INTRODUCTION
Autoimmune pancreatitis (AIP) is a rare cause of recurrent acute pancreatitis and chronic pancreatitis. First described by Sarles in 1960, AIP is characterised by a marked infiltration of lymphocytes and plasma cells in pancreatic tissue (lymphoplasmocytic sclerosing pancreatitis)[1-5]. It can be associated with extrapancreatic lesions (including sclerosing cholangitis, retroperitoneal fibrosis, nephritis, sialoadenitis, chronic inflammatory bowel disease) and is the pancreatic manifestation of a systemic inflammatory disease called IgG4-associated systemic disease in about one third of cases[6-9]. Two radiological types are described: a diffuse form (the most frequent, 70% of cases) and a focal form (30% of cases) that may mimic pancreatic adenocarcinoma[7,10,11].
Most cases have been reported in male patients (sex ratio = 3:1), and in patients older than 55 years (average age: 65 years); less than 10 pediatric cases have been reported[9,12-19]. We report the case of a 15-year-old girl who had a focal AIP and associated cholangitis, with atypical clinicoradiological features, that mimicked a pancreatic endocrine tumor.
CASE REPORT
A 15-year-old girl was referred to our unit for the exploration of a pancreatic mass. She had a 1-mo history of epigastric pain and nausea, associated with jaundice and pruritus for 5 d, without either fever or weight loss. She had a personal history of asthma in childhood, and no family medical history. She was not taking any drugs. Over the previous 3 years, she had had several episodes of weakness, with fainting, tremor, sweating and hunger, which resolved after eating.
Examination was normal except for the finding of jaundice. Total bilirubin was 142 μmol/L (conjugated: 104 μmol/L), aspartate aminotransferase was 6 times the upper limit of normal (ULN), alanine aminotransferase was 16 ULN and γ-GT was 4 ULN; blood ionogram results including serum creatinine level, blood cell counts and prothrombin time were normal.
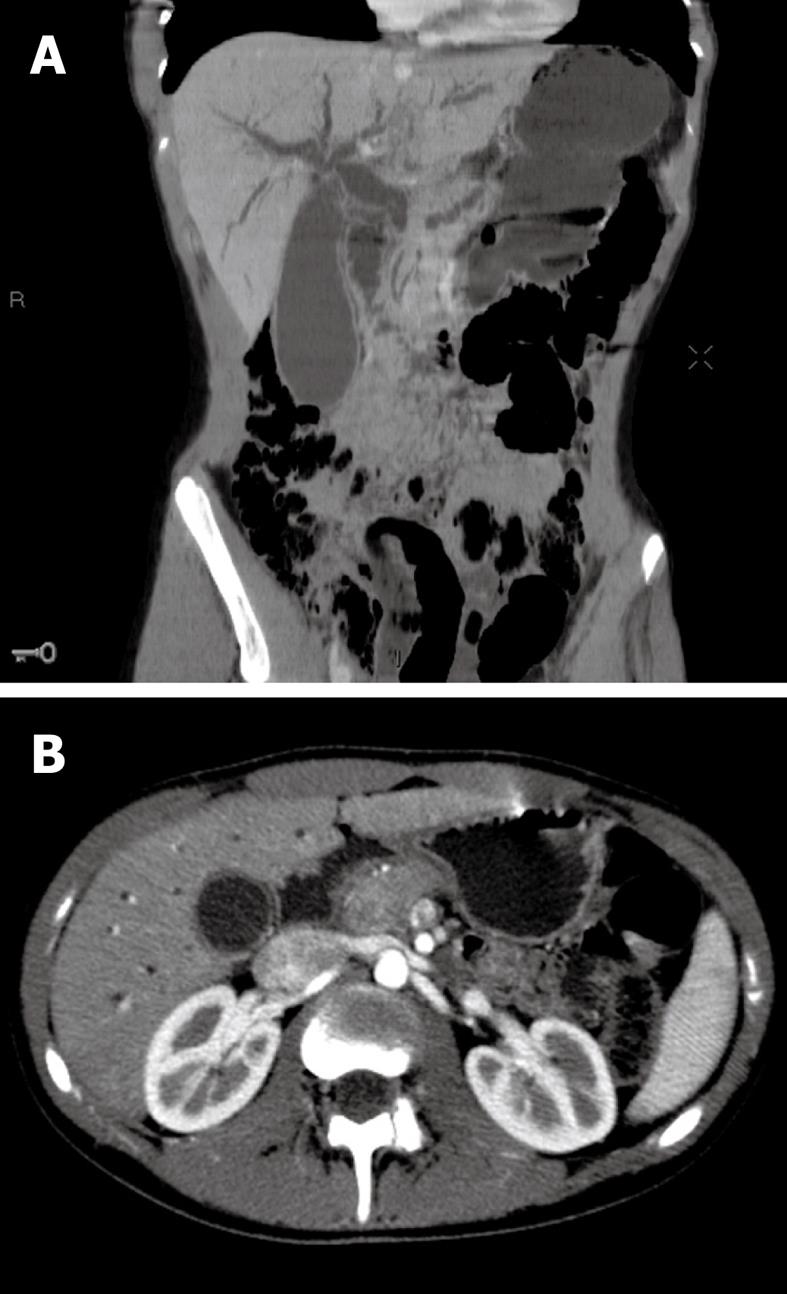
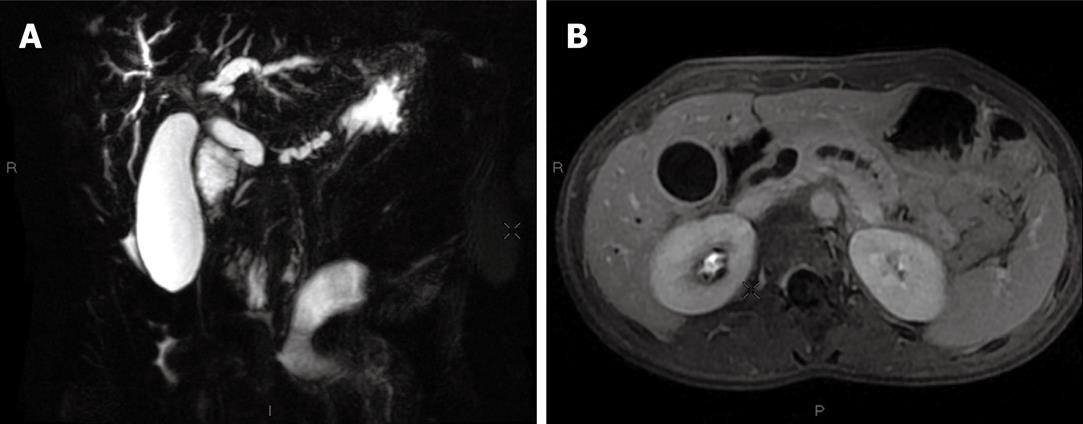
Abdominal ultrasonography showed enlargement of the intrahepatic and common bile ducts (common bile duct diameter: 11 mm), an enlarged gallbladder without either lithiasis or thick wall, and enlargement of the main pancreatic duct due to an oval mass located in the head of the pancreas which was quite well limited, hypoechogenic and homogeneous, measuring 22 mm × 15 mm. Abdominal multi-detector computed tomography (MDCT) revealed a lesion of the head of the pancreas, isodense before intravenous contrast medium injection and that moderately enhanced after injection, measuring 20 mm in diameter, with bicanalar dilation and gallbladder enlargement, without invasion of the duodenum or the vascular structures, and with neither suspicious lymph nodes nor liver metastasis (Figure 1A and B). Bilio-pancreatic magnetic resonance imaging (MRI) showed that the lesion was hypointense in T1-weighted sequences and isointense in T2-weighted compared with surrounding pancreatic parenchyma, and enhanced after intravenous contrast medium injection in arterial and portal venous phases; the main pancreatic duct was enlarged, irregular, and the terminal part of the common bile duct had a thick enhanced wall suggesting cholangitis (Figure 2A and B).
Figure 1 Initial multi-detector computed tomography scan imaging.
A: CT scan before contrast injection, coronal reconstruction: enlargement of the main pancreatic duct and the biliary ducts, with enlarged gallbladder; B: CT scan after contrast injection, arterial phase, axial slice: lesion of the head of the pancreas, moderately enhanced after contrast injection.
Figure 2 Initial magnetic resonance imaging (MRI) imaging.
A: T2-weighted MRI, coronal slice: bicanalar dilation and enlarged gallbladder; B: T1-weighted MRI, axial slice: main biliary duct and gallbladder enlargement with a thick wall evocative of cholangitis; the main pancreatic duct is also irregularly enlarged.
Because the mass was hypervascular on imaging and the patient reported episodes of weakness suggesting hypoglycemia, we considered the diagnosis of pancreatic insulinoma. A 72-h fast test and the blood levels of insulin and C-peptide were normal. Chromogranin A and neuron specific enolase levels were also normal.
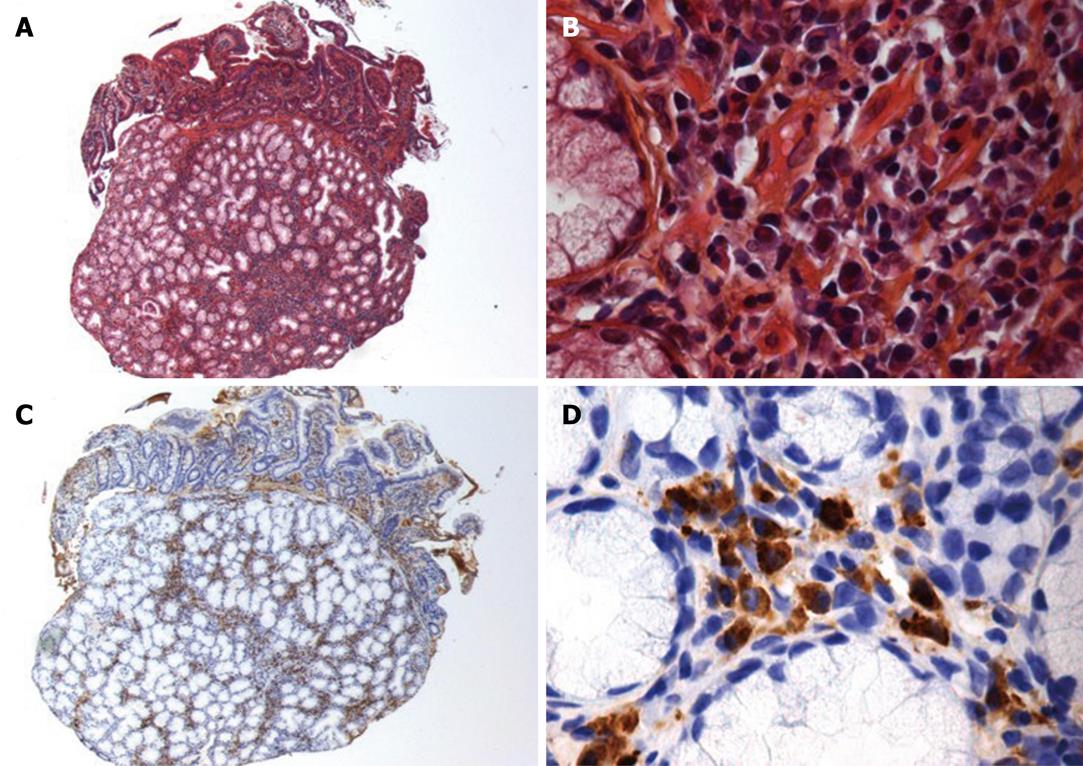
Due to all these atypical features, we preferred performing a pancreatic biopsy rather than a somatostatin-receptor scintigraphy at this stage of investigation. Endoscopic ultrasonography (EUS) showed a heterogeneous area in the head of the pancreas measuring 23 mm × 35 mm. The main pancreatic duct was much enlarged (maximal diameter: 6 mm) along its whole length, with a moniliform aspect; the accessory pancreatic duct was also abnormally visible. The terminal part of the common bile duct had a thick wall. There was a narrowing of the terminal part of the superior mesenteric vein, without invasion of its wall. All these features were not evocative of a malignant tumor. The fine needle aspiration specimen was moderately cellular and consisted of cellular stromal fragments in a mesenchymal and lymphocytic background. At higher magnification, the stromal fragments contained bland spindled mesenchymal cells with a lymphocytic infiltrate. Rare plasma cells were also present in the stromal fragments. No granulomas were seen. There were no malignant cells. Histological examination of the duodenal biopsy showed normal mucosa with scattered plasma cells; examination of the adjacent pancreatic parenchyma revealed chronic pancreatitis with atrophic acini surrounded by a fibrous stroma, associated with periductal lymphoplasmocytic infiltration and obliterative phlebitis (Figure 3A and B). The plasma cells expressed IgG4 (Figure 3C and D).
Figure 3 Histological examination of periampular biopsies (notice that both duodenal mucosa and pancreatