Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4428
Revised: May 31, 2010
Accepted: June 7, 2010
Published online: September 21, 2010
AIM: To investigate the relationship between exercise-provoked esophageal motility disorders and the prognosis for patients with chest pain.
METHODS: The study involved 63 subjects with recurrent angina-like chest pain non-responsive to empirical therapy with proton pump inhibitor (PPI). In all, a coronary artery angiography, panendoscopy, 24-h esophageal pH-metry and manometry, as well as a treadmill stress test with simultaneous esophageal pH-metry and manometry monitoring, were performed. Thirty-five subjects had no significant coronary artery lesions, and 28 had more than 50% coronary artery narrowing. In patients with hypertensive esophageal motility disorders, a calcium antagonist was recommended. The average follow-up period was 977 ± 249 d.
RESULTS: The prevalence of esophageal disorders, such as gastroesophageal reflux or diffuse esophageal spasm, was similar in patients both with and without significant coronary artery narrowing. Exercise prompted esophageal motility disorders, such as a decrease in the percentage of peristaltic and effective contractions and their amplitude, as well as an increase in the percentage of simultaneous and non-effective contractions. In 14 (22%) patients the percentage of simultaneous contractions during the treadmill stress test exceeded the value of 55%. Using Kaplan-Meier analysis and the proportional hazard Cox regression model, it was shown that the administration of a calcium channel antagonist in patients with such an esophageal motility disorder significantly decreased the risk of hospitalization as a result of a suspicion of acute coronary syndrome after the 2.7-year follow-up period.
CONCLUSION: In patients with chest pain non-responsive to PPIs, a diagnosis of exercise-provoked esophageal spasm may have the effect of lowering the risk of the next hospitalization.
- Citation: Budzyński J. Exercise-provoked esophageal motility disorder in patients with recurrent chest pain. World J Gastroenterol 2010; 16(35): 4428-4435
- URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4428
Chest pain, including its angina-like form, is a common problem in health care because of its frequency, recurrence, the utilization of resources consistent with costs of medical procedures, and diagnostic difficulties[1-7]. There are many causes of chest pain and in respective patients they may coexist, and even overlap[8,9]. For this reason, chest pain in patients with coronary artery disease (CAD), including that which is exercise-provoked, may originate not only from the myocardium, but also from noncardiac sources. The most frequent causes of noncardiac chest pain (NCCP) are diseases of the upper part of the digestive tract, such as gastroesophageal reflux disease (GERD), esophageal motility disorders, and gastric and duodenal ulcer disease[2]. Their prevalence in patients both with and without cardiovascular diseases seems to be similar[9,10], although some authors have reported a lower percentage of esophageal disorder diagnoses in patients with CAD than in individuals without CAD[11,12].
The importance of esophageal motility disorders in exercise-provoked, angina-like chest pain pathogenesis is still uncertain[5]. Pharmacological provocative tests have not been diagnostically useful[1,5], whereas the use of exercise in order to provoke symptoms in patients with NCCP suspected of being related to esophageal motility disorders has not been sufficiently investigated[13,14]. However, it is known that exercise can induce myocardial ischemia as well as alterations in esophageal motility and gastroesophageal reflux (GER), and in such a way reproduce chest pain[14]. Therefore, the simultaneous monitoring of esophageal function and electrocardiography (ECG) during a treadmill stress test, besides potentially shortening the diagnostic procedure time, seems to have additional benefits in providing an opportunity to carry out cardiac and esophageal investigations at the same time. It is also of great importance to exclude the possibility of life-threatening conditions (by revealing potential ischemic ECG changes), and to simulate the circumstances of angina-like (effort-provoked) chest pain appearance.
The aim of this study was to compare the effect of a treadmill stress test on esophageal motility in patients with and without angiographic signs of CAD. The objective was also to evaluate the influence of recommended treatment on the risk of hospitalization due to a suspicion of acute coronary syndrome over a 2.7-year follow-up period. To the author’s knowledge, this is the first report concerning this aspect of recurrent angina-like chest pain.
The analysis was carried out in 63 consecutive patients hospitalized in order to perform scheduled diagnostic procedures because of recurrent angina-like chest pain, defined as precordial symptoms induced by exercise and receding after rest or the taking of nitroglycerine. The symptoms were diagnosed as being noncardiac in origin by the cardiologist, who was not related to the researcher, and who had referred his patients to a gastroenterologist because of recurrent, angina-like symptoms. These symptoms had been resistant to treatment orientated towards coronary reserve improvement (in patients with CAD) and empirical therapy with proton pump inhibitors (PPIs). In all individuals, a coronary angiography was performed prior to all gastroenterological procedures. The studied group consisted of 28 (44%) patients who had significant angiographic changes, with > 50% of the coronary vessels being narrowed but not suitable for revascularization, and 35 (66%) subjects having normal coronary angiograms or no obstructive lesions. The purpose of referring patients with CAD to a gastroenterologist was to diagnose possibly overlapping gastroenterological and cardiological chest pain causes. This was of particular concern in subjects with CAD who did not present significant ST interval ECG changes accompanying chest pain occurrence during an ambulatory stress test, or who did not suffer from esophageal symptoms other than chest pain. Patients with CAD and those without significant coronary artery narrowing did not differ according to the majority of demographic and clinical data (Table 1).
| Parameter | CAD- (n = 35) | CAD+ (n = 28) | ||
| EPES (+) (n = 8, 23%) | EPES (-) (n = 27, 77%) | EPES(+) (n = 6, 21%) | EPES (-) (n = 22, 79%) | |
| Male gender | 2 (25) | 8 (30) | 3 (50) | 10 (45) |
| Age (yr) | 56.3 ± 10.0 | 52.9 ± 7.3 | 54.5 ± 11.1 | 54.8 ± 8.8 |
| Body mass index (kg/m2) | 31 ± 3.5 | 27.6 ± 4.8 | 30.2 ± 6.2 | 27.7 ± 4.0 |
| Waist to hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| > 50% narrowing of coronary vessels | 0 | 0 | 6 (100) | 22 (100) |
| Smoking | 0 | 3 (11) | 1 (16) | 1 (5) |
| History of myocardial infarction | 0 | 0 | 1 (16) | 6 (27) |
| History of hypertension | 3 (38) | 10 (38) | 3 (50) | 8 (36) |
| History of diabetes (n) | 1 (13) | 1 (4) | 2 (33) | 4 (18) |
| Blood glucose (mg/dL) | 106 ± 18.7 | 95.5 ± 13.7 | 91 ± 6.3 | 116.7 ± 22.2 |
| LDL cholesterol (mg/dL) | 127 ± 15.8 | 125.3 ± 44.2 | 116.6 ± 38 | 128 ± 34.3 |
| Triglycerides (mg/dL) | 120 ± 22 | 134.4 ± 58.3 | 154.8 ± 69.2 | 181.4 ± 104 |
| Angina-like chest pain during the stress test | 4 (50) | 2 (15)c | 4 (67) | 4 (18) |
| Horizontal ST interval depression ≥ 1 mm | 6 (75) | 15 (56) | 4 (75) | 8 (36) |
| Duration of stress test (s) | 477 ± 145 | 408 ± 158a | 501 ± 186 | 607 ± 175 |
| Echocardiographically determined EF (%) | 60 ± 7 | 63 ± 3 | 65 ± 3 | 69 ± 8 |
| Helicobacter pylori infection | 4 (50) | 12 (44) | 2 (33) | 11 (50) |
| Esophagitis | 0 | 4 (15) | 2 (33) | 2 (9) |
| Pathological acid GER | 0 | 6 (22) | 2 (33) | 5 (23) |
| Acid epGER | 0 | 2 (7) | 6 (100) | 0 |
| Calcium antagonist recommendation | 8 (100) | 20 (74) | 6 (100) | 16 (73) |
| Event-free period (d) | 904 ± 352a | 866 ± 356 | 887 ± 374a | 541 ± 400 |
| Hospitalization | 0 | 10 (37) | 3 (50) | 11 (50) |
| Time to first hospitalization (d)1 | 494 ± 372 | 226 ± 124b | 604 ± 335 | 309 ± 210 |
The inclusion criteria were as follows: (1) aged between 40 and 70 years; (2) prior coronarography performance; (3) angina-like chest pain to the degree of class II or III according to the Canadian Cardiovascular Society (CCS); and (4) persistent symptoms despite adequate anti-angina treatment and therapy for at least 1 mo with a double dose of omeprazole. The exclusion criteria were as follows: (1) changes in the ECG which made it impossible to estimate ischemic signs (e.g. left bundle branch block or pre-excitation syndrome); and (2) the taking of medicines which would affect gastric acid secretion or digestive tract motility up to 2 wk prior to the examination, with the exception of the ad hoc use of nitroglycerine tablets.
The well-being of all 63 individuals, diagnosed during 2004-2007, was followed in outpatient cardiology clinics. The mean observation period between the day of gastroenterological diagnostic performance and 31 September 2008, when the follow-up period finished, amounted to 977 ± 249 d. The data concerning date, duration and cause of eventual hospitalization were obtained from the National Health Foundation (NHF) on the basis of social security numbers. Fortunately, the standard primary end-points, such as death or myocardial infarction, did not occur within the observation period, so hospitalization due to suspected acute coronary syndrome was established as the end-point of the analysis. The author had no influence on the decision of patients’ hospitalization. The mean time before the first hospitalization due to suspected acute coronary syndrome was 437 ± 356 d (in hospitalized individuals). None of the patients died during the course of their hospitalization and in none were observed signs of myocardial infarction (i.e. an increase in troponin level). None of the subjects needed an emergency coronarography or percutaneous coronary intervention (PCI).
In all subjects the medical history, physical examination, 24-h esophageal pH-metry and manometry, and a panendoscopy with gastric and esophageal biopsy (after the removal of pH-metric and manometric probes) were performed. An investigation of ambulatory esophageal function was conducted by means of a multi-use antimony probe (Synetics Medical AB, Sweden), and a manometry catheter (Synectics, Medtronic) with three pressure sensors (separated by 5 cm) and a Synectic Digitrapper. An esophageal pH-metric sensor, after calibration to pH 7 and 1, and following nasal and esophageal intubation, was positioned 5 cm above the monometrically-determined lower esophageal sphincter (LES). A pressure probe was placed through the other nostril at 3, 8 and 13 cm above the LES. During esophageal pH and pressure monitoring, all patients eventually recorded the occurrence of symptoms. None of the patients reported disturbances in nasal breathing. The following day, during continuous esophageal pH-metry and manometry monitoring, a treadmill stress test on a running track was carried out at approximately 7 am, after patients had become accustomed to the pH-metric and manometric probes. The exercise test was performed using a device manufactured by Schiller, Switzerland, according to the Bruce protocol (the speed and gradient of the running track were increased every 3 min, respectively: 2.7, 4, 5.5, 6.8 km/h; and by 10°, 12°, 14° and 16°).
