Guida Portela-Gomes, MD, PhD, Professor, Faculty of Medicine, University of Lisbon, Rua Domingos Sequeira-128, Estoril 2765-525, Portugal; Robert Jensen, MD, Digestive Disease Branch, National Institutes of Health, Building 10, Rm 9C-103, Bethesda, MD 20892, United States
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
| 1. | Imamura M, Takahashi K, Adachi H, Minematsu S, Shimada Y, Naito M, Suzuki T, Tobe T, Azuma T. Usefulness of selective arterial secretin injection test for localization of gastrinoma in the Zollinger-Ellison syndrome. Ann Surg. 1987;205:230-239. |
| 2. | Krenning EP, Kwekkeboom DJ, Oei HY, de Jong RJ, Dop FJ, Reubi JC, Lamberts SW. Somatostatin-receptor scintigraphy in gastroenteropancreatic tumors. An overview of European results. Ann N Y Acad Sci. 1994;733:416-424. |
| 3. | Soga J. The term "carcinoid" is a misnomer: the evidence based on local invasion. J Exp Clin Cancer Res. 2009;28:15. |
| 4. | Klöppel G. Tumour biology and histopathology of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:15-31. |
| 5. | Plöckinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, Goede A, Caplin M, Oberg K, Reubi JC. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology. 2004;80:394-424. |
| 6. | Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, Haglund C, Knigge U, Vatn MH, Välimäki M. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I-general overview. Acta Oncol. 2004;43:617-625. |
| 7. | Tomassetti P, Campana D, Piscitelli L, Casadei R, Nori F, Brocchi E, Santini D, Pezzilli R, Corinaldesi R. Endocrine tumors of the ileum: factors correlated with survival. Neuroendocrinology. 2006;83:380-386. |
| 8. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. |
| 9. | Ito T, Tanaka M, Sasano H, Osamura YR, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K. Preliminary results of a Japanese nationwide survey of neuroendocrine gastrointestinal tumors. J Gastroenterol. 2007;42:497-500. |
| 10. | Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234-243. |
| 11. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. |
| 12. | Grant CS. Insulinoma. Surgical Endocrinology. Philadelphia: Lippincott Williums & Wilkins 2001; 345-360. |
| 13. | Service FJ, O'Brien PC, Kao PC, Young WF Jr. C-peptide suppression test: effects of gender, age, and body mass index; implications for the diagnosis of insulinoma. J Clin Endocrinol Metab. 1992;74:204-210. |
| 14. | Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;85:295-330. |
| 15. | Berna MJ, Hoffmann KM, Long SH, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore). 2006;85:331-364. |
| 16. | Imamura M, Shimada Y, Kato M, Doi R, Okada N, Hashimoto M. Usefulness of selective arterial calcium injection test and secretin test in patients with insulinoma. J Hep Bil Pancr Surg. 1994;1:530-534. |
| 17. | Imamura M, Hattori Y, Nishida O, Honda T, Shimada Y, Miyahara T, Wagata T, Baba N, Tobe T. Unresponsiveness of insulinoma cells to secretin: significance of the secretin test in patients with insulinoma. Pancreas. 1990;5:467-473. |
| 18. | Isenberg JI, Walsh JH, Passaro E Jr, Moore EW, Grossman MI. Unusual effect of secretin on serum gastrin, serum calcium, and gastric acid secretion in a patient with suspected Zollinger-Ellison syndrome. Gastroenterology. 1972;62:626-631. |
| 19. | Hattori Y, Imamura M, Tobe T. Gastrin release from antral G cells stimulated with secretin. Am J Gastroenterol. 1992;87:195-200. |
| 20. | Imamura M, Komoto I, Ota S. Changing treatment strategy for gastrinoma in patients with Zollinger-Ellison syndrome. World J Surg. 2006;30:1-11. |
| 21. | Imamura M, Takahashi K, Isobe Y, Hattori Y, Satomura K, Tobe T. Curative resection of multiple gastrinomas aided by selective arterial secretin injection test and intraoperative secretin test. Ann Surg. 1989;210:710-718. |
| 22. | Turner JJ, Wren AM, Jackson JE, Thakker RV, Meeran K. Localization of gastrinomas by selective intra-arterial calcium injection. Clin Endocrinol (Oxf). 2002;57:821-825. |
| 23. | Alexander HR, Fraker DL, Norton JA, Bartlett DL, Tio L, Benjamin SB, Doppman JL, Goebel SU, Serrano J, Gibril F. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg. 1998;228:228-238. |
| 24. | Noda S, Norton JA, Jensen RT, Gay WA Jr. Surgical resection of intracardiac gastrinoma. Ann Thorac Surg. 1999;67:532-533. |
| 25. | Doppman JL, Miller DL, Chang R, Shawker TH, Gorden P, Norton JA. Insulinomas: localization with selective intraarterial injection of calcium. Radiology. 1991;178:237-241. |
| 26. | Krenning EP, Kwekkeboom DJ, Oei HY, de Jong RJ, Dop FJ, Reubi JC, Lamberts SW. Somatostatin-receptor scintigraphy in gastroenteropancreatic tumors. An overview of European results. Ann N Y Acad Sci. 1994;733:416-424. |
