INTRODUCTION
Epidemiological and intervention studies in both humans and animals have shown that regular consumption of fruits, vegetables, and tea is associated with decreased risk of cancer[1]. Fruits, vegetables, and tea provide essential nutrients and many diet-derived phenolics, in particular flavonoids, which have been demonstrated to exert potential anticarcinogenic activities[2]. Chrysin (5,7-dihydroxyflavone, ChR), a natural and biologically active flavone extracted from many plants, honey, and bee propolis, has been shown to inhibit cell proliferation and induce apoptotic cell death in a variety of cancer cells. Investigations into the molecular mechanisms underlying the inhibition of cell proliferation and induction of apoptosis by ChR have shown that ChR inhibited the growth by downregulating expression of proliferating cell nuclear antigen in HeLa cells[3], induced apoptosis through caspase activation and Akt inactivation in leukemia cells[4-7], and induced cell cycle arrest in human colon carcinoma cells, human esophageal adenocarcinoma OE33 cells and human lung adenocarcinoma cells[8-10]. Nevertheless, poor oral bioavailability has been a major limitation for the successful use of dietary flavonoids as cancer chemotherapeutic agents[11]. It has been reported that ChR halogenated derivatives had stronger bioactivities than the lead compound[12]. The higher hepatic metabolic stability and intestinal absorption of the methylated polyphenols make them more favorable than the unmethylated polyphenols for development as potential cancer chemopreventive agents[13]. Our previous study showed that the effect of 8-bromo-7-methoxychrysin (BrMC) on the inhibition of proliferation and induction of apoptosis in a colon cancer cell line, HT-29, and a gastric cancer cell line, SGC-7901, was stronger than that of ChR[14-17]. However, the molecular mechanisms of induced apoptosis in human hepatocellular carcinoma cells (HCC) by BrMC were not clear.
Although flavonoids are generally considered as antioxidants, they can also generate reactive oxygen species (ROS) depending on their structure and molecular environment[18]. A number of flavonoids exert direct and indirect pro-oxidant effects by inhibiting the mitochondrial respiratory chain complexes I by inducing glutathione (GSH) depletion[19-23]. In addition, Kachadourian et al[19-24] have reported that chrysin is a potent inducer of ROS generation and GSH depletion in A549, HL-60, and PC-3 cells. We here demonstrate that BrMC induces apoptosis of human HCC at least partly by promoting generation of ROS, and ROS-dependent sustained activation of c-Jun N-terminal kinase (JNK).
MATERIALS AND METHODS
Cell culture and reagents
Human HCC HepG2 [AFP(+), no tumorigenicity in immunosuppressed mice], Bel-7402 [AFP(+),with high frequency of tumorigenicity], and human embryo liver L-02 cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 mg/L penicillin, and 100 mg/L streptomycin, and incubated in a humidified atmosphere of 5% CO2 at 37°C. BrMC was synthesized as described previously[14]. ChR was purchased from the Sigma Chemical Co. (St Louis, MO, USA). BrMC and ChR were dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 0.1% in media. N-acetylcysteine (NAC), and GW9662 were obtained from Sigma. Caspase-3 substrate N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA), and the caspase-3 specific inhibitor Z-Asp-Glu-Val-Asp-CH2F (Z-DEVD-fmk), were obtained from Calbiochem (La Jolla, CA, USA). Dichlorodihydrofuorescein diacetate (DCHF-DA) was from Molecular Probes Inc. (Eugene, OR, USA). Rabbit anti-human total JNK was from Cell Signaling Technology (Beverly, MA, USA); mouse anti-human phospho-JNK, phospho-c-Jun, total c-Jun and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies were from Cell Signaling Technology. The commercial anti-hepatoma agent 5-flurouracil (5-FU) was obtained from Sigma Chemical Co. and was used as an apoptotic inducer positive control whereas 0.1% DMSO was used as a negative control.
Flow cytometry with propidium iodide staining
As previously described[25], cells were treated with serum-free medium for 24 h, followed by treatment with media containing different concentrations of test agents for 48 h. Cells were collected and prepared as a single cell suspension by mechanical blowing with phosphate-buffered saline (PBS), washed with cold PBS twice, fixed with 700 mL/L alcohol at 4°C for 24 h, stained with propidium iodide (PI), and cell apoptosis was detected using flow cytometry (FCM; American BD Company, FACS 420).
DNA agarose gel electrophoresis
As previously described[26], cells were treated with serum-free medium for 24 h, followed by treatment with media containing different concentrations of test agents for 48 h. Cells were washed twice with PBS and DNA was extracted with Apoptotic DNA Ladder Detection Kit (Bodataike Company, Beijing) according to the manufacturer’s instructions. The extracted DNA was kept at 4°C overnight. Then 8.5 μL of DNA sample was mixed with 1.5 μL of 6 × buffer solution, electrophoresed on 20 g/L agarose gel containing ethidium bromide at 40 V, and observed through the DBT-08 gel image analysis system.
Caspase-3 activity assay
To evaluate caspase-3 activity, cell lysates were prepared after treatment with test agents. Assays were performed in 96-well microtiter plates by incubating 20 μg cell lysates in 100 μL reaction buffer (1% NP-40, 20 mmol/L Tris-HCl (pH 7.5), 137 mmol/L NaCl, 10% glycerol) containing the 5 μmol/L caspase-3 substrate (DEVD-pNA). Lysates were incubated at 37°C for 2 h. Thereafter, the absorbance at 405 nm was measured with an enzyme-labeling instrument (ELX-800 type). In the caspase inhibitors assay, cells were pretreated with a caspase-3 specific inhibitor (10 μmol/L, Z-DEVD-fmk) for 1 h prior to addition of test agents.
Determination of ROS
Intracellular ROS accumulation was measured by FCM using the fluorescent probe DCHF-DA. Briefly, cells were incubated with 10 μmol/L of DCHF-DA for 30 min at 37°C in the dark after treatment with various concentrations of test agents. After incubation, the cells were washed with PBS and analyzed within 30 min by FCM equipped with an air-cooled argon laser tuned to 488 nm. The specific fluorescence signals corresponding to DCHF-DA were collected with a 525 nm band pass filter. As a rule, 10 000 cells were counted in each determination.
Western blotting analysis
As previously described[27], cells were collected, washed 3 times with PBS, lysed in cell lysate containing 0.1 mol/L NaCl, 0.01 mol/L Tris-Cl (pH 7.6), 0.001 mol/L EDTA (pH 8.0), 1 μg/mL aprotinin, 100 μg/mL PMSF, and then centrifuged at 13 000 ×g for 10 min at 4°C. Extracted protein sample (25 μg total protein/lane) was added in the same volume of sample buffer solution and subjected to denaturation at 100°C for 10 min, then electrophoresed on 100 g/L or 60 g/L sodium dodecyl sulfate polyacrylamide gel electrophoresis at 100 mA for 3 h, and finally transferred onto polyvinylidene fluoride membrane (PVDF) (Millipore). The PVDF membrane was treated with Tris-Buffered Saline Tween-20 (TBST) containing 50 g/L skimmed milk at room temperature for 2 h, followed by incubation with the first antibodies phospho-JNK, total JNK, phospho-c-Jun, total c-Jun and β-actin (1:1000 dilution), respectively, at 37°C for 2 h. After being washed with TBST for 30 min, the corresponding secondary antibody was added and incubated at room temperature for 1 h. Bound antibody was visualized using chemiluminescent substrate (ECL; Amersham, Arlington Heights, IL, USA). Total JNK, total c-Jun and β-actin (1:1000 dilution) were used as an internal control. Images were scanned, followed by densitometry analysis with UN-SCAN-IT software (Silk Scientific).
Statistical analysis
The database was set up with the SPSS 15.0 software package (SPSS Inc., Chicago, IL, USA) for analysis. Data were represented as mean ± SD. The means of multiple groups were compared with one-way analysis of variance, after the equal check of variance, and two-two comparisons of the means were performed using the least significant difference method. Statistical comparison was also performed with two-tailed t-test when appropriate. P < 0.05 was considered statistically significant.
RESULTS
Effects of BrMC on apoptosis in human HCC
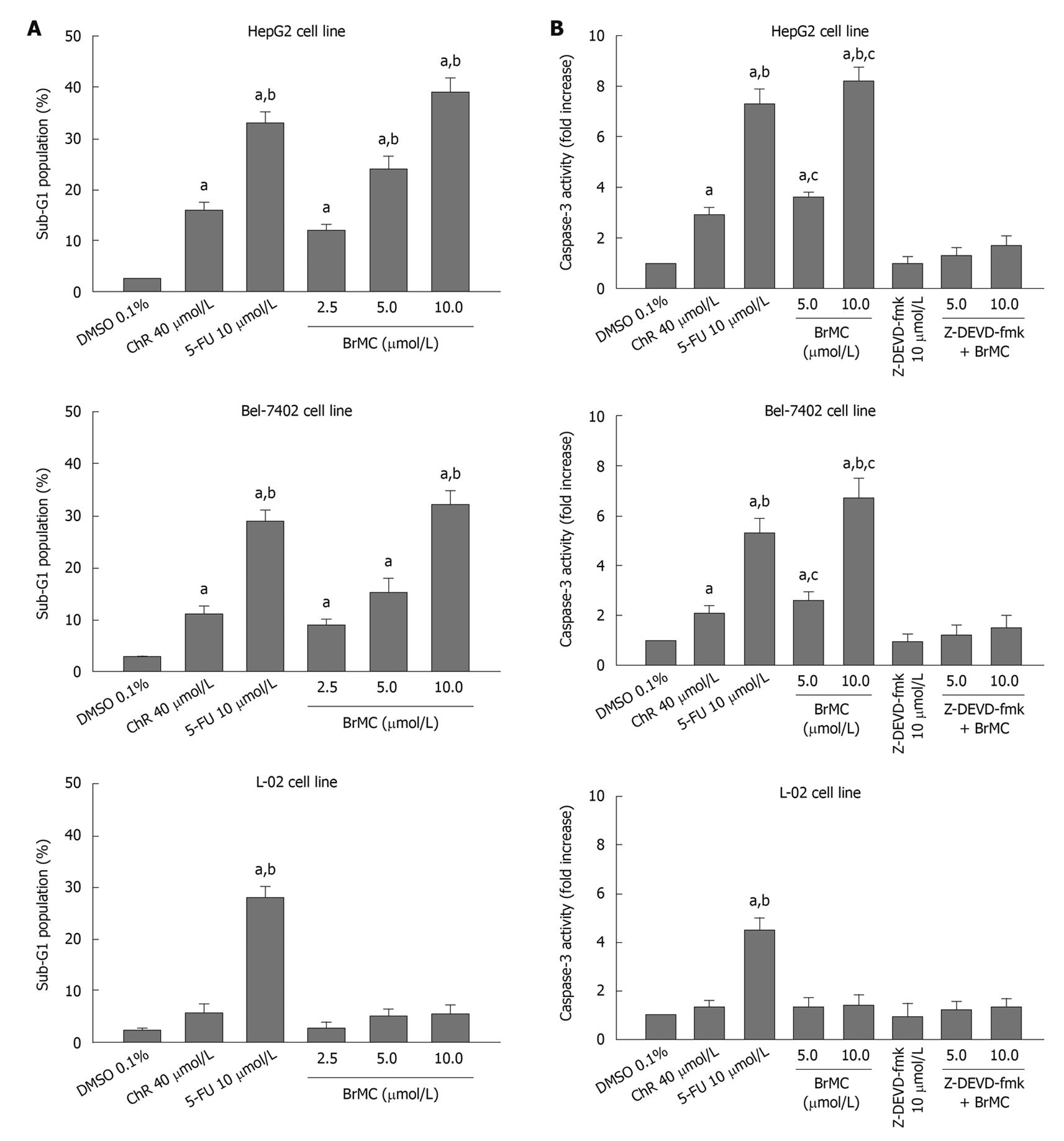
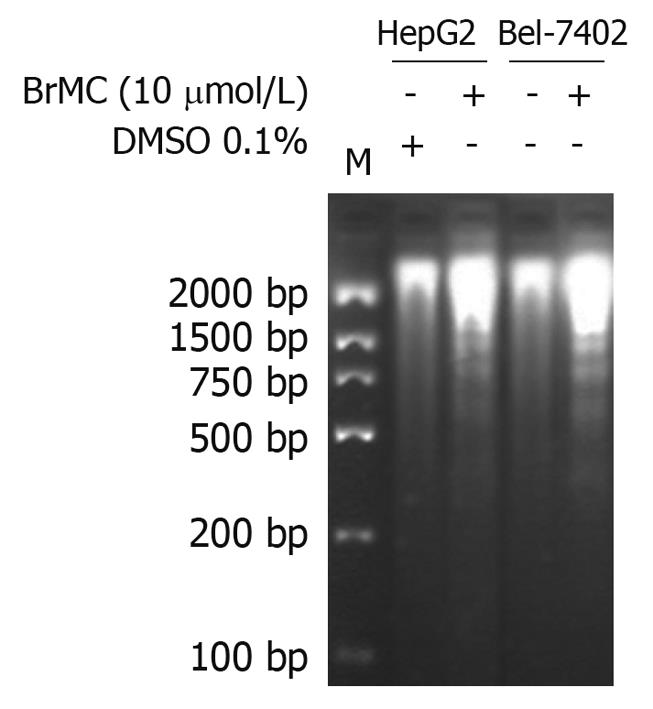
To determine whether BrMC selectively induces apoptosis of human HCC, the human HCC lines HepG2 and Bel-7402 and human embryo liver L-02 cells were treated with increasing concentrations of BrMC for 48 h. Apoptotic cell death was examined by: (1) cell population with sub-G1 contents of DNA using FCM after PI staining; (2) caspase-3 activity determined by enzyme-linked immunosorbent assay; and (3) DNA fragmentation observed by DNA agarose gel electrophoresis. Figure 1A shows that there is a dose-dependent increase in the percentage of sub-G1 cell population (P < 0.05), reaching 39.0% ± 2.8% of HepG2 cells after 48 h of treatment with BrMC at 10 μmol/L. The potency of BrMC in HepG2 and Bel-7402 (32.1% ± 2.6%) cells was found to be more effective than the lead compound, chrysin (ChR, 16.2% ± 1.6% for HepG2 cells and 11.0% ± 1.3% for Bel-7402 cells) at 40 μmol/L and similar to 5-FU (33.0% ± 2.1% in HepG2 cells and 29.3% ± 2.3% in Bel-7402 cells) at 10 μmol/L. BrMC had little effect in human embryo liver L-02 cells, with the percentage of the sub-G1 cell population 5.4% ± 1.8%. Compared with HepG2 cells, Bel-7402 cells were less sensitive to BrMC. Parallel to the cell lethal effect and the enhanced caspase-3 activity, treatment of HepG2 cells with BrMC for 48 h increased the levels of active caspase-3, in a concentration-dependent manner (Figure 1B). Requirement of caspase activity for BrMC-induced apoptosis was examined using a caspase-3-specific inhibitor, z-DEVD-fmk. The data showed that z-DEVD-fmk was able to prevent activation of caspase-3 (Figure 1B). Similarly, treatment with BrMC at 10 μmol/L for 48 h resulted in the formation of a DNA ladder (Figure 2). These results indicate that BrMC selectively induced apoptotic cell death of HCC in a caspase-dependent fashion.
Figure 1 Effects of 8-bromo-7-methoxychrysin on the percentage sub-G1 cell population (A), caspase-3 activity (B) in human hepatocellular carcinoma HepG2, Bel-7402 and human embryo liver L-02 cells.
aP < 0.05 vs treatment with dimethyl sulfoxide (DMSO); bP < 0.05 vs treatment with chrysin (ChR); cP < 0.05 vs treatment with Z-Asp-Glu-Val-Asp-CH2F (Z-DEVD-fmk) plus BrMC. 5-FU: 5-flurouracil.
Figure 2 Effects of 8-bromo-7-methoxychrysin on DNA fragmentation in human hepatocellular carcinoma HepG2 and Bel-7402 cells.
DMSO: Dimethyl sulfoxide.