After the stress test, the data obtained during esophageal function monitoring were downloaded to a PC and analyzed using GASTROSOFT software. Standard parameters of esophageal pH-metry and manometry were calculated according to the software settings. As the normal values of exercise-induced esophageal disorders were unknown, the researcher’s own definitions were proposed as follows. GER in 24-h pH-metry was defined as the time that intraesophageal pH < 4 exceeded 4.5% of the total duration of the examination. Gastroesophageal acid reflux provoked by exercise (epGER) was defined as a decrease in esophageal pH < 4 for more than 10 s during an exercise stress test. Simultaneous contractions were determined as being contractions when the delay between adjacent transducers separated by 5 cm was less than 0.25 s (with a propagation speed higher than 20 cm/s). Peristaltic contractions were defined as the increase of esophageal pressure in which the delay between contractions beginning on the adjacent transducers was in the range 0.25-7 s. Effective contractions were defined as complete (detected by all three sensors) peristaltic contractions with adequate amplitude. This last definition, according to the GASTROSOFT settings, is represented by the following: 20 mmHg at 13 cm above the LES, 25 mmHg at 8 cm above the LES, and 30 mmHg at 3 cm above the LES[15]. Ineffective esophageal motility (IEM) was defined as more than 30% of water swallows (during a meal) provoking contractions with an amplitude of less than 30 mmHg and/or with a non-transmission rate to the distal esophagus[16]. In patients with a diagnosis of hypertensive esophageal motility disorders (nutcracker esophagus or diffuse esophageal spasm), a calcium channel antagonist - amlodipine 1 × 10 mg or diltiazem retard 2 × 120-180 mg, depending on the subject’s resting heart rate - was recommended.
The study protocol was approved by the local Bioethics Committee of Nicolaus Copernicus University in Toruń and Ludwik Rydygier Collegium Medicum in Bydgoszcz, Poland. All subjects gave their informed consent prior to the start of the investigation. All procedures have been conducted in compliance with the Declaration of Helsinki.
Statistical analysis was conducted using a licensed version of statistical software STATISTICA PL 8.0 for Windows. The results were mainly presented as the mean ± SD or n, %. The statistical significance of differences between patients with and without CAD, as well as with a diagnosis of exercise-provoked esophageal spasm (EPES) and those without EPES, was checked using an unpaired t-Student test, χ2 or Fisher’s exact test (Table 1). The statistical significance of differences between values of esophageal motility parameters obtained from whole-day monitoring and those from exercise tests using the nonparametric Wilcoxon test were estimated. Survival analysis was carried out. The Cox’s F test in the Kaplan-Meier method for two and many groups and the Cox proportional hazard analysis were used.
Gradual exercise during a treadmill stress test, in comparison to the values obtained during the whole monitoring process (24 h) and daily activity period, provoked epGER in 8/63 (13%) subjects. This included four in whom neither acid reflux within the 24-h monitoring was found, nor motility disorders, such as a decrease in the percentage of peristaltic and effective contractions, a decrease in esophageal contraction amplitude, or an increase in the percentage of simultaneous and non-effective contractions (Table 2). In 14/63 (22%) patients, a percentage of simultaneous contractions during the treadmill stress test exceeded the arbitrarily established cut-off value of > 55%, according to the diffuse esophageal spasm (DES) definition by Stein et al[17]. This esophageal motor disorder was termed EPES. Patients with such an esophageal motility disorder had no special characteristics in comparison with the remaining subjects (Table 1). The appearance of EPES features was not predicted by the occurrence of chest pain during the 24-h esophageal function examination (P = 0.62), by Helicobacter pylori (H. pylori) infection (P = 0.42), or by a history of hypertension (P = 0.23) or diabetes (P = 0.23). However, an EPES diagnosis was significantly related to angina-like chest pain presence (P = 0.01) during the treadmill stress test (Table 1).
