Published online Jul 21, 2010. doi: 10.3748/wjg.v16.i27.3465
Revised: February 22, 2010
Accepted: March 1, 2010
Published online: July 21, 2010
AIM: To establish a predictive algorithm which may serve for selecting optimal candidates for interferon-α (IFN-α) treatment.
METHODS: A total of 474 IFN-α treated hepatitis B virus e antigen (HBeAg)-positive patients were enrolled in the present study. The patients’ baseline characteristics, such as age, gender, blood tests, activity grading (G) of intrahepatic inflammation, score (S) of liver fibrosis, hepatitis B virus (HBV) DNA and genotype were evaluated; therapy duration and response of each patient at the 24th wk after cessation of IFN-α treatment were also recorded. A predictive algorithm and scoring system for a sustained combined response (CR) to IFN-α therapy were established. About 10% of the patients were randomly drawn as the test set. Responses to IFN-α therapy were divided into CR, partial response (PR) and non-response (NR). The mixed set of PR and NR was recorded as PR+NR.
RESULTS: Stratified by therapy duration, the most significant baseline predictive factors were alanine aminotransferase (ALT), HBV DNA level, aspartate aminotransferase (AST), HBV genotype, S, G, age and gender. According to the established model, the accuracies for sustained CR and PR+NR, respectively, were 86.4% and 93.0% for the training set, 81.5% and 91.0% for the test set. For the scoring system, the sensitivity and specificity were 78.8% and 80.6%, respectively. There were positive correlations between ALT and AST, and G and S, respectively.
CONCLUSION: With these models, practitioners may be able to propose individualized decisions that have an integrated foundation on both evidence-based medicine and personal characteristics.
- Citation: Mao QG, Pan JS, Fang KN, Zhang RM, Hong QY, Song MN, Zhu JP, Huang WQ, Chen LM, Hong MZ. Precise prediction model and simplified scoring system for sustained combined response to interferon-α. World J Gastroenterol 2010; 16(27): 3465-3471
- URL: https://www.wjgnet.com/1007-9327/full/v16/i27/3465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i27.3465
Chronic hepatitis B (CHB) is one of the most refractory diseases that mankind faces and is a serious global public health problem. Hepatitis B virus (HBV) infection often leads to acute or chronic hepatitis B, hepatocellular carcinoma (HCC) and other complications. Of approximately 350 million carriers of HBV worldwide, about 1 million die from chronic complications, such as cirrhosis, HCC or both every year[1]. According to Liu et al[2], there is a 9% rate of HBV surface antigen (HBsAg) in the general population in China. In the United States, there are an estimated 1.25 million HBV carriers[3]. Currently, available antiviral options can be divided into 2 types, interferon-α (IFN-α) and nucleoside/nucleotide analogues, including conventional IFN-α, pegylated IFN-α (PEG-IFN), lamivudine, adefovir dipivoxil, entecavir, telbivudine, etc. IFN-α is one of the major choices; however, some factors greatly hinder its wide range of applications. First of all, the expense of antiviral therapy is considerable in either underdeveloped or developing countries; in addition, there are several side effects, such as fatigue, flu-like syndrome and others; ultimately, the most important aspect is that only a proportion of patients may achieve a response after therapy[4,5]. Thus, antiviral therapy is not generally accepted by patients.
Predicting the efficacy of IFN-α is crucial before attempting treatment for CHB patients. Some factors, such as HBV genotype A or B, lower viral load, higher serum alanine aminotransferase (ALT) levels, higher grading (G) of intrahepatic inflammation, and lower staging (S) of liver fibrosis, have been identified to be the predictors of the outcome of IFN-α therapy in HBV e antigen (HBeAg)-positive patients[3,6,7]. Female gender, short course of disease, having mild liver fibrosis, having good compliance with therapy, absence of co-infection and an early virological response at the 12th wk also indicate a good therapeutic outcome[8]. Sometimes, however, patients do not have all the “positive” predictors. They may also have one or more “negative” predictors. For example, a patient infected by HBV of genotype C, has a high viral load, accompanied by high ALT and high G. Is he suitable for IFN-α treatment? As pointed out by the European Association for the Study of the Liver, the HBV genotype has a poor individual predictive value, and currently, genotype alone should not define the choice of treatment[7]. These types of issues may be challenging for both practitioners and patients. Owing to the trials that had rigorous designs, many patients have benefited from evidence-based medicine developed in the last several decades. However, evidence-based medicine aims at the resolution of issues which came from individuals with a common background whereas individual information is not always taken into account. The current research aims at making a sensible decision that has an integrated foundation in both evidence-based medicine and personal characteristics.
We therefore conducted the present study to determine (1) baseline predictive factors for the response to IFN-α; (2) what was the relationship between these predictive factors; (3) whether a predictive algorithm for IFN-α treatment of CHB can be derived from these factors; and (4) what was the efficacy of the model.
During the period between July 2005 and November 2008, all HBeAg-positive CHB patients were followed up for their response to IFN-α if they initially started IFN-α treatment in our Liver Division, the 174th Hospital of the PLA, the Traditional Chinese Medicine Hospital of Xiamen, Zhongshan Hospital Xiamen University, Xiamen, or Macheng Hospital, Hubei. Patients were recruited according to the guidelines in China[8], and were administrated with 5 MU of conventional IFN-α every other day for 24 wk or longer. The patients’ baseline information was collected, including age, gender, blood tests, G, S, HBV DNA, genotype, etc. Several studies have indicated the potential benefits of extended duration of IFN-α or PEG-IFN therapy regarding a sustained response[9,10] or suppression of chronic complications[11]. Thus, duration was also recorded for balancing the effect of therapy span. Patients were excluded if they had HCC on presentation or other concomitant diseases including hepatitis A, C or D virus infection, autoimmune hepatitis, Wilson’s disease, primary biliary cirrhosis and alcoholic liver disease. Patients with the following conditions were also excluded: pregnancy, mental disorders (such as severe depression), uncontrolled epilepsy, alcohol abuse, narcotic abuse, uncontrolled autoimmune disorders, decompensated liver cirrhosis, symptomatic heart disease, neutrophil count below 1.0 × 109/L and/or platelet count below 50 × 109/L before treatment, had received or were receiving any other form of established treatment for CHB. Finally, 474 patients were included in the current study. For the treatment of HBeAg-positive CHB, a combined response (CR) was defined as ALT levels returning to normal, undetectable HBV DNA, and HBeAg seroconversion; partial response (PR) was defined as ALT levels returning to normal, HBV DNA < 105 copies/mL, but no seroconversion; whereas non-response (NR) refers to no CR or PR observations[8]. The mixed set of PR and NR was recorded as PR+NR. A sustained response was defined as the response at the 24th wk after cessation of IFN-α treatment.
Patients were followed up every 1-2 mo by monitoring HBsAg status, HBeAg/anti-HBe status, HBV DNA level, ALT, aspartate aminotransferase (AST), α-fetoprotein (AFP), complete blood count and mental status. Complete blood counts were taken once every 1-2 wk for the first month, then once per month until cessation of treatment. Other tests, such as thyroid function, blood glucose, routine urinalysis, were taken once every 3 mo. For patients who had abnormal thyroid function at baseline, appropriate therapy was initiated, and thyroid function was closely monitored during antiviral therapy. If there was evidence of a depressive disorder or suicidal tendency, treatment was stopped and patients were closely monitored. Ultrasound of the liver was scheduled for patients with AFP levels greater than 20 ng/mL. Patients were suggested to stop IFN-α administration if CR or NR occurred after therapy for 24 wk, or if severe side effects developed during the course of treatment. Patients’ choices were also taken into account.
Sera from patients on presentation were taken for the following tests: (1) HBV genotyping performed by the polymerase chain reaction (PCR)-fluorescence detection kit for HBV genotype B, C according to the manufacturer’s instructions (Bioselex, Hangzhou, China); and (2) HBV DNA levels were determined by quantitative fluorescence PCR on the ABI 7000 (Applied Biosystems), with a lower limit of detection of 1000 copies/mL. HBV DNA levels below the lower detection limit were regarded as negative for statistical calculations.
Statistical analyses were performed using version R 2.8.1 (a language and environment for statistical computing, Vienna, Austria, ISBN 3-900051-07-0, http://www.R-project.org). The inter-variable correlation was determined by the Spearman rank correlation coefficient. The Gini index based on random forest methodology was used to determine whether the identified variables were associated with therapy outcomes. In the present study, the response to IFN-α treatment (dependent variable) was ordinal data. If the independent variable was ordinal data (such as ALT, AST, G, S, etc.), Kendall’s tau-b test was adopted to test the statistical significance between independent variable and dependent variable. For the nominal independent variable (gender, genotype, etc.), the Pearson χ2 test was used.
About 10% of patients were randomly selected as the test set, and the remaining patients were employed as the training set. The predictive model was constructed with a support vector machine (SVM) package for the R platform. Accuracies for CR and PR+NR in the training set and test set were calculated. The above process was repeated 300 times and mean accuracy was calculated. Performance of the constructed predictive algorithm was evaluated by the mean accuracies for CR and PR+NR for the training set and test set. The scoring system for sustained CR (SCR) was derived from our observations (Table 1) with computer-aided minor adjustment according to other data[3,7,12,13]. The weight for every level in each factor was rounded to the nearest integer.
| Factor | n (range) |
| Sex (M:F) | 345:129 |
| Age (yr) | 29.8 (10-58) |
| ALT (U/L) | 250 (16-1908) |
| AST (U/L) | 146 (24-1304) |
| Genotype (A:B:C)1 | 51:212:211 |
| HBV DNA (log copies/mL) | 7.35 (5.00-9.83) |
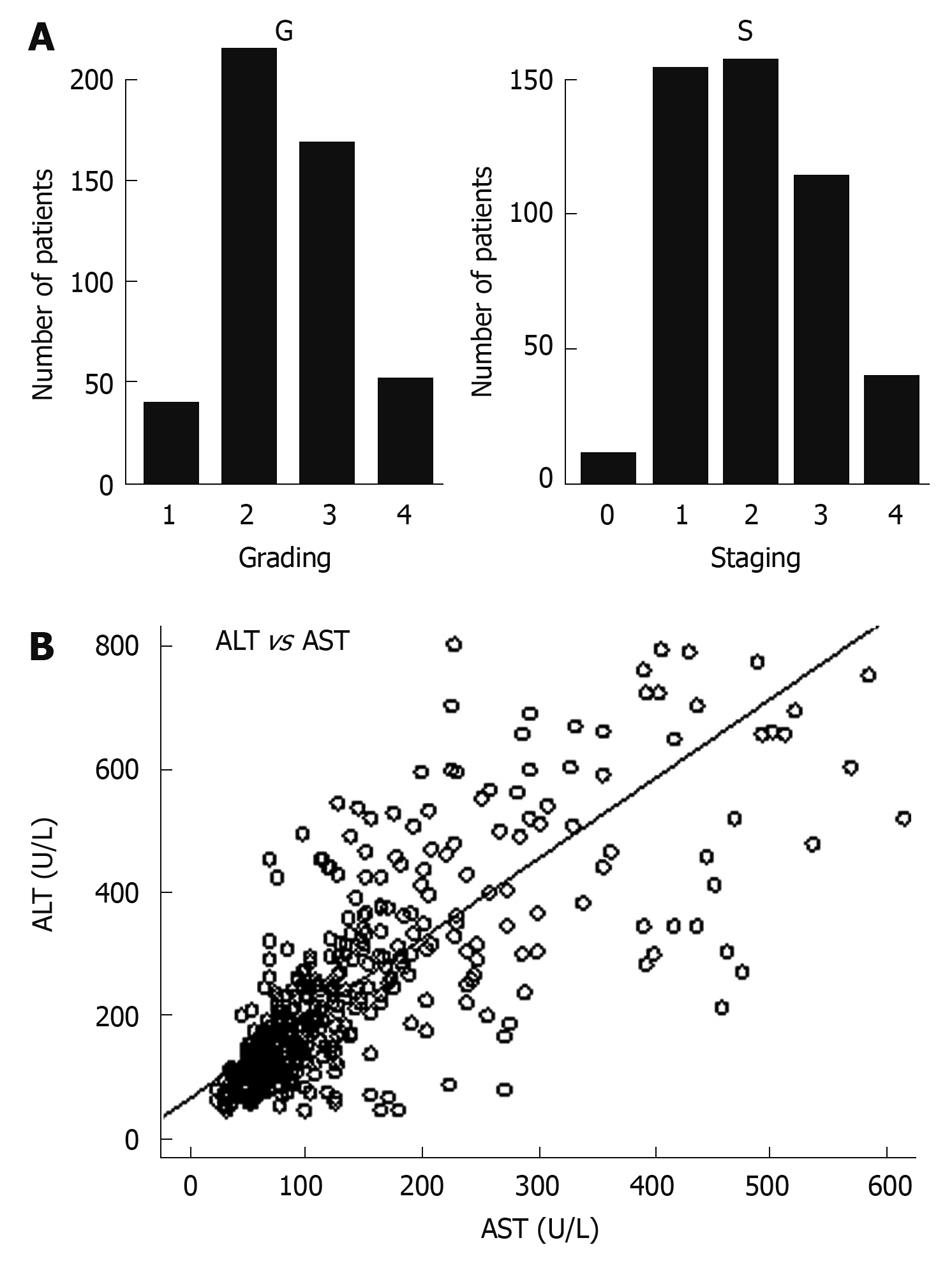
| Fibrosis staging, S (0:1:2:3:4) | 10:154:157:114:39 |
| Histology activity index, G (1:2: 3:4) | 39:215:169:51 |
The area under the curve (AUC) was then calculated for measuring the overall prediction accuracy. A 95% confidence interval (CI) for an AUC was obtained by sampling the 474 patients for 1000 bootstrap samples with the confidence limits calculated as the 2.5th and 97.5th percentiles. The scoring system was assessed by the leave-one-out cross-validation in order to assess the performance of new data[14]. To ease clinical employment of the SCR score, cut-off values were determined by maximizing the Youden index, i.e. sensitivity + specificity - 1, calculated from the receiver operating characteristic curves analysis. Accuracy of using the optimal cut-off values was assessed by the sensitivity, specificity, predictive values and likelihood ratios. Their 95% CIs were obtained from 1000 bootstrap samples. The cut-off values were also cross-validated by the leave-one-out method.
