Published online Sep 7, 2010. doi: 10.3748/wjg.v16.i33.4193
Revised: June 8, 2010
Accepted: June 15, 2010
Published online: September 7, 2010
AIM: To analyze the possible protective role of Arctium lappa L. (AL) in a murine model of ulcerative colitis (UC).
METHODS: BALB/c mice were administered 100 mg/kg AL powder orally each day. After 7 d, colitis was induced by administration of dextran sulfate sodium (DSS) (5% W/V) in drinking water for a further 8 consecutive days. Diarrhea and bloody stools as well as colonic histology were observed. The level of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in colonic sections were detected by immunohistochemistry.
RESULTS: There were significant differences in mean body weight values and disease activity indices between controls and AL-treated animals. Moreover, the histological findings showed that AL treatment can prevent mucosal edema, submucosal erosions, ulceration, inflammatory cell infiltration and colon damage. In addition, immunohistochemistry analysis showed that the levels of the inflammatory cytokines, IL-6 and TNF-α were also decreased in AL-treated groups.
CONCLUSION: We suggest that AL can prevent intestinal damage and decrease inflammatory cytokines in mice with DSS-induced colitis. Thus, AL could prove to be a useful food for UC.
-
Citation: Huang TC, Tsai SS, Liu LF, Liu YL, Liu HJ, Chuang KP. Effect of
Arctium lappa L. in the dextran sulfate sodium colitis mouse model. World J Gastroenterol 2010; 16(33): 4193-4199 - URL: https://www.wjgnet.com/1007-9327/full/v16/i33/4193.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i33.4193
Ulcerative colitis and Crohn’s disease are chronic inflammatory bowel diseases (IBD). Although these two diseases have some characteristics in common, the exact etiology and pathogenesis of these disorders remain unclear. However, in recent years, epidemiologic and genetic studies in man and particularly, in IBD-related animal models, have suggested that a combination of genetic susceptibility factors and altered immune response driven by microbial factors in the enteric environment contributes to the initiation and chronification of these diseases. On the other hand, there is substantial evidence that intestinal inflammation is likely to depend on cytokines including interleukin (IL)-1, IL-6, IL-12p40, IL-23p19, IL-10, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ)[1]. When chosen appropriately, animal models can be used to investigate pathophysiological mechanisms and are valuable tools for testing emerging therapeutic strategies in the preclinical phase. Hence, animal models of colitis resemble some of the important immunological and histopathological aspects of IBD in humans.
The dextran sulfate sodium (DSS) challenge-induced ulcerative colitis (UC) model has been well characterized morphologically and biochemically. DSS induces an acute colitis characterized by bloody stools, ulcerations and infiltration of inflammatory cells[2]. Histologically, DSS produces submucosal erosions, ulceration, inflammatory cell infiltration and crypt abscesses as well as epithelioglandular hyperplasia. It is generally believed that DSS is directly toxic to gut epithelial cells of the basal crypts and affects the integrity of the mucosal barrier. The lumen bacteria induce the production of inflammatory cytokines, IL-6 and TNF-α, and cause colitis. Hence, the DSS-induced colitis model is particularly useful for studying the contribution of inflammatory mechanisms in colitis.
The “Gobo”, the roots of edible burdock [Arctium lappa L. (AL)], is a food in Asia and contains a higher amount of polysaccharides and residues than other vegetables and is easily obtained all year round. AL has been extensively analyzed due to its reserve and cell-wall polysaccharides[3]. Arctium lappa has been reported to have antimicrobial activity[4] as well as antioxidant activity[5]. The chloroform extract fraction of the roots from AL protects animals from chronic gastric ulceration by reducing gastric acid secretion via inhibition of gastric H+, K+-ATPase[6]. However, the effects of AL on colitis are not fully understood.
The AL extract was able to significantly reduce the release of inflammatory mediators through inhibition of degranulation and cys-leukotriene release[7]. This research indicated that AL has anti-inflammatory activity and may have therapeutic or prophylactic effects on colitis. A mouse model of distal colitis induced by DSS that histologically resembles human UC was used in this study to evaluate the anti-colitis activity of AL powder.
Fresh burdock (Cheer Mean Industrial Co., Ltd.) was sliced with a hand knife and spread in an oven (Gallenkamp, UK) at 60°C for 9 h. The oven-dried AL was then crushed into tiny pieces in a mortar and then pulverized in an osterizer blender (Hitachi, Tokyo, Japan) to produce powdered AL.
BALB/c mice (12 wk old, 24-28 g) were bred under standard conditions and maintained in a 12-h light/12-h dark cycle at 22°C plus or minus 1°C and given food and tap water ad libitum in accordance with Taiwan Office Regulations.
Colitis was induced by modification of the method of DSS-induced colonic inflammation as previously described[2]. Colitis was induced in BALB/c mice by adding DSS (molecular weight: 36-50 kDa; MP Biomedicals) to drinking water at a level of 5% during the experiment period. Water consumption was comparable between the different groups. Mice were monitored daily for weight loss as well as signs of rectal bleeding and diarrhea. The animals received 200 μL of water (control group) or 100 mg/kg per 200 μL AL powder (treatment group) orally each day. After 7 d, colitis was induced by administration of DSS (5% W/V) in drinking water for a further 8 consecutive days. At day 8 of DSS administration, the mice were sacrificed and sections were taken from the colon for histological assessment.
Body weight loss, stool consistency, and blood in the stool were monitored daily to assess the severity of colitis. Body weight, rectal bleeding and stool consistency were monitored daily. Blood in the stool was scored as 0, normal; 2, slight bleeding; and 4, gross bleeding. Diarrhea was scored as 0, normal; 2, loose stools; and 4, watery diarrhea.
For microscopic histological evaluation, formalin-fixed tissues were embedded in paraffin and 5 μm sections were stained with hematoxylin and eosin and evaluated using light microscopy by a pathologist in the Animal Hospital of NPUST who was blinded to the experimental protocol.
For immunohistochemistry evaluation, formalin-fixed tissues were embedded in paraffin and 5 μm sections were preincubated in PBS with 20% goat serum and 0.05% Saponin (Sigma) or 0.5% Triton X-100 for 1 h, and then incubated overnight with primary antibodies diluted in PBS with 2% goat serum at room temperature (10 μg/mL). The following antibodies were used: rat anti-mouse TNF-α (Southern Biotech, Birmingham, AL, USA), rat anti-mouse IL-6 (Southern Biotech, Birmingham, AL, USA). The second antibody step was performed using HRP-conjugated species-specific antibodies (Jackson