| Parameter | CAD- (n = 35) | CAD+ (n = 28) | ||||
| 24 h | ext | P | 24 h | ext | P | |
| Contractions per minute | 1.5 ± 0.8 | 2.7 ± 1.7 | 0.001 | 1.9 ± 1.5 | 2.7 ± 2.2 | 0.04 |
| Peristaltic contractions (%) | 64.3 ± 9.6 | 47.2 ± 8.4 | 0.001 | 67.7 ± 28.6 | 56.9 ± 18.7 | 0.03 |
| Complete peristalsis (%) | 43.9 ± 15.3 | 35.6 ± 18.5 | 0.140 | 51.9 ± 10.9 | 49.3 ± 26.5 | 0.70 |
| Reduced peristalsis (%) | 18.3 ± 13.2 | 16.2 ± 14.8 | 0.630 | 13.3 ± 6.3 | 10.1 ± 7.2 | 0.30 |
| Interrupted peristalsis (%) | 37.8 ± 10.8 | 40.1 ± 30.0 | 0.690 | 34.8 ± 8.5 | 36.8 ± 24.1 | 0.70 |
| Simultaneous/mixed contractions (%) | 35.7 ± 9.6 | 49.3 ± 23.8 | 0.010 | 32.3 ± 8.6 | 43.0 ± 18.7 | 0.02 |
| Complete contractions (%) | 16.6 ± 5.2 | 35.6 ± 28.5 | 0.010 | 14.9 ± 4.4 | 30.6 ± 30.1 | 0.03 |
| Simultaneous contractions (%) | 83.4 ± 5.3 | 71.1 ± 28.7 | 0.040 | 85.1 ± 4.9 | 69.4 ± 30.1 | 0.03 |
Moreover, in the subjects studied, erosive esophagitis occurred in eight (12%); features of gastroesophageal acid reflux in 24-h esophageal pH-metry during daily activity appeared in 13 (21%); in 24-h esophageal manometry, features of DES with a > 30% cut-off value percentage of simultaneous contractions[18] during daily activity were experienced by 17 (27%); and features of ineffective esophageal motility (IEM) were found in five (8%). The prevalence of the esophageal disturbances mentioned, in patients with CAD and without significant (> 50%) coronary artery narrowing, was similar; the exceptions being that features of IEM were not found in patients with CAD (Table 1). Moreover, neither patient group differed significantly in relation to the values of the majority of the demographic and clinical parameters or in non-invasive cardiac examinations (Table 1). Only CAD-EPES- patients had a significantly longer duration of the stress test than respective subjects with CAD (CAD+EPES-).
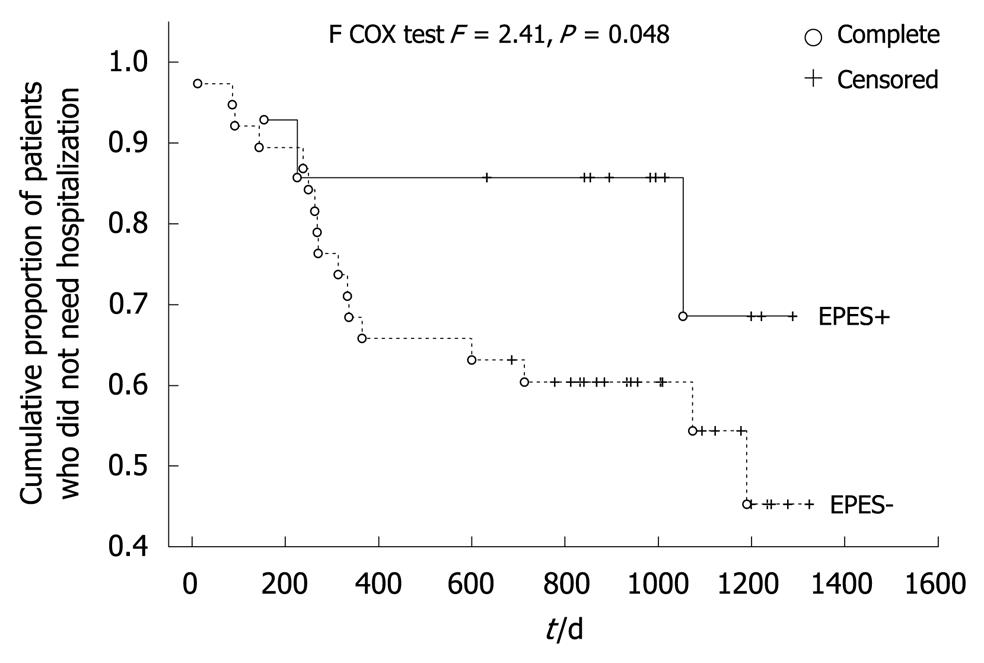
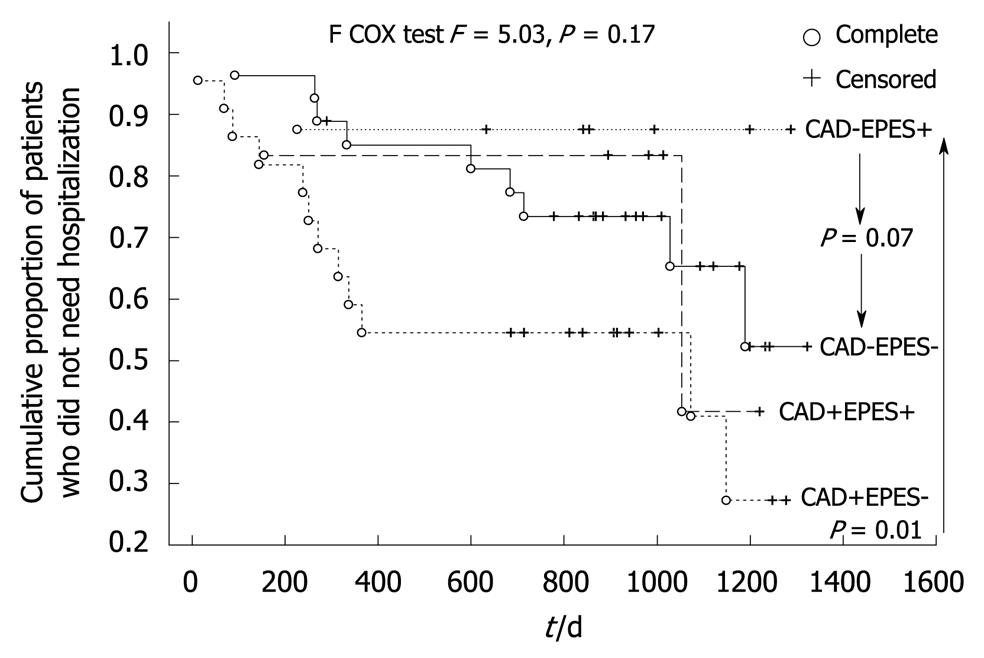
After the 2.7-year follow-up period, all subjects remained alive. Twenty-four patients (38%) were hospitalized because of acute coronary syndrome suspicion. Patients with an EPES diagnosis made up only 12.5% of this group. The percentage of patients with EPES who needed hospitalization in the follow-up period (3/14 = 21%) was two times lower than subjects without such a diagnosis (21/49 = 42%, P > 0.05). Patients with an EPES diagnosis also had a significantly longer event-free period and time to the first hospitalization episode within the follow-up period (Table 1). The favorable effect of an EPES diagnosis on the risk of hospitalization due to suspected acute coronary syndrome was also confirmed by the Kaplan-Meier survival analysis (F = 2.41, P = 0.048, Figure 1). Kaplan-Meier survival analysis was also performed (Figure 2) because the investigated group consisted of patients both with and without significant coronary artery narrowing and this variable plays a known role as a predictor of events in patients with recurrent chest pain. The common (four-way) effect of coronary artery narrowing and EPES presence did not reveal a statistically significant influence on the risk of hospitalization (F = 5.03, P = 0.17). However, CAD-EPES+ patients had a more favorable course of the Kaplan-Meier curve than CAD-EPES- patients (statistically borderline, F = 2.56, P = 0.07) and CAD+EPES- (F = 6.89, P = 0.006). The detailed Kaplan-Meier curve analysis shows that the differences mentioned had already appeared after an observation period of about 1 year.