A total of 474 CHB patients were enrolled. The baseline demographics, liver function tests, liver biochemistry, histology data and virological data are listed in Table 1. As shown in Table 2, the ratios of CR, PR and NR at the 24th wk after cessation of IFN-α therapy were 34.4%, 45.1% and 20.5%, respectively. It should be pointed out that genotype A in the current research refers to co-infection of genotype B and C, and other genotypes beside B and C.
| CR | PR | NR | |
| Sex (M:F) | 110:53 | 163:51 | 72:25 |
| Age [0-14):(15-24):(25-44):(≥ 45), yr] | 3:64:123:5 | 5:47:148:14 | 2:21:71:3 |
| ALT [(1-2):(2-3):(3-5):(5-10):(≥ 10), ULN] | 7:5:27:75:49 | 27:41:71:44:31 | 19:31:31:13:3 |
| AST [(0-1):(1-2):(2-3):(3-5):(5-10):(≥ 10), ULN] | 1:22:30:49:43:18 | 12:76:54:38:24:10 | 9:50:18:14:6:0 |
| Genotype (A:B:C) | 14:96:53 | 30:101:83 | 7:15:75 |
| HBV DNA [(5-5.99):(6-6.99):(7-7.99):(8-8.99):(≥ 9), log copies/mL] | 21:55:56:26:5 | 19:65:79:49:2 | 5:31:37:21:3 |
| Fibrosis staging, S (0:1:2:3:4) | 2:54:51:48:8 | 7:68:71:50:18 | 1:32:35:16:13 |
| Histology activity index, G (1:2:3:4) | 11:71:60:21 | 22:95:77:20 | 6:49:32:10 |
| Responses (CR:PR:NR) | 163 | 214 | 97 |
As shown in Table 2, female patients had a higher chance of a CR compared to male patients (41.1% vs 31.9%, P < 0.001; Kendall’s tau-b test). Genotype B had a preferential effect on CR (45.3% and 25.1% for genotype B and genotype C, respectively, P < 0.001, Pearson χ2 test). ALT and AST had a positive reciprocal relationship with treatment response (P < 0.001; Kendall’s tau-b test) (Table 2).
The correlations between variables were determined by the Spearman rank correlation coefficient. As shown in Table 3, there were positive reciprocal relationships between G and S (0.74, P < 0.01), ALT and AST (0.73, P < 0.01). Baseline ALT (0.39, P < 0.01), AST (0.35, P < 0.01) and genotype (0.25, P < 0.01) had a substantial predictive effect on the sustained response. The correlations between G and S, ALT and AST are illustrated in Figure 1A and B.