Effects of BrMC on ROS generation in HepG2 cells
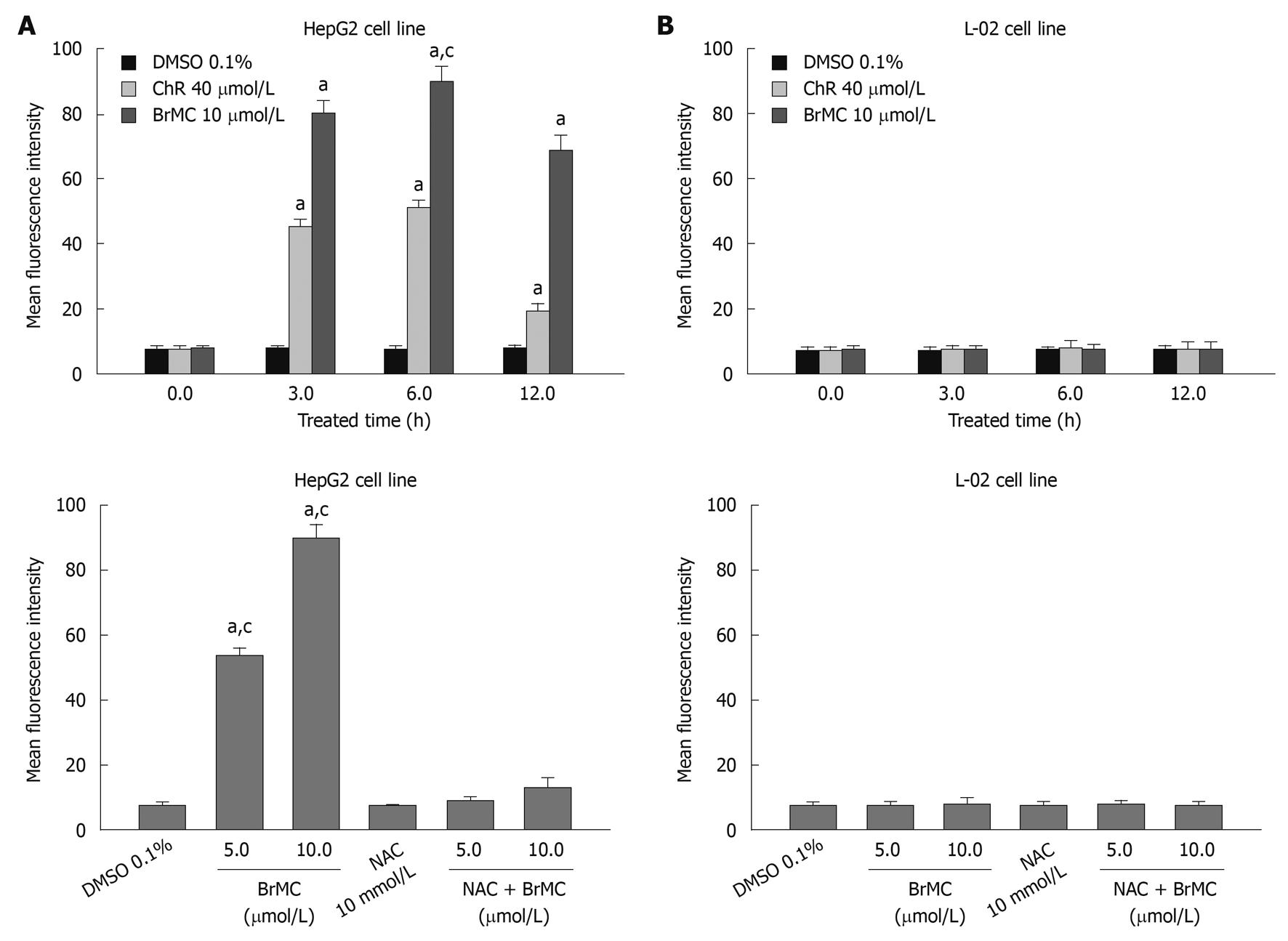
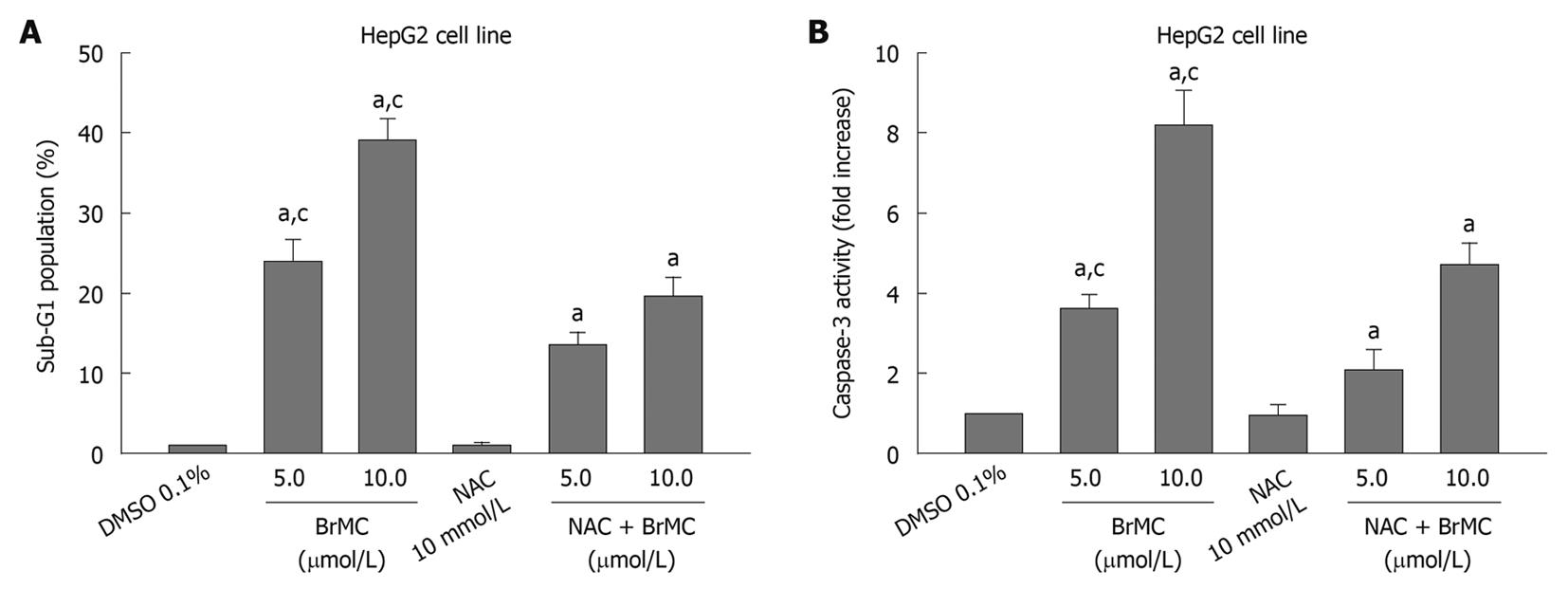
Because oxidative damage plays an important role in anticancer effects of ChR[19], we subsequently examined the level of intracellular ROS in HepG2 and L-02 cells after treatment with BrMC using an oxidation-sensitive fluorescent probe DCHF-DA, which is oxidized to 2’,7’-dichlorofluorescein (DCF) in the presence of ROS. Figure 3A shows that treatment of cells with BrMC (10 μmol/L) increased mean fluorescence intensity of DCF in HepG2 cell from 7.2 ± 1.12 at 0 h to 79.8 ± 3.9 at 3 h and 89.7 ± 4.7 for 6 h. However, BrMC did not affect ROS generation in L-02 cells. BrMC treatment failed to induce ROS generation in HepG2 cells pretreated with 10 mmol/L NAC. We next investigated whether generation of ROS induced by BrMC was accompanied by apoptotic cell death after BrMC treatment. To determine a link between elevation of the intracellular ROS level and apoptotic cell death in BrMC-treated cells, HepG2 cells were pre-incubated with the thiol-containing antioxidant NAC before treatment with BrMC. BrMC treatment failed to induce cell death and caspase-3 activation in cells pretreated with 10 mmol/L NAC (Figure 4). These observations suggest that a selective increase in the intracellular ROS level after BrMC treatment in HCC is required in the cell death pathway, accompanied by activation of caspase-3.
Figure 3 Effects of 8-bromo-7-methoxychrysin on reactive oxygen species generation in human hepatocellular carcinoma HepG2 (A) and human embryo liver L-02 cells (B).
aP < 0.05 vs baseline or treatment with dimethyl sulfoxide (DMSO); cP < 0.05 vs treatment with N-acetylcysteine (NAC) plus 8-bromo-7-methoxychrysin (BrMC).
Figure 4 Effects of N-acetylcysteine on 8-bromo-7-methoxychrysin-induced apoptosis rate (A) and caspase-3 activity (B) in HepG2 cells.
aP < 0.05 vs treatment with medium (0 h) or dimethyl sulfoxide (DMSO); cP < 0.05 vs treatment with N-acetylcysteine (NAC) plus 8-bromo-7-methoxychrysin (BrMC).
Effects of BrMC on JNK activation in HepG2 cells
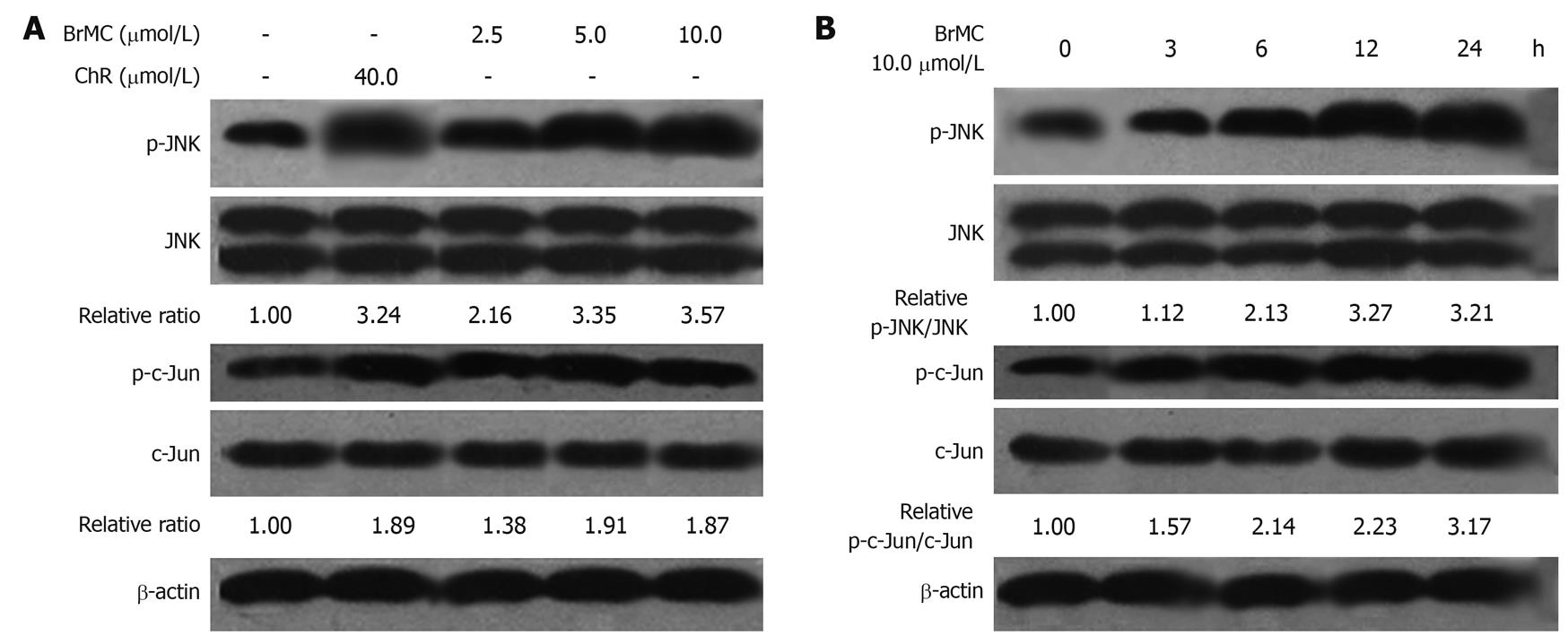
It is well known that many therapeutic agents trigger apoptosis via activation of stress-related signaling pathways including JNK-mediated ones[28]. JNK plays distinct roles in cell death. Transient activation of JNK is believed to be antiapoptotic whereas persistent activation is proapoptotic[29,30]. Here we examined the effect of BrMC-induced JNK activation. JNK activation was measured by Western blotting analysis of phosphorylated JNK and its downstream target c-Jun. In HepG2 cells treated with BrMC (2.5, 5.0, 10.0 μmol/L) for 12 h, JNK activation was observed (Figure 5A). Time course experiments revealed peak JNK activation at 12 h post-treatment and this activation persisted for up to 24 h (Figure 5B).
Figure 5 Effects of 8-bromo-7-methoxychrysin on the level of phosphorylated Jun N-terminal kinase and phosphorylated c-Jun in HepG2 cells (Western blotting, mean ± SD, n = 3).
8-bromo-7-methoxychrysin (BrMC) elevated the level of phosphorylated Jun N-terminal kinase (JNK) and phosphorylated c-Jun in a concentration-dependent manner (A) and in a time-dependent manner (B). The ratio of p-JNK/JNK or p-c-Jun/c-Jun was normalized to 0 h or the untreated group.
Effects of BrMC-stimulated JNK activation on induction of apoptosis and caspase-3 activation in HepG2 cells
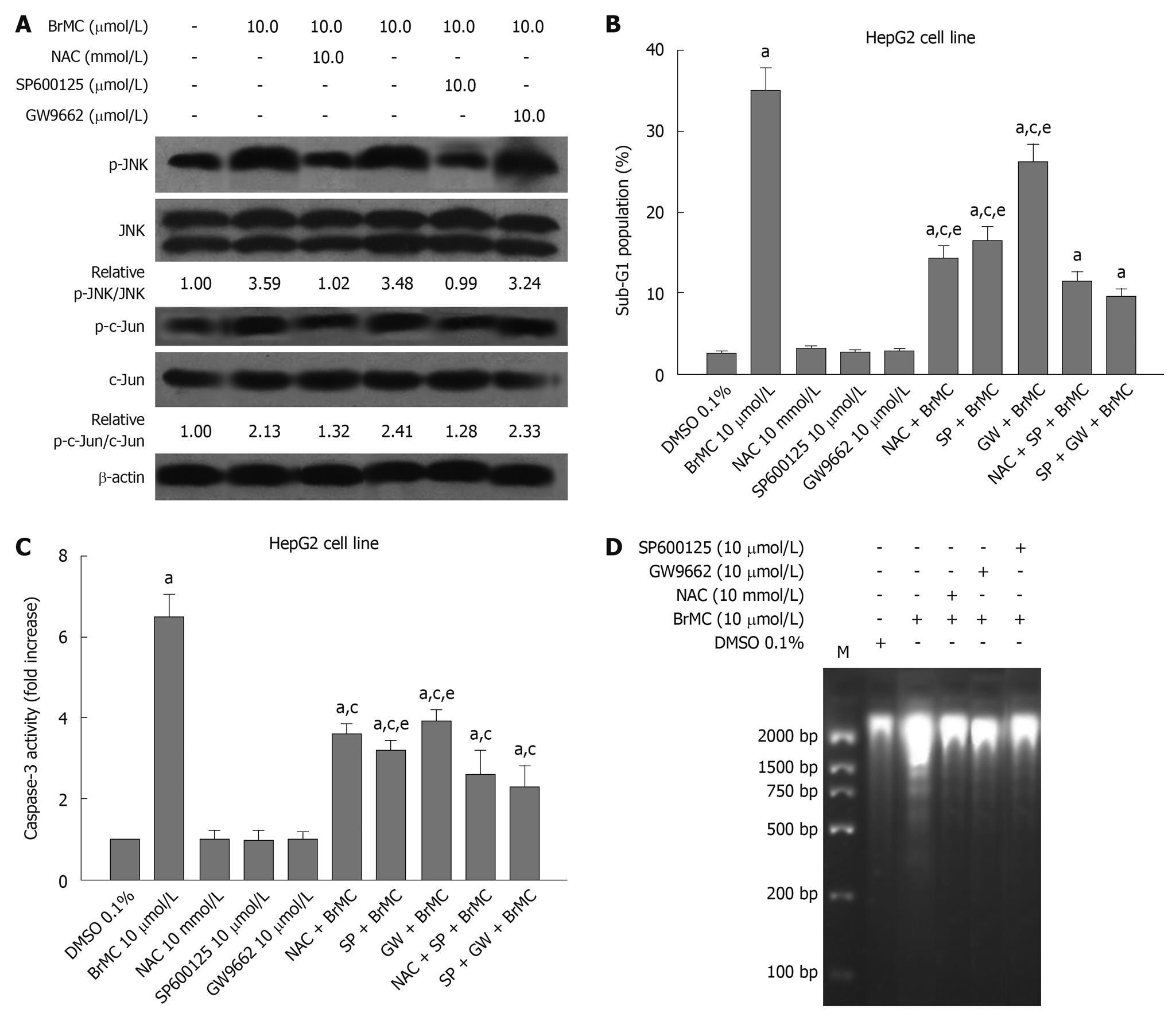
It has been reported that ChR derivatives induce apoptosis of human HCC and human gastric cancer cells by activating peroxisome proliferator-activated receptor-γ (PPARγ)[17,26]. One of the important components in ROS signaling is JNK activation[31]. These results prompted us to investigate whether NAC, an antioxidant, and GW9662, a blocker of PPARγ, and JNK inhibitor SP600125 affected the phosphorylated JNK protein level in HepG2 cells treated with BrMC. Figure 6A shows that the expression of phosphorylated JNK protein after 12 h with BrMC-treated cells was inhibited by NAC and SP600125 pre-treatment, but GW9662 had no effect. To examine the effects of BrMC-stimulated JNK activation on induction of apoptosis and caspase-3 activation in HepG2 cells, we used the JNK inhibitor SP600125 to investigate the role of JNK in BrMC-induced cell death and caspase-3 activation. SP600125 substantially reduced BrMC-induced cell death and caspase-3 activation of HepG2 cells (Figure 6B-D). In addition, NAC and GW9662 also inhibited induction of cell death and caspase-3 activation in HepG2 cells treated with BrMC (Figure 6B-D). These results suggest that ROS production and JNK activation are required for BrMC-induced cell death and caspase-3 activation in HepG2 cells.
Figure 6 Effect of N-acetylcysteine, an antioxidant, and GW9662, a blocker of peroxisome proliferator-activated receptor-γ, and Jun N-terminal kinase inhibitor SP600125 on 8-bromo-7-methoxychrysin-induced activation of Jun N-terminal kinase (A), apoptosis (B), activation of caspase-3 (C), and DNA fragmentation (D) in HepG2 cells.
The ratio of p-Jun N-terminal kinase (JNK)/JNK or p-c-Jun/c-Jun was normalized to 0 h or the untreated group. aP < 0.05 vs treatment with dimethyl sulfoxide (DMSO); cP < 0.05 vs treatment with 8-bromo-7-methoxychrysin (BrMC) alone; eP < 0.05 vs treatment with N-acetylcysteine (NAC) in combination with SP600125 and BrMC or GW9662 in combination with SP600125 and BrMC.
DISCUSSION
Our previous study showed that the effect of BrMC on the inhibition of proliferation and induction of apoptosis in a colon cancer cell line HT-29 and gastric cancer cell line SGC-7901 was stronger than that of ChR[14,16]. In addition, it has been reported that ChR is a potent inducer of ROS generation and GSH depletion in A549, HL-60, and PC-3 cells[19,20]. In this study, we firstly showed that BrMC selectively induced apoptotic cell death of human HCC in a caspase-dependent fashion, with little effect on human embryo liver L-02 cells (Figures 1 and 2). The potency of BrMC in HepG2 and Bel-7402 cells was found to be greater than ChR and similar to 5-FU. Secondly, we indicated that BrMC selectively induced apoptosis of HepG2 cells and was accompanied by ROS generation. However, BrMC did not affect ROS generation of L-02 cells (Figures 3 and 4). Furthermore, we demonstrated that BrMC induced sustained activation of JNK in HepG2 cells in a ROS-dependent manner (Figures 5 and 6).