ImmunoResearch, West Grove, PA, USA), 1:200 for 20-40 min developed in DAB for 10 min. Finally, sections were counter-stained with hematoxylin and mounted. Stained sections were examined and photographed using light microscopy by a pathologist in the Animal Hospital of NPUST who was blinded to the experimental protocol.
Data are expressed as mean ± SD groups of data (histological scores, body weight) were analyzed using Student’s t test.
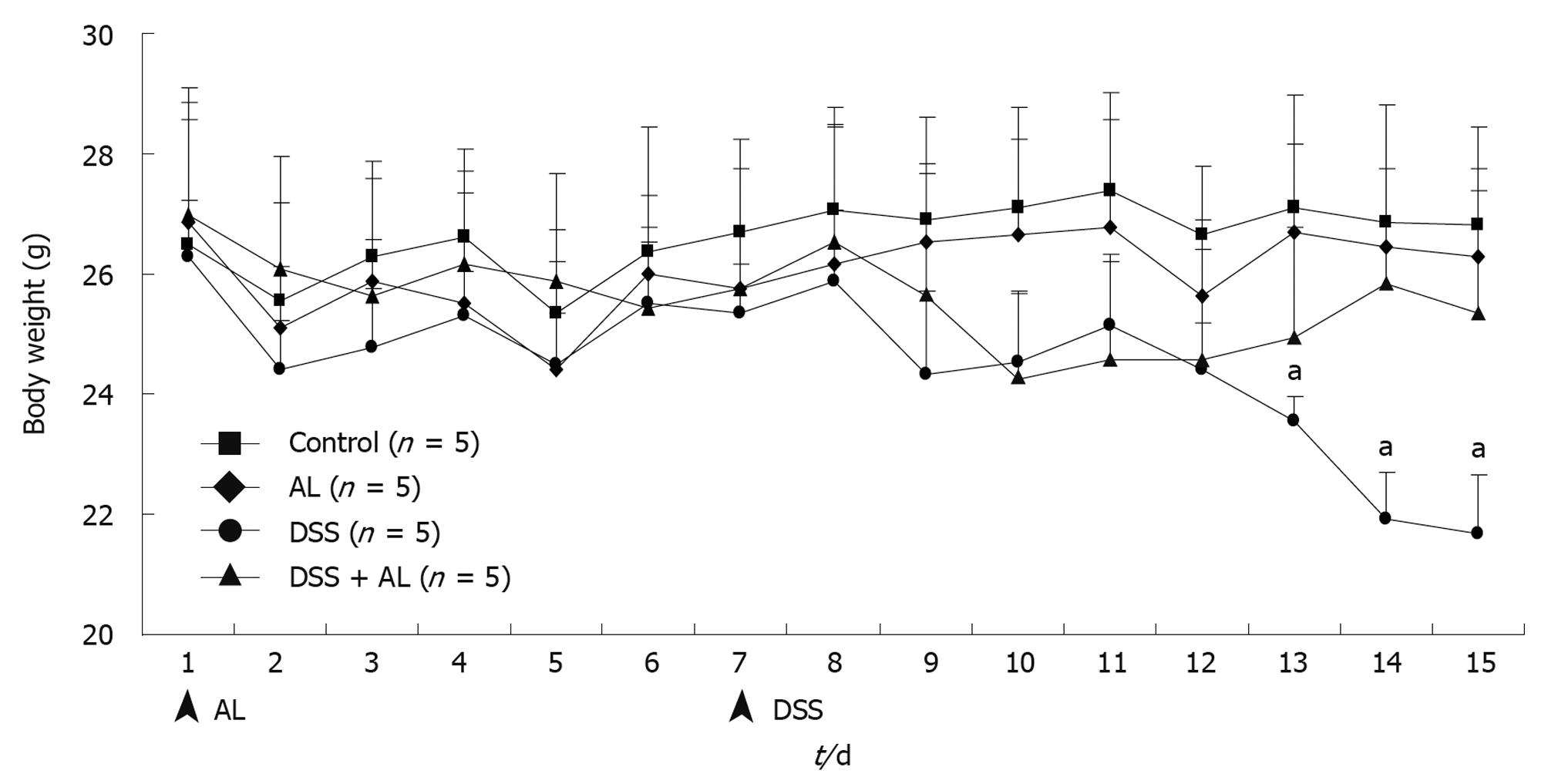
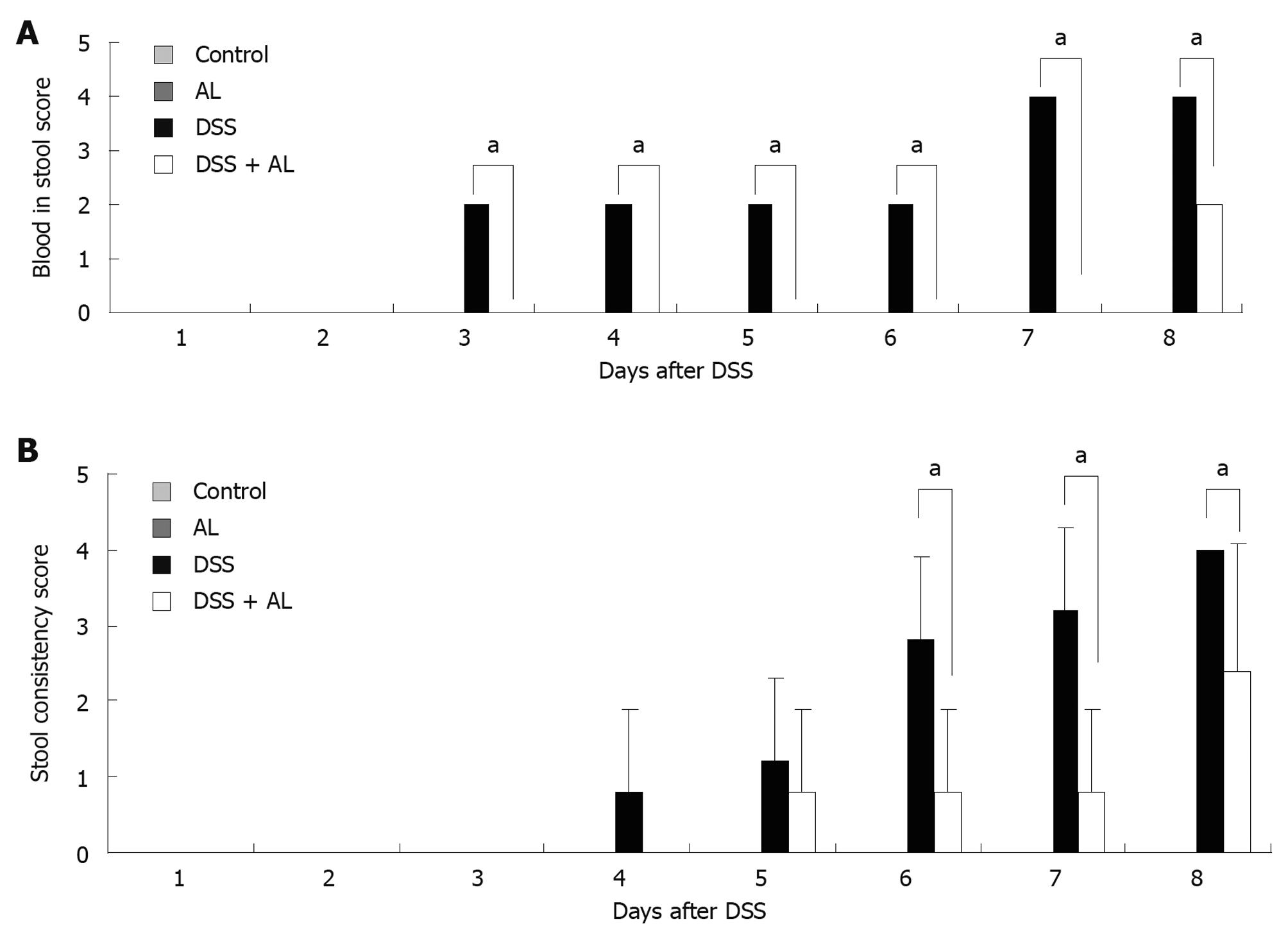
Symptomatic colitis parameters such as weight loss and disease activity index (DAI) score were monitored each day. Mice given 5% DSS in their drinking water for 7 d developed symptoms of colitis without mortality. Compared with vehicle-treated controls, the DSS-administered groups had significantly decreased body weight (Figure 1). Oral AL treatment obviously improved weight loss. Fecal characteristics and fecal occult blood were evaluated individually. Macroscopic examination revealed that no significant morphological changes were observed following water or AL administration. The