| Gender | Age | Grading | Staging | ALT | AST | DNA1 | Genotype | Duration | Y F6 m2 | |
| Gender | 1.00 | |||||||||
| Age | 0.06 | 1.00 | ||||||||
| Grading | -0.11a | 0.05 | 1.00 | |||||||
| Staging | -0.06 | 0.08 | 0.74b | 1.00 | ||||||
| ALT | 0.05 | -0.01 | 0.13b | 0.02 | 1.00 | |||||
| AST | -0.13b | -0.04 | 0.25b | 0.17b | 0.73b | 1.00 | ||||
| DNA1 | -0.09 | 0.04 | -0.04 | -0.10a | 0.07 | 0.09a | 1.00 | |||
| Genotype | 0.09a | 0.00 | -0.01 | 0.01 | 0.09 | 0.03 | -0.12b | 1.00 | ||
| Duration | 0.05 | -0.03 | -0.02 | 0.03 | 0.00 | -0.04 | 0.03 | 0.02 | 1.00 | |
| Y F6 m | 0.05 | -0.01 | -0.03 | 0.00 | -0.39b | -0.35b | 0.06 | 0.25b | -0.07 | 1.00 |
Stratified by duration of IFN-α therapy, Gini index analysis showed that baseline predictors, from highly significant to least significant, were ALT, HBV DNA in log copies/mL, AST, genotype, S, G, age and gender (Table 4).
| Variable | CR | PR | NR | Mean decrease accuracy | Mean decrease Gini |
| ALT | 2.242 | 0.860 | 2.611 | 1.363 | 42.806 |
| Duration | 0.536 | 0.599 | 0.251 | 0.507 | 36.340 |
| HBV DNA | 0.603 | 0.742 | 0.015 | 0.553 | 35.713 |
| AST | 1.045 | -0.243 | 0.955 | 0.502 | 35.488 |
| Genotype | 1.101 | 1.083 | 3.095 | 1.269 | 30.462 |
| Staging | 0.737 | -0.058 | -0.147 | 0.205 | 29.400 |
| Grading | -0.033 | 0.330 | -0.035 | -0.182 | 23.283 |
| Age | -0.069 | 0.094 | 0.160 | 0.050 | 20.944 |
| Gender | -0.125 | 0.478 | -0.084 | 0.186 | 14.396 |
Based on SVM, a predictive model was developed for the SCR to IFN-α therapy. According to the established model, the accuracies for SCR and PR+NR respectively were 86.4% and 93.0% for the training set, 81.5% and 91.0% for the test set.
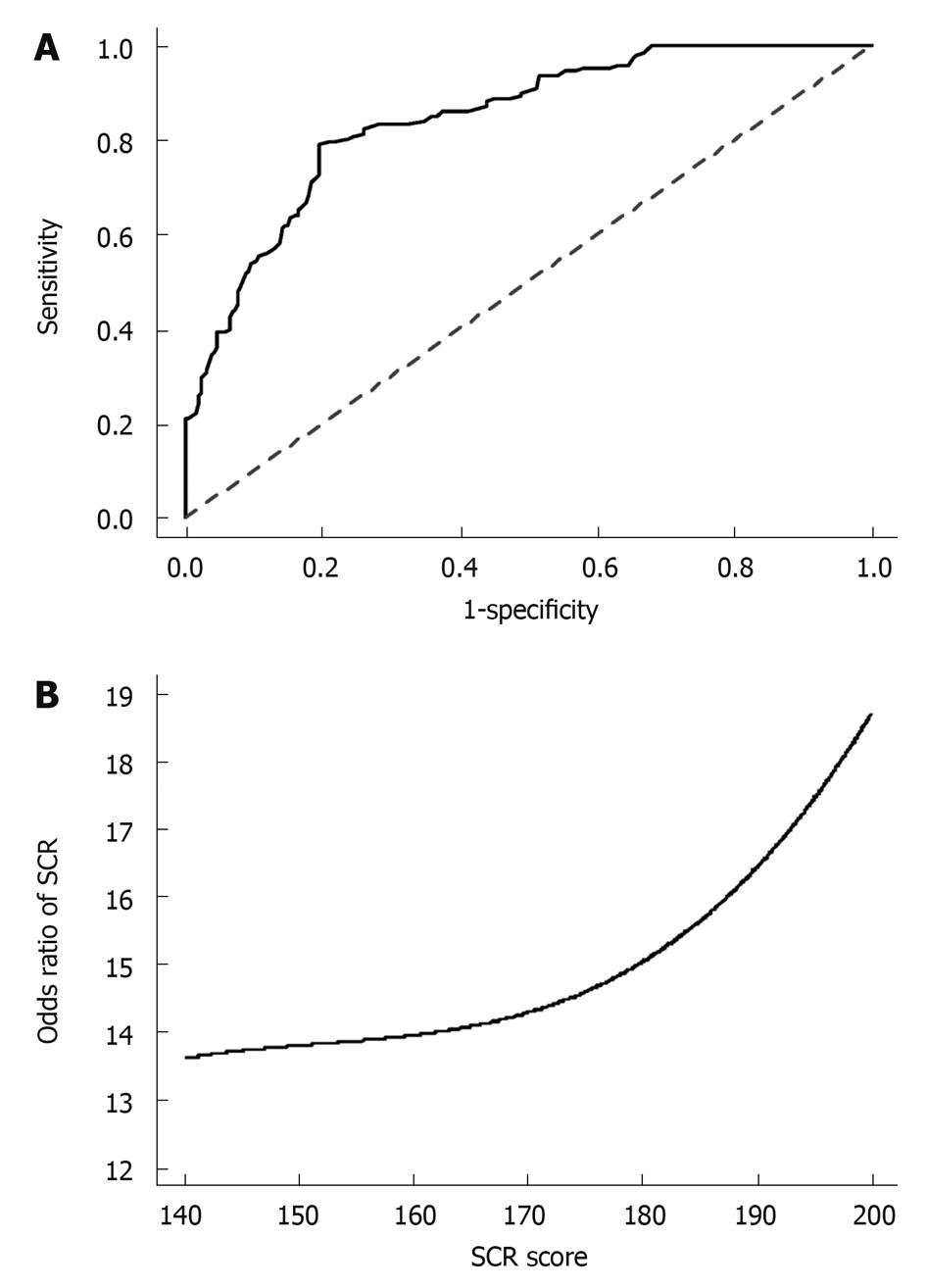