| 27. | Jensen RT. Zollinger-Ellison syndrome. Surgical Endocrinology: Clinical syndromes. Philadelphia: Lippincott William & Wilkins 2001; 291-343. |
| 28. | Portela-Gomes GM, Stridsberg M, Grimelius L, Rorstad O, Janson ET. Differential expression of the five somatostatin receptor subtypes in human benign and malignant insulinomas - predominance of receptor subtype 4. Endocr Pathol. 2007;18:79-85. |
| 29. | Grant CS, van Heerden J, Charboneau JW, James EM, Reading CC. Insulinoma. The value of intraoperative ultrasonography. Arch Surg. 1988;123:843-848. |
| 30. | Kato M, Imamura M, Hosotani R, Shimada Y, Doi R, Itami A, Komoto I, KosakaM TT, Konishi J. Curative resection of microgastrinomas based on the intraoperative secretin test. World J Surg. 2000;24:1425-1430. |
| 31. | Imamura M. Surgical Treatment. The Pancreas. 2nd edition. Massachusetts: Blackwell Publishing 2008; 818-822. |
| 32. | Norton JA, Fraker DL, Alexander HR, Gibril F, Liewehr DJ, Venzon DJ, Jensen RT. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410-419. |
| 33. | Goretzki PE, Röher HD. Islet tumors. The Pancreas. 2nd edition. Massachusetts: Blackwell Publishing 2008; 794-801. |
| 34. | Gibril F, Venzon DJ, Ojeaburu JV, Bashir S, Jensen RT. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab. 2001;86:5282-5293. |
| 35. | Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807-1843. |
| 36. | Goudet P, Murat A, Binquet C, Cardot-Bauters C, Costa A, Ruszniewski P, Niccoli P, Ménégaux F, Chabrier G, Borson-Chazot F. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d'Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34:249-255. |
| 37. | Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease. Results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243:495-500. |
| 38. | Lairmore TC, Piersall LD, DeBenedetti MK, Dilley WG, Mutch MG, Whelan AJ, Zehnbauer B. Clinical genetic testing and early surgical intervention in patients with multiple endocrine neoplasia type 1 (MEN 1). Ann Surg. 2004;239:637-645; discussion 645-647. |
| 39. | O'Riordain DS, O'Brien T, van Heerden JA, Service FJ, Grant CS. Surgical management of insulinoma associated with multiple endocrine neoplasia type I. World J Surg. 1994;18:488-493; discussion 493-494. |
| 40. | Pipeleers-Marichal M, Donow C, Heitz PU, Klöppel G. Pathologic aspects of gastrinomas in patients with Zollinger-Ellison syndrome with and without multiple endocrine neoplasia type I. World J Surg. 1993;17:481-488. |
| 41. | Imamura M, Kanda M, Takahashi K, Shimada Y, Miyahara T, Wagata T, Hashimoto M, Tobe T, Soga J. Clinicopathological characteristics of duodenal microgastrinomas. World J Surg. 1992;16:703-709; discussion 709-710. |
| 42. | Bartsch DK, Fendrich V, Langer P, Celik I, Kann PH, Rothmund M. Outcome of duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg. 2005;242:757-764, discussion 764-766. |
| 43. | Imamura M, Komoto I, Doi R. Resection surgery for gastrinomas in patients with MEN 1 and ZES guided by selective arterial secretagogue injection test. World J Surg. 2009;33 Suppl 1:S67. |
| 44. | Imamura M, Komoto I, Doi R, Onodera H, Kobayashi H, Kawai Y. New pancreas-preserving total duodenectomy technique. World J Surg. 2005;29:203-207. |
| 45. | Klöppel G, Anlauf M, Perren A. Endocrine precursor lesions of gastroenteropancreatic neuroendocrine tumors. Endocr Pathol. 2007;18:150-155. |
| 46. | Steinmüller T, Kianmanesh R, Falconi M, Scarpa A, Taal B, Kwekkeboom DJ, Lopes JM, Perren A, Nikou G, Yao J. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87:47-62. |
| 47. | Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432-445. |
| 48. | Chen H, Hardacre JM, Uzar A, Cameron JL, Choti MA. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88-92; discussion 92-93. |
| 49. | Bettini R, Boninsegna L, Mantovani W, Capelli P, Bassi C, Pederzoli P, Delle Fave GF, Panzuto F, Scarpa A, Falconi M. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19:903-908. |
| 50. | Bilchik AJ, Rose DM, Allegra DP, Bostick PJ, Hsueh E, Morton DL. Radiofrequency ablation: a minimally invasive technique with multiple applications. Cancer J Sci Am. 1999;5:356-361. |
| 51. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. |
| 52. | Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, Hoosen S, St Peter J, Haas T, Lebwohl D. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69-76. |
| 53. | Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311-4318. |
| 54. | Ekeblad S, Sundin A, Janson ET, Welin S, Granberg D, Kindmark H, Dunder K, Kozlovacki G, Orlefors H, Sigurd M. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986-2991. |