DSS-administered groups had loose stools or diarrhea, occult or gross rectal bleeding which markedly increased the DAI scores (Figure 2A and B). Oral administration of AL before DSS obviously decreased colonic bloody diarrhea.
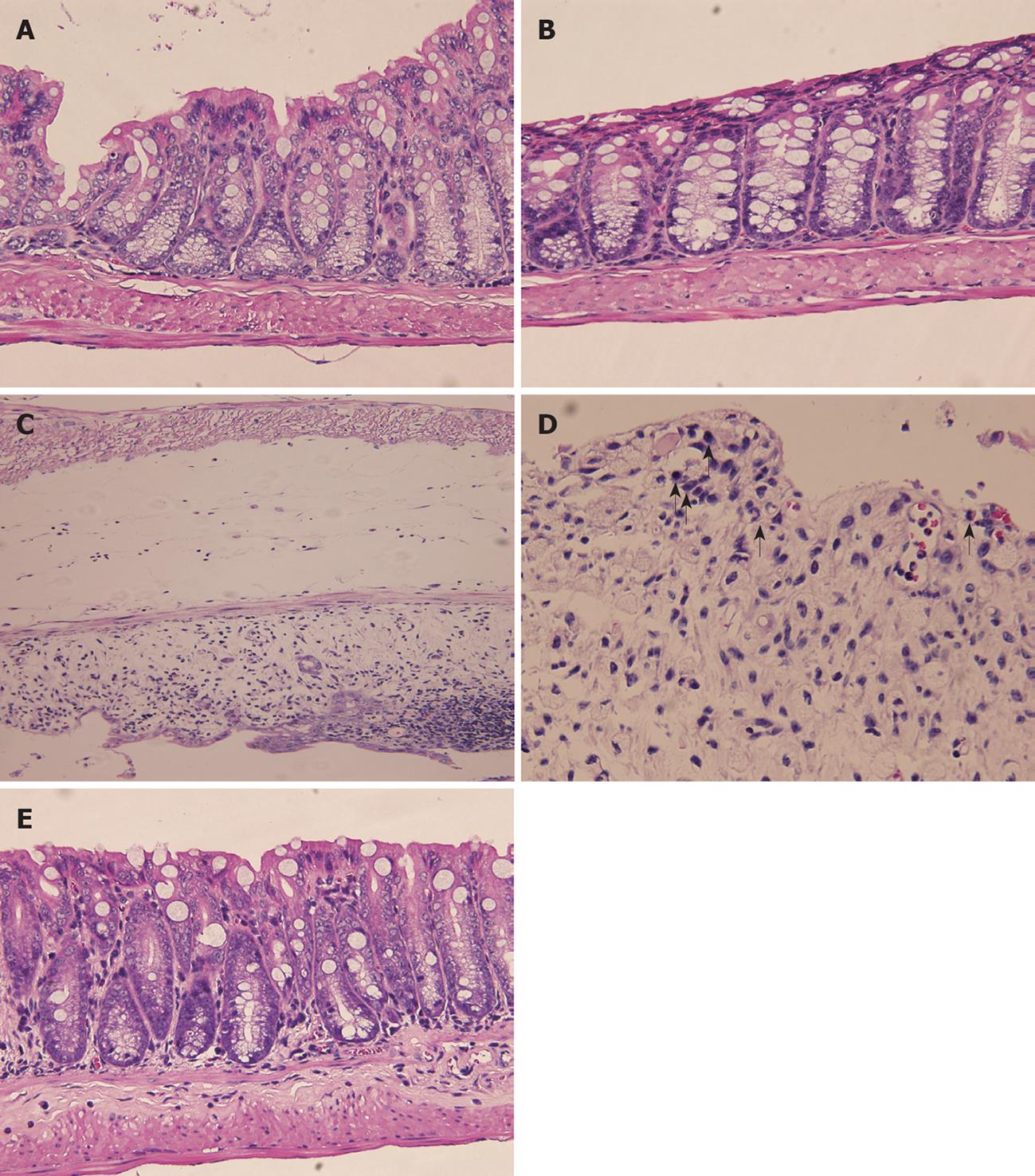
Microscopically, normal colonic tissue architecture is shown in Figure 3A. The control group given water showed no histological alterations as seen in Figure 3C. Similarly, mice given AL exhibited virtually the same normal histology with no inflammatory cell infiltration, edema or crypt abscesses (Figure 3B). Severe submucosal edema, erosion, ulceration, inflammatory cell infiltration and crypt abscesses as well as epithelial glandular hyperplasia were observed in the mucosa of DSS-treated animals (Figure 3C and D). Prior treatment with AL before the DSS challenge produced a slight inflammatory reaction in the colonic mucosa with no submucosal edema or crypt cell abnormalities (Figure 3E). On the other hand, many polymorphonuclear cells and mononuclear cells infiltrated the apical side of DSS-treated mouse intestine (Figure 3D arrows).
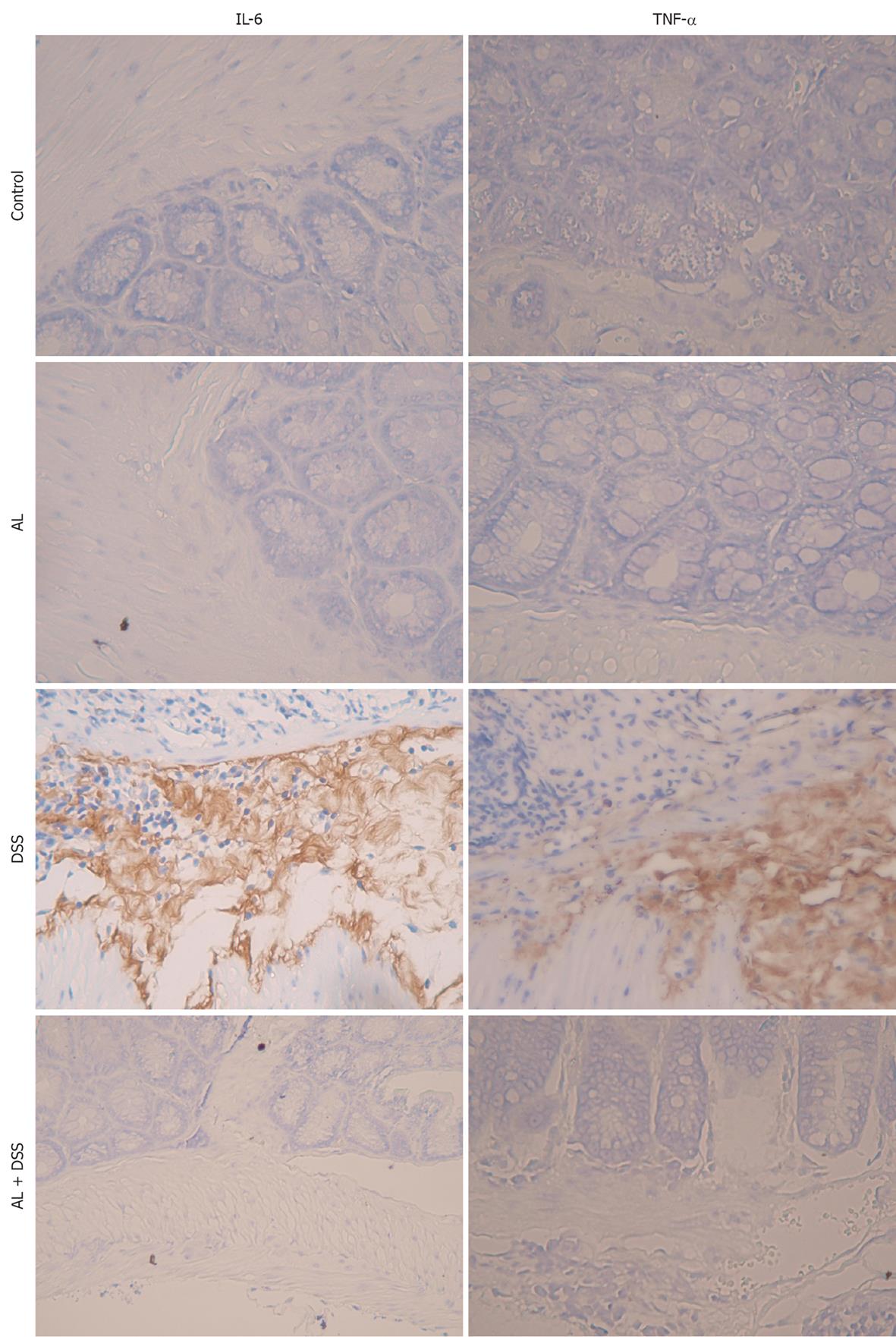
IL-6 and TNF-α are considered important inflammatory mediators that play a key role in the pathogenesis of IBD. To determine the effect of AL on major inflammatory cytokines in the colon, we determined the levels of IL-6 and TNF-α (Figure 4). After 8 d of DSS administration, the levels of IL-6 and TNF-α increased significantly. AL administration prevented significant elevations in IL-6 and TNF-α at day 8. These results indicated that AL has anti-inflammatory effects in the DSS-induced colitis mouse model.
The results presented herein clearly indicate that AL efficiently suppresses DSS-induced colitis in mice without any adverse effects. In this study, we showed that AL prevents body weight loss and an increase in DAI scores in mice with DSS-induced colitis. Furthermore, AL alleviates the symptoms of DSS-induced colitis and improves colitis-induced histological damage by reducing the infiltration of immune cells and the production of various inflammatory cytokines, such as IL-6 and TNF-α. These results suggest that AL has potentially clinically useful anti-inflammatory and therapeutic effects in IBD.
The steroid agent, glucocorticoid, has been used clinically as an anti-inflammatory drug for some time for the treatment of IBD, but is frequently associated with serious side effects, such as liver damage, cancers, stroke, and growth inhibition, and there is a long-standing dilemma regarding the use of clinical steroid anti-inflammatory therapy[8,9]. New anti-inflammatory drugs, as well as prophylactic or therapeutic foods are important for IBD patients. Comprehensive data in previous reports show that IBD are fairly common chronic inflammatory conditions of the gastrointestinal tract. The main pathologic feature of IBD is an infiltration of polymorphonuclear neutrophils and mononuclear cells into the intestinal tissues. Both IL-6 and TNF-α play crucial roles in the DSS-mediated inflammatory response in mice and pigs[10,11]. Mice administered oral AL showed less infiltration of immune cells into the colon epithelium (Figure 3E). AL decreased inflammatory cytokine production and reduced elevated immune cell numbers. The therapeutic effect exhibited by AL may thus be explained by its ability to blunt inflammatory cytokine production in DSS-induced murine IBD.