A multi-factorial Cox proportional hazard analysis was also performed (Table 3) because hospitalization due to acute coronary syndrome suspicion may be an effect of many factors. It showed that in the researcher’s group the independent significant variables which decreased the risk of the appearance of chest pain needing hospitalization were as follows: EPES, recommendation of eradicative triple therapy due to H. pylori infection, female gender and younger age (Table 3).
| Independent variable | β | Standard error | P |
| Number of hospitalizations before gastrological diagnostic performance | 0.01 | 0.06 | 0.87 |
| Spontaneous chest pain during 24-h monitoring | 1.05 | 0.56 | 0.06 |
| Eradicative treatment recommendation due to Helicobacter pylori infection | -1.36 | 0.62 | 0.03 |
| EPES | -2.38 | 0.93 | 0.01 |
| epGER | -0.012 | 1.01 | 0.99 |
| Significant (> 50%) coronary vessel narrowing | 1.01 | 0.73 | 0.17 |
| History of myocardial revascularization | -0.78 | 0.67 | 0.24 |
| DUKE score | 0.03 | 0.06 | 0.62 |
| Gender (male/female) | -1.42 | 0.72 | 0.049 |
| Smoking | 0.06 | 1.38 | 0.96 |
| Canadian Cardiovascular Society Classification | 0.74 | 0.76 | 0.33 |
| History of myocardial infarction | 0.30 | 0.99 | 0.76 |
| Hypertension | -0.47 | 0.96 | 0.63 |
| Diabetes mellitus | -0.22 | 0.80 | 0.78 |
| Age | 0.085 | 0.04 | 0.047 |
| BMI | 0.10 | 0.09 | 0.27 |
| WHR | -4.69 | 5.07 | 0.35 |
| Fasting blood glucose | -0.074 | 0.05 | 0.11 |
| LDL cholesterol | -0.015 | 0.01 | 0.18 |
| Triglycerides | 0.005 | 0.04 | 0.277 |
This preliminary observational investigation was undertaken to estimate the clinical usefulness of esophageal manometry examination during graded exercise, as a provocative test in the diagnosis of angina-like chest pain of suspected noncardiac origin, both in patients with CAD and in subjects without significant coronary artery narrowing. As a criterion of clinical usefulness of such a diagnostic strategy, the risk of hospitalization due to the suspicion of acute coronary syndrome over a long follow-up period was assumed. The main observation of this study is that graded exercise during a treadmill test may induce esophageal motility disturbances leading to a decrease in esophageal mechanical clearance, expressed more in patients without CAD (Table 2). This particular kind of motility disorder, in which a percentage of simultaneous contractions exceed the cut-off value of 55%, was named EPES by the author. The prevalence of EPES in the studied group was rare (n = 14/63, 22%), but similar to the presence of the other esophageal disorders (GER, epGER, DES and IEM) in patients both with and without significant coronary artery narrowing (Table 1). The presence of this esophageal motility disorder was related to angina-like chest pain occurrence with the treadmill stress test. To my knowledge this is the first paper to present such a view of esophageal motility disorder.