Based on our data provided in Table 2 with computer-aided minor adjustment according to other data[3,7,12,13], a predictive scoring system was developed for the SCR to IFN-α therapy. The odds ratio of SCR score was 15.25 (95% CI: 9.65-24.68, P < 0.001) indicating that the scoring system had an excellent prediction performance. By optimizing with the Youden’s index, the optimal cut-off for the prediction of SCR was 169. This cut-off had good sensitivity and specificity and had been accurately validated by the leave-one-out validation (Table 5). The AUCs were as high as 0.797 (95% CI: 0.773-0.812) for SCR prediction (Figure 2A). The odds ratio of SCR according to the scoring system is depicted in Figure 2B.
| Value | 95% CI | |
| Total study population | ||
| Optimal cut-off | 169 | |
| Sensitivity (%) | 78.79 | 71.93-84.33 |
| Specificity (%) | 80.58 | 75.81-84.61 |
| Positive predictive value (%) | 68.42 | 61.50-74.61 |
| Negative predictive value (%) | 87.68 | 83.34-91.00 |
| Positive likelihood ratio | 4.06 | 3.19-5.16 |
| Negative likelihood ratio | 0.26 | 0.20-0.36 |
| Odds ratio | 15.25 | 9.65-24.68 |
| Accuracy (%) | 79.96 | 76.12-83.31 |
| Youden index | 0.594 | 0.5913-0.5961 |
| AUC | 0.797 | 0.773-0.812 |
| Leave-one-out cross-validation | ||
| Optimal cut-off | 169 | |
| Sensitivity (%) | 78.18 | 71.28-83.80 |
| Specificity (%) | 79.94 | 75.11-84.02 |
| Positive predictive value (%) | 67.54 | 60.61-73.78 |
| Negative predictive value (%) | 87.28 | 82.89-90.67 |
| Positive likelihood ratio | 3.90 | 3.08-4.94 |
| Negative likelihood ratio | 0.27 | 0.20-0.37 |
| Odds ratio | 14.13 | 8.98-22.73 |
| Accuracy (%) | 79.32 | 75.45-82.73 |
| Youden index | 0.581 | 0.5787-0.5836 |
| AUC | 0.79 | 0.779-0.807 |
In summary, the main findings and results of the current research include: (1) development of an accurate predictive model for the SCR to IFN-α therapy; (2) deduction of a scoring system for SCR as the data mining method may be unavailable to most the clinicians; (3) identification of positive reciprocal relationships between G and S, ALT and AST; (4) a predictive role of baseline ALT, AST and genotype for SCR; and (5) baseline predictive factors, listed from the greatest to the least significant, are ALT, HBV DNA, AST, genotype, S, G, age and gender.