The mouse model of distal colitis induced by DSS histologically resembles human ulcerative colitis[12]. The exact mechanism of DSS-induced mucosal injury is not fully understood, but a topical toxic effect of DSS on colonic epithelial cells has been proposed[13]. This breach of barrier function would likely result in increased uptake of luminal antigens (bacteria and bacterial products) as well as activation of lamina propria immune cells and the inflammatory response[1,12,14]. Recent studies pointed out the possible effect of inulins in plant materials on the functions of the immune system in relation to the regulation of differentiation and proliferation of intestinal epithelial cells[15-17]. AL is an inulin-rich food and is easily obtained all year round. Moreover, inulin can also stabilize the gut mucosal barrier[18]. In our data, the oral administration of AL powder maintained the architecture of colonic intestinal cells and the mucosal layer (Figure 3).
Oral (1% in drinking water, or 400 mg/d) administration of inulin in rats was found to ameliorate DSS-induced colitis[19]. It was found that daily administration of inulin by the oral route induced an acidic environment (pH < 7.0) from the cecum to the left colon and increased lactobacilli counts. Recently, an increase in the number of fecal bifidobacteria and lactobacilli in the cecal content of rats was reported[20]. These results indicated that a significant increase in propionic, succinic and butyric acid was observed in inulin-fed Sprague-Dawley rats. The authors postulated that oral inulin reduces the severity of DSS-induced colitis mediated by modification of the intracolonic milieu.
In addition to inulin, the role of chlorogenic acid should not be ignored. Chlorogenic acid, one of the most common polyphenols in the human diet, inhibits staphylococcal exotoxin-induced production of IL-1β, TNF, IL-6, INF-γ, monocyte chemotactic protein-l, macrophage inflammatory protein (MIP)-lα, and MIP-lβ in human peripheral blood mononuclear cells[21]. Chlorogenic acid also inhibits lipopolysaccharide (LPS)-induced inflammatory response in RAW 264.7 cells. Shan et al[22] (2009) indicated that chlorogenic acid significantly decreased LPS-induced up-regulation of cyclooxygenase-2 at the protein and mRNA levels resulting in the inhibition of prostaglandin E2 release from LPS-treated RAW 264.7 cells. Further studies showed that LPS-induced activation of nuclear factor-κB and c-JunN-terminal kinase-c-Jun-activator protein-1 pathway were significantly suppressed by chlorogenic acid.
According to these studies, we suggest that both inulins and chlorogenic acid in burdock powder play an important role in the AL-mediated prophylactic effect in DSS-induced colitis, however, the exact mechanisms still need to be investigated further. AL may have multiple functions including anti-inflammatory activity, modification of the content of colonic microorganisms, protection of epithelial cells and stabilization of the mucosal barrier.
In conclusion, oral AL improves body weight loss, histological scores, maintains the colon architecture, and decreases the release of inflammatory mediators in DSS-induced colitis in mice. Our findings may be relevant for future pharmacological or dietary interventions in patients with ulcerative colitis.
Ulcerative colitis and Crohn’s disease (CD) are chronic inflammatory bowel diseases (IBD). Although these two diseases have some characteristics in common, the exact etiology and pathogenesis of these disorders remain unclear. Research has indicated that Arctium lappa L. (AL) has anti-inflammatory activity and may have therapeutic or prophylactic effects on colitis. However, the effects of AL on colitis are not fully understood.
In this study, the authors examined whether AL had an effect on colitis. A mouse model of distal colitis induced by dextran sulfate sodium (DSS) that histologically resembles human ulcerative colitis (UC) was used in this study to evaluate the anti-colitis activity of AL powder.
The results presented herein clearly indicate that AL efficiently suppresses DSS-induced colitis in mice without any adverse effects. In this study, the authors showed that AL prevents body weight loss and an increase in disease activity index scores in mice with DSS-induced colitis. Furthermore, AL alleviates the symptoms of DSS-induced colitis and improves colitis-induced histological damage by reducing the infiltration of immune cells and the production of various inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α.
These findings may be relevant for future pharmacological or dietary interventions in patients with ulcerative colitis.
This study investigated the effect of AL, a vegetable commonly used in Asian, on DSS-induced colitis in mice. It showed that pretreatment of the animals with AL exerted some protective effect on DSS-induced gut damage. This is a study with some practical value and clinical significance.
Peer reviewer: Xiaofa Qin, MD, PhD, Department of Surgery, UMDNJ-New Jersey Medical School, 185 South Orange Avenue, Newark, NJ 07103, United States
S- Editor Wang YR L- Editor Webster JR E- Editor Lin YP
| 1. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. |
| 2. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. |
| 3. | Kato Y, Watanabe T. Isolation and characterization of a xyloglucan from gobo (Arctium lappa L.). Biosci Biotechnol Biochem. 1993;57:1591-1592. |
| 4. | Holetz FB, Pessini GL, Sanches NR, Cortez DA, Nakamura CV, Filho BP. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz. 2002;97:1027-1031. |
| 5. | Lin CC, Lu JM, Yang JJ, Chuang SC, Ujiie T. Anti-inflammatory and radical scavenge effects of Arctium lappa. Am J Chin Med. 1996;24:127-137. |
| 6. | Dos Santos AC, Baggio CH, Freitas CS, Lepieszynski J, Mayer B, Twardowschy A, Missau FC, dos Santos EP, Pizzolatti MG, Marques MC. Gastroprotective activity of the chloroform extract of the roots from Arctium lappa L. J Pharm Pharmacol. 2008;60:795-801. |
| 7. | Knipping K, van Esch EC, Wijering SC, van der Heide S, Dubois AE, Garssen J. In vitro and in vivo anti-allergic effects of Arctium lappa L. Exp Biol Med (Maywood). 2008;233:1469-1477. |
| 8. | Tremaine WJ. Refractory IBD: medical management. Neth J Med. 1997;50:S12-S14. |
| 9. | Maser EA, Deconda D, Lichtiger S, Ullman T, Present DH, Kornbluth A. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol. 2008;6:1112-1116. |
| 10. | Kim CJ, Kovacs-Nolan JA, Yang C, Archbold T, Fan MZ, Mine Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21:468-475. |
| 11. | Kwon KH, Murakami A, Hayashi R, Ohigashi H. Interleukin-1beta targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem Biophys Res Commun. 2005;337:647-654. |
| 12. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. |
| 13. | Ni J, Chen SF, Hollander D. Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut. 1996;39:234-241. |
| 14. | Joo YE, Karrasch T, Mühlbauer M, Allard B, Narula A, Herfarth HH, Jobin C. Tomato lycopene extract prevents lipopolysaccharide-induced NF-kappaB signaling but worsens dextran sulfate sodium-induced colitis in NF-kappaBEGFP mice. PLoS One. 2009;4:e4562. |
| 15. | Kurita-Ochiai T, Fukushima K, Ochiai K. Volatile fatty acids, metabolic by-products of periodontopathic bacteria, inhibit lymphocyte proliferation and cytokine production. J Dent Res. 1995;74:1367-1373. |
| 16. | Kvale D, Brandtzaeg P. Constitutive and cytokine induced expression of HLA molecules, secretory component, and intercellular adhesion molecule-1 is modulated by butyrate in the colonic epithelial cell line HT-29. Gut. 1995;36:737-742. |
| 17. | LeLeiko NS, Walsh MJ. The role of glutamine, short-chain fatty acids, and nucleotides in intestinal adaptation to gastrointestinal disease. Pediatr Clin North Am. 1996;43:451-470. |
| 19. | Videla S, Vilaseca J, Antolín M, García-Lafuente A, Guarner F, Crespo E, Casalots J, Salas A, Malagelada JR. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96:1486-1493. |
| 20. | Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. |
| 21. | Krakauer T. The polyphenol chlorogenic acid inhibits staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol Immunotoxicol. 2002;24:113-119. |
| 22. | Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo L, Yin Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int Immunopharmacol. 2009;9:1042-1048. |