The second important observation of this investigation is that patients with a diagnosis of EPES had an independently (Table 3) and significantly lower risk of hospitalization due to the suspicion of acute coronary syndrome in the 2.7-year long follow-up period (Figure 1). This independent effect, although confirmed by the multi-factorial Cox proportional hazard analysis (Table 3), was less apparent when all subjects were divided into four groups in relation to the presence of EPES and CAD (Figure 2), although a more favorable trend for EPES was perceptible. These observations might have resulted from pharmacotherapy because calcium antagonists had been recommended in all patients with hypertensive esophageal disorders (Table 1). The reported effects of a calcium antagonist in the treatment of hypertensive esophageal motility disorders diagnosed using stationary manometry have been ambiguous. Some studies have shown a favorable outcome for this group of drugs; some have not confirmed this[1,5]. However, no reports concerning the usefulness of calcium antagonists in the treatment of IEM or the other esophageal motility disorders diagnosed on the basis of ambulatory 24-h manometry were found. Moreover, it is also possible that calcium channel blockers only have an influence on exercise-provoked esophageal motility disorder. It might also be that my observations of a good prognosis for patients with EPES treated with calcium antagonists resulted not from the effects of the medicine but from the diagnosis of an extra-cardiac and non-life-threatening source of their chest pain. Such an interpretation concurs with the results of a study by Spencer et al[19], who showed that patients with dysphagia or chest pain and a diagnosis of esophageal motility disorder (such as achalasia, diffuse esophageal spasm, “nutcracker” hypercontracting esophagus, and hypocontracting esophagus) reported clinical improvement in a 3-year follow-up. However, in the patients’ opinion, the amelioration of their symptoms was not an effect of the recommended treatment but a belief in the benign character of their discomfort. Consideration of other than a drug-related mechanism of better prognosis in patients with EPES treated with calcium antagonists resulted from an open-label, uncontrolled study design. In this study the course of angina-like chest pain in patients with a diagnosis of EPES was also compared with patients for whom calcium antagonists were also recommended because of hypertensive esophageal motility disorders other than EPES (Table 1). However, in the latter patient group the recommendation of a calcium antagonist had no favorable effect on the risk of hospitalization.
The potential pathomechanism for EPES, which is a particular kind of IEM, is unknown. According to the results and conclusions of Adamek et al[20], it may be supposed that EPES could be a marker of general smooth muscle readiness for contractive reactions. This hypothesis would be confirmed by the coexistence of esophageal motility disorders with other vasospastic syndromes, such as migraine, Raynaud’s phenomenon, Prinzmetal’s angina, and hypertension and/or a hypertensive (exaggerated) reaction of blood pressure to exercise[21,22]. However, in the present study no relationship between EPES manometric features and the above-mentioned blood pressure reactions or signs of myocardial ischemia (significant ST interval depression) was found. In addition, no patients had a history of vasospasm during coronarography. The other possible explanations for the appearance of EPES features during the treadmill stress test, which occurred in only 14/63 (22%) of subjects, may be as follows: an individually related difference of exercise-related changes in sympathetic autonomic nervous system activity; different sensitivity of smooth muscle to noradrenalin; a decrease in vagally-mediated regulation of esophageal motility; the inhibition of nitric oxide synthase activity; as well as esophageal ischemia induced by a decrease in splanchnic blood flow during exercise observed in 50%-80% of subjects[23]. All these factors may also have an effect on the presence of visceral hypersensitivity and symptom intensity[18,24-27]. On the other hand, Tipnis et al[28] have shown that distension of the esophagus itself plays an important role in symptom modulation in patients with GERD. It seems that non-propulsive motility disorders, amongst which is EPES, may lead to similar esophageal conditions.
The third observation of my study is that patients with recurrent angina-like chest pain with CAD and without significant coronary artery narrowing were similar with regard to the majority of estimated demographic and clinical data, including parameters of esophageal manometry, especially those obtained from examination during the treadmill stress test (EPES) (Table 1). These observations corroborate data published by other authors, who have reported a similar prevalence of esophageal disorders in patients both with and without cardiovascular diseases[9,10]. However, some authors have also reported a lower percentage of diagnoses of esophageal disorders in patients with CAD than in individuals without CAD[11,12]. The clinical importance of this observation is that there is no clinical or demographic factor which could help to predict the origin of chest pain in respective patients, especially when they have not responded to guided therapy. On the other hand, my results have confirmed the possibility of overlapping noncardiac and cardiac chest pain sources, and justified the common analysis of patients with and without significant coronary artery narrowing. A simple explanation for the coexistence of esophageal and cardiac disorders might be the actions of viscero-visceral and viscero-somatic reflexes, which may lead to a decrease in myocardial perfusion in response to a decrease in intraesophageal pH[24,26]. It might also relate to esophageal function disorders within myocardial ischemia[29] and/or to the increase of back or precordial muscle tension in response both to myocardial ischemia and esophageal function disorders[30].