ROS have been associated with carcinogenesis but also, paradoxically, with mitochondrial-mediated cell death in cancer cells. The overproduction of ROS as a central event in mitochondrial-mediated apoptosis is now well documented[32-35]. The antioxidant properties of flavonoids have been associated with their cardioprotective and neuroprotective properties, yet such an association is much less certain concerning their cancer preventive properties. In the case of flavonoids, however, their chemopreventive properties may rather rely on eliminating precancerous cells because of their prooxidant properties in vivo. This is likely the case of apigenin and ChR, where their cytotoxicity may result from a combination of interference with the mitochondrial respiratory chain and multidrug resistance protein-mediated GSH depletion[36,37]. It is worth noting that the bee product propolis, which is known to exert antimicrobial, antiviral, and cancer preventive properties, contains ChR, a poor antioxidant, as one of its major components[38]. Intracellular ROS mediate multiple cellular responses, including protein kinase activation[39], cell cycle progression[40], myeloid cell differentiation[41,42], and apoptotic and necrotic cell death[43]. It has been reported in several studies that depletion of intracellular GSH plays a critical role in initiating apoptosis by ChR[10,19]. This is likely caused by the reversible interaction between ChR and GSH. In present study, we found that the ChR derivative, BrMC promoted accumulation of ROS products in a concentration-dependent manner in HepG2 cells but not in L-02 cells (Figure 3). NAC is an antioxidant agent and mainly known as a ROS scavenger. It reduces ROS generation and protects the cells from oxidative stress. BrMC-induced apoptosis of HepG2 cells was accompanied by ROS generation. It has been reported that arsenic trioxide-induced ROS generation was inhibited by NAC treatment[44]. We used NAC as an antioxidant to investigate the ROS generation induced by BrMC. NAC treatment not only reduced ROS generation but also attenuated induction of apoptosis in HepG2 cells (Figures 3 and 4). All together, these results indicate that induction of ROS generation contributes to BrMC-induced apoptosis in HepG2 cell line.