The predictive factors for the response to IFN-α therapy have been extensively investigated; however, response prediction for individual patients remains uncertain. First, evidence-based medicine aims at a therapeutic strategy for patients with a similar background rather than a given patient. Additionally, a given individual may have “positive” predictive factors and “negative” predictive factors at the same time. Thus, patients or even clinicians may be perplexed by the probability estimation of therapy outcome. This leads to significant profligacy of health resources and a delay in treating patients who need an appropriate antiviral intervention. Fortunately, the present study may facilitate the management of these difficulties. One of the limitations of the current study is that most of the genotypes are B and C, or co-infection of B and C, which is in accordance with the report by Zeng et al[15]. There are not enough patients infected by other genotypes of HBV for statistical analysis. Another limitation is that treatment with conventional IFN-α rather than PEG-IFN was evaluated in the present research. Although PEG-IFN was extensively prescribed in developed countries, its application was greatly hindered by its high cost in developing countries. In China, there is great disparity in the prescription costs of IFN-α and PEG-IFN. We cannot recruit enough patients administrated with PEG-IFN for statistical analysis. However, IFN-α and PEG-IFN share the same bioactive molecule in vivo and similar baseline predictors[16]. Therefore, using our scoring system, which was easily employed in clinical practice, the response to PEG-IFN therapy may be predicted with reasonable accuracy. If statistical packages were available, higher predictive accuracy could be achieved.
In line with several studies[12,17], genotypes B or C have dramatically different effects on treatment response. Apart from genotypes, the present study also found that increasing HBV DNA levels were associated with a stepwise decrease in the response, which was similar to that of S; in contrast, increasing ALT, AST and G played an opposite role. Patients with higher ALT, AST and G tended to have better outcomes. According to the inter-variable correlation analysis, there were significantly positive reciprocal relationships between ALT and AST, G and S, respectively. The correlation between ALT and AST sounds reasonable, which may be a result of parallel release of intracellular contents after immune injury. Repeated intrahepatic immunologic inflammation triggers fibrinogen secretion from hepatic stellate cells and eventually leads to cirrhosis, which may partly explain the correlation between G and S. The performed study indicated the most important predictive factor was baseline ALT, followed by HBV DNA level, AST, genotype, S, G, age and gender. Interestingly, the Gini index of S was slightly higher than that of G, which may be due to the higher variation of G than that of S though they have a high correlation as mentioned before. Baseline AST also had a considerable predictive role for SCR. Although ALT is regarded as a specific index for HBV-induced inflammation, AST rather than ALT manifests a correlation with fibrosis stage[18]. Data by Brook et al[19] also indicated AST > 85 U/L is a predictive factor for the response to IFN-α therapy.
We developed a predictive model that was shown to have parallel accuracies for both a training set and test set by adjusting kernel parameters, which ensured that satisfactory sensitivity may be achieved for samples out of the observation pool. In other words, the established model would have a reasonable predictive accuracy for patients who were not enrolled in the current research. In addition, a scoring system for SCR was developed to identify patients who may have SCR if the score was greater or equal to the optimal cut-off value of 169. This score was validated by the stringent leave-one-out statistical analysis with high sensitivity and specificity of 78.2% and 79.9%, respectively, for the prediction of SCR. Using these SCR scores, the practitioner can calculate the prognosis of a patient on presentation, which is important for devising individual management of the patient. The practitioner can also identify very high-risk patients who should be recommended for treatment by nucleoside/nucleotide analogues to obtain good results.
In clinical practice, we are not aware of any predictive score for the SCR to IFN-α therapy in HBeAg-positive CHB patients with the integration of potential predictive factors. Our novel predictive algorithm and SCR score may serve as an excellent reference for clinicians to decide who should undergo IFN-α therapy. With these models, practitioners would be able to propose individualized treatment paradigms that have an integrated foundation in both evidence-based medicine and personal characteristics. It has extensive potential clinical use to identify CHB patients who have a high potential of a SCR to IFN-α therapy. These patients should be suggested to be treated by IFN-α to delay or prevent lethal complications of CHB such as liver cirrhosis and HCC.
Predicting the efficacy of interferon-α (IFN-α) is crucial before attempting treatment of chronic hepatitis B (CHB) patients. Though predictors of the responses to IFN-α have been well identified, patients frequently have “positive” and “negative” predictors at the same time. Therefore, it is very difficult to predict the treatment response before IFN-α therapy for a specific patient.
Evidence-based medicine aims at the resolution of issues which came from individuals with a common background whereas individual information can not always be taken into account. The current research aims at sensible decision-making that has an integrated foundation on both evidence-based medicine and personal characteristics.