My observations, like many others, have some limitations. Firstly, the number of patients in the studied group was small. However, in the PubMed database I could only find a few works concerning the gastroenterological aspects of chest pain which had a number of subjects greater than in the present study. However, the number of subjects in my study was enough to reach statistical significance in some important comparisons (Tables 2 and 3, Figure 1). The inclusion in the study of consecutive patients helped to avoid, or at least reduce, selection bias. Secondly, the follow-up period was relatively short but similar to that in the recent study by Eslick et al[31]. Thirdly, swallowing was not monitored during manometric examination, although this limitation seems only to carry importance in the definition of diffuse esophageal spasm. This resulted from the methodology (ambulatory motility monitoring) and the kind of manometric probe used (distal sensors only). In my opinion, the appearance of EPES in only 22% of individuals, all patients having been submitted to the same conditions on the treadmill, justified a new dysmotility diagnosis, independent of its primary, secondary or tertiary character. Fourthly, patients were recommended to take amlodipine or diltiazem in this study, depending on their resting heart rate. Such dual therapy might have influenced the results obtained, especially as a more favorable effect of the dihydropyridine class of calcium-channel blockers on vascular endothelial function in patients with coronary spastic angina has been reported[32]. Although the studied patient group did not present with vasospasm during the coronarography, this indicates a necessity to retry the investigation using another design in a homogeneous patient group. Fifthly, a similar conclusion applies to the lack of a placebo-controlled study design. However, in this study calcium antagonists were recommended in open-label design in all patients with hypertensive esophageal motility disorders (Table 1), not only those with EPES. Thus, patients without EPES and treated with a calcium antagonist should be recognized as a control group; this may even suggest that an improved prognosis in patients with EPES did not only result from a drug effect.
In conclusion, patients with recurrent angina-like chest pain non-responsive to treatment with PPIs, both with and without significant coronary artery narrowing, had a similar prevalence of potential noncardiac causes of precordial symptoms, including exercise-related esophageal motility disorders. However, patients with a diagnosis of EPES and a recommendation of a calcium antagonist showed significantly lower risk of hospitalization due to suspected acute coronary syndrome in the 2.7-year follow-up period than others, but this aspect needs further study.
Noncardiac chest pain (NCCP) due to esophageal motility disorders can occur both in patients with and without coronary artery disease (CAD). This symptom is frequently resistant to treatment with a recurrence rate of about 80%. NCCP significantly decreases patients’ health-related quality of life and may predispose to invasive chest pain diagnostic procedures. However, the influence of diagnosis and treatment of esophageal motility disorders during the course of NCCP in patients both with and without CAD is still unclear. Moreover, the outcome of therapy with calcium channel inhibitors in patients with noncardiac chest pain and/or esophageal motility disorders is ambiguous.
The results of this research were biased by the possibility of chest pain sources overlapping, including those of exercise-provoked angina-like chest pain. This fact should be considered both by cardiologists and gastroenterologists. The other limitations are listed in the article.
This study has shown that the use of exercise as a provocative test may not only help to distinguish cardiac and esophageal chest pain source, but may also offer the possibility of diagnosing exercise-provoked esophageal motility disorders having a favorable outcome with calcium antagonists. Moreover, it was found that calcium antagonists might not show a favorable effect in patients with all hypertensive esophageal motility abnormalities because their efficacy may be limited only to patients with exercise-provoked esophageal dysmotility. This investigation also confirmed a similar prevalence of esophageal disorders in patients both with and without CAD.
The results of this investigation should be the premise for further studies on the use of exercise as a provocative test in NCCP diagnosis and the application of calcium channel inhibitors in the treatment of esophageal motility disorders and NCCP. The outcome may be a change in the diagnostic and therapeutic strategy for patients with recurrent chest pain of suspected noncardiac origin.
Ischemic heart disease is an effect of the imbalance between blood supply and myocardial demand. CAD is a form of heart disease in which myocardial ischemia is due to coronary artery narrowing. Esophageal motility disorder is a condition in which uncoordinated esophageal contractions with increased or decreased pressure amplitude occur. Gastroesophageal reflux disease is a disorder resulting from regurgitation of stomach content into the esophagus.
The introduction is adequate. The materials and methods are really well written and specific. The clinical experiment has been well thought out and conducted. Conclusions are supported by data, and discussion addressed specific points.
Peer reviewer: Piero Marco Fisichella, MD, Assistant Professor of Surgery, Medical Director, Swallowing Center, Loyola University Medical Center, Department of Surgery, Stritch School of Medicine, 2160 South First Avenue, Room 3226, Maywood, IL 60153, United States