In this study, we investigated the signaling pathways affected by BrMC in HepG2 cells. We show that BrMC persistently activates JNK and induces apoptosis of HepG2 cells (Figures 5 and 6). BrMC-activated signaling pathways appear to activate executioner caspases because caspase 3 activity was enhanced in cells exposed to BrMC (Figure 1). JNK is implicated in mediating endoplasmic reticulum stress-induced apoptosis[45]. It has been shown that triterpenoids activate JNK leading to apoptosis[46,47]. In the present study, we detected both JNK activation and apoptotic cell death in cells exposed to BrMC. In addition, the presence of the JNK inhibitor SP600125 attenuated BrMC-induced apoptosis of HepG2 cells, indicating that BrMC-induced apoptosis is also JNK dependent (Figures 5 and 6). Collectively, we conclude that JNK activation mediates BrMC-induced apoptosis in the HepG2 cell line. We noted that SP600125 inhibited BrMC-induced c-Jun phosphorylation completely, but only partially prevented induction of apoptosis by BrMC in HepG2 cells (Figure 5). Thus, we cannot role out the possibility that other mechanisms also participate in BrMC-induced apoptosis in the HepG2 cell line.
It has been documented in several studies that depletion of intracellular GSH plays a critical role in initiating apoptosis by chrysin[10,19]. Zou et al[46]recently showed that triterpenoids deplete intracellular GSH, resulting in JNK-dependent apoptosis in human lung cancer A549 cells. In this study, we found that the presence of NAC blocked the effects of BrMC not only in generating ROS but also in activating JNK and triggering apoptotic cell death (Figure 6).
In summary, the present study has shown that BrMC promotes accumulation of intracellular ROS, resulting in sustained activation of JNK, leading to apoptosis in human HCC but not in human embryo liver L-02 cells. While further investigation is required to provide evidence for the efficacy of this HCC therapy in a nude mouse model and whether it reaches an effective dose in vivo, these results highlight a new mechanism responsible for BrMC-induced apoptosis, and raise the possibility that BrMC may be promising as a candidate for human HCC therapy.
COMMENTS
Background
Chrysin (5,7-dihydroxyflavone), a natural and biologically active flavone extracted from many plants, honey, and propolis, has been shown to inhibit cell proliferation and induce apoptotic cell death in a variety of cancer cells. Nevertheless, poor oral bioavailability has been a major limitation for the successful use of dietary flavonoids as cancer chemopreventative agents. The authors have synthesized 8-bromo-7-methoxyhysin (BrMC), a novel chrysin analogue. BrMC has been demonstrated to inhibit proliferation and induction of apoptosis in a colon cancer cell line HT-29 and gastric cancer cell line SGC-7901 and its effect was stronger than that of chrysin.
Research frontiers
Epidemiological and intervention studies in both humans and animals have shown that regular consumption of fruits, vegetables, and tea is associated with decreased risk of cancer. Fruits, vegetables, and tea provide essential nutrients and many diet-derived phenolics, in particular flavonoids, which have been demonstrated to exert potential anticarcinogenic activities. Flavonoids are plant polyphenolic compounds, which comprise several classes including flavonols, flavanones, flavanols, and flavans. Chrysin is a natural flavonoid contained in many plant extracts, honey, and propolis. Several studies in recent years have shown that chrysin and its derivatives have multiple biological activities, such as anti-inflammatory, anti-cancer, and anti-oxidative effects. However, the cellular and molecular mechanisms underlying chrysin and derivatives induced apoptosis of cancer cells are not clearly understood.
Innovations and breakthroughs
The authors firstly showed that BrMC, a novel chrysin analogue induced apoptotic cell death of human hepatocellular carcinoma cells (HCC) in a caspase-dependent fashion. BrMC-induced apoptosis of HepG2 cells was accompanied by ROS generation. Induction of reactive oxygen species (ROS) generation contributes to BrMC-induced apoptosis in a HepG2 cell line. In addition, they demonstrated that BrMC induced sustained activation of Jun N-terminal kinase (JNK) in HepG2 cells in a ROS-dependent manner.
Applications
The present study has shown that BrMC promotes accumulation of intracellular ROS, resulting in sustained activation of JNK, leading to apoptosis in human HCC. These results suggest that BrMC is a promising candidate for human HCC therapy.
Peer review
This is an original article by Jian-Guo Cao’s group that investigated the effect of BrMC on HCC. They have found that BrMC induces apoptosis in HepG2 and Bel-7402 cells by generation of ROS, JNK activation, and activation of caspase-3. Overall the experiments were conducted appropriately, and the content is interesting.