The current research successfully integrated the patients’ personal characteristics into the foundations of evidence-based medicine. A precise prediction model and a simplified scoring system for a sustained combined response (SCR) to IFN-α were generated.
It has extensive clinical use to identify CHB patients who have a high potential of a SCR to IFN-α therapy. With these predictive models, practitioners would be able to propose individualized treatments that have an integrated foundation in both evidence-based medicine and personal characteristics.
This is an interesting report of a prediction model and simplified scoring system for SCR to IFN.
Peer reviewer: Toru Ishikawa, MD, Department of Gastroenterology, Saiseikai Niigata Second Hospital, Teraji 280-7, Niigata, Niigata 950-1104, Japan
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH
| 1. | Hepatitis B. Fact sheet WHO/204. Geneva: World Health Organization, October 2000. Accessed on Nov 25 2009; Available from: http://www.who.int/mediacentre/factsheets/fs204/en/index.html. |
| 3. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. |
| 4. | Lampertico P, Del Ninno E, Manzin A, Donato MF, Rumi MG, Lunghi G, Morabito A, Clementi M, Colombo M. A randomized, controlled trial of a 24-month course of interferon alfa 2b in patients with chronic hepatitis B who had hepatitis B virus DNA without hepatitis B e antigen in serum. Hepatology. 1997;26:1621-1625. |
| 5. | Pastore G, Santantonio T, Milella M, Monno L, Mariano N, Moschetta R, Pollice L. Anti-HBe-positive chronic hepatitis B with HBV-DNA in the serum response to a 6-month course of lymphoblastoid interferon. J Hepatol. 1992;14:221-225. |
| 6. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. |
| 7. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. |
| 8. | Guideline on prevention and treatment of chronic hepatitis B in China (2005). Chin Med J (Engl). 2007;120:2159-2173. |
| 9. | Luo K, Mao Q, Karayiannis P, Liu D, Liu Z, Zhou Y, Feng X, Zhu Y, Guo Y, Jiang R. Tailored regimen of interferon alpha for HBeAg-positive chronic hepatitis B: a prospective controlled study. J Viral Hepat. 2008;15:684-689. |
| 10. | Gish RG, Lau DT, Schmid P, Perrillo R. A pilot study of extended duration peginterferon alfa-2a for patients with hepatitis B e antigen-negative chronic hepatitis B. Am J Gastroenterol. 2007;102:2718-2723. |
| 11. | Lampertico P, Del Ninno E, Viganò M, Romeo R, Donato MF, Sablon E, Morabito A, Colombo M. Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology. 2003;37:756-763. |
| 12. | Zhao H, Kurbanov F, Wan MB, Yin YK, Niu JQ, Hou JL, Wei L, Wang GQ, Tanaka Y, Mizokami M. Genotype B and younger patient age associated with better response to low-dose therapy: a trial with pegylated/nonpegylated interferon-alpha-2b for hepatitis B e antigen-positive patients with chronic hepatitis B in China. Clin Infect Dis. 2007;44:541-548. |
| 13. | Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol. 2008;49:634-651. |
| 14. | Hastie T, Tibshirani R, Friedman J. The elements of statistical learning. 1st ed. New York: Springer 2001; 214-217. |
| 15. | Zeng G, Wang Z, Wen S, Jiang J, Wang L, Cheng J, Tan D, Xiao F, Ma S, Li W. Geographic distribution, virologic and clinical characteristics of hepatitis B virus genotypes in China. J Viral Hepat. 2005;12:609-617. |
| 16. | Piratvisuth T. Reviews for APASL guidelines: immunomodulator therapy of chronic hepatitis B. Hepatol Int. 2008;2:140-146. |
| 17. | Flink HJ, van Zonneveld M, Hansen BE, de Man RA, Schalm SW, Janssen HL. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am J Gastroenterol. 2006;101:297-303. |
| 18. | Chen YP, Dai L, Wang JL, Zhu YF, Feng XR, Hou JL. Model consisting of ultrasonographic and simple blood indexes accurately identify compensated hepatitis B cirrhosis. J Gastroenterol Hepatol. 2008;23:1228-1234. |
| 19. | Brook MG, Karayiannis P, Thomas HC. Which patients with chronic hepatitis B virus infection will respond to alpha-interferon therapy? A statistical analysis of predictive factors. Hepatology. 1989;10:761-763. |