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Dickman R, Fass R. Noncardiac chest pain. Clin Gastroenterol Hepatol. 2006;4:558-563. |
| 2. | Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol. 2007;102:1173-1179. |
| 3. | Eslick GD, Coulshed DS, Talley NJ. Diagnosis and treatment of noncardiac chest pain. Nat Clin Pract Gastroenterol Hepatol. 2005;2:463-472. |
| 4. | Eslick GD. Classification, natural history, epidemiology, and risk factors of noncardiac chest pain. Dis Mon. 2008;54:593-603. |
| 5. | Fass R, Dickman R. Non-cardiac chest pain: an update. Neurogastroenterol Motil. 2006;18:408-417. |
| 6. | Katerndahl D. Panic plaques: panic disorder & coronary artery disease in patients with chest pain. J Am Board Fam Pract. 2004;17:114-126. |
| 7. | Panju A, Farkouh ME, Sackett DL, Waterfall W, Hunt R, Fallen E, Somers S, Stevenson G, Walter S. Outcome of patients discharged from a coronary care unit with a diagnosis of “chest pain not yet diagnosed”. CMAJ. 1996;155:541-546. |
| 8. | Mudipalli RS, Remes-Troche JM, Andersen L, Rao SS. Functional chest pain: esophageal or overlapping functional disorder. J Clin Gastroenterol. 2007;41:264-269. |
| 9. | Cooke RA, Anggiansah A, Chambers JB, Owen WJ. A prospective study of oesophageal function in patients with normal coronary angiograms and controls with angina. Gut. 1998;42:323-329. |
| 10. | Budzyński J, Kłopocka M, Pulkowski G, Suppan K, Fabisiak J, Majer M, Swiatkowski M. The effect of double dose of omeprazole on the course of angina pectoris and treadmill stress test in patients with coronary artery disease--a randomised, double-blind, placebo controlled, crossover trial. Int J Cardiol. 2008;127:233-239. |
| 11. | Adamek RJ, Roth B, Zymanski CH, Hagemann D, Pfaffenbach B. Esophageal motility patterns in patients with and without coronary heart disease and healthy controls. Hepatogastroenterology. 1999;46:1759-1764. |
| 12. | Battaglia E, Bassotti G, Buonafede G, Serra AM, Dughera L, Orzan F, Casoni R, Chistolini F, Morelli A, Emanuelli G. Noncardiac chest pain of esophageal origin in patients with and without coronary artery disease. Hepatogastroenterology. 2005;52:792-795. |
| 13. | Bovero E, Torre F, Poletti M, Faveto M, De Iaco F. Exertional gastroesophageal pH-metry: a new provocative physiological test in the diagnosis of chest pain. Gastroenterol Clin Biol. 1993;17:4-8. |
| 14. | Ravi N, Stuart RC, Byrne PJ, Reynolds JV. Effect of physical exercise on esophageal motility in patients with esophageal disease. Dis Esophagus. 2005;18:374-377. |
| 15. | Awad RA, Camacho S. Reference values for stationary and 24-hour ambulatory esophageal manometry and pH data in Hispanic population. Arch Med Res. 2003;34:388-393. |
| 16. | Kim KY, Kim GH, Kim DU, Wang SG, Lee BJ, Lee JC, Park DY, Song GA. Is ineffective esophageal motility associated with gastropharyngeal reflux disease? World J Gastroenterol. 2008;14:6030-6035. |
| 17. | Stein HJ, DeMeester TR, Eypasch EP, Klingman RR. Ambulatory 24-hour esophageal manometry in the evaluation of esophageal motor disorders and noncardiac chest pain. Surgery. 1991;110:753-761; discussion 761-763. |
| 18. | Lacima G, Grande L, Pera M, Francino A, Ros E. Utility of ambulatory 24-hour esophageal pH and motility monitoring in noncardiac chest pain: report of 90 patients and review of the literature. Dig Dis Sci. 2003;48:952-961. |
| 19. | Spencer HL, Smith L, Riley SA. A questionnaire study to assess long-term outcome in patients with abnormal esophageal manometry. Dysphagia. 2006;21:149-155. |
| 20. | Adamek RJ, Bock S, Pfaffenbach B. Oesophageal motility patterns and arterial blood pressure in patients with chest pain and normal coronary angiogram. Eur J Gastroenterol Hepatol. 1998;10:941-945. |
| 21. | Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, Bacher AC, Dobrosielski DA, DeRegis JR, Shapiro EP. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314-320. |
| 22. | Baster T, Baster-Brooks C. Exercise and hypertension. Aust Fam Physician. 2005;34:419-424. |
| 23. | van Nieuwenhoven MA, Brouns F, Brummer RJ. The effect of physical exercise on parameters of gastrointestinal function. Neurogastroenterol Motil. 1999;11:431-439. |
| 24. | Chauhan A, Petch MC, Schofield PM. Cardio-oesophageal reflex in humans as a mechanism for "linked angina". Eur Heart J. 1996;17:407-413. |
| 25. | Makk LJ, Leesar M, Joseph A, Prince CP, Wright RA. Cardioesophageal reflexes: an invasive human study. Dig Dis Sci. 2000;45:2451-2454. |
| 26. | Dobrzycki S, Baniukiewicz A, Korecki J, Bachórzewska-Gajewska H, Prokopczuk P, Musial WJ, Kamiński KA, Dabrowski A. Does gastro-esophageal reflux provoke the myocardial ischemia in patients with CAD? Int J Cardiol. 2005;104:67-72. |
| 27. | Tougas G, Spaziani R, Hollerbach S, Djuric V, Pang C, Upton AR, Fallen EL, Kamath MV. Cardiac autonomic function and oesophageal acid sensitivity in patients with non-cardiac chest pain. Gut. 2001;49:706-712. |
| 28. | Tipnis NA, Rhee PL, Mittal RK. Distension during gastroesophageal reflux: effects of acid inhibition and correlation with symptoms. Am J Physiol Gastrointest Liver Physiol. 2007;293:G469-G474. |
| 29. | Caldwell MT, Byrne PJ, Marks P, Walsh TN, Hennessy TP. Bradykinin, coronary artery disease and gastro-oesophageal reflux. Br J Surg. 1994;81:1462-1464. |
| 30. | Jou CJ, Farber JP, Qin C, Foreman RD. Convergent pathways for cardiac- and esophageal-somatic motor reflexes in rats. Auton Neurosci. 2002;99:70-77. |
| 31. | Eslick GD, Talley NJ. Natural history and predictors of outcome for non-cardiac chest pain: a prospective 4-year cohort study. Neurogastroenterol Motil. 2008;20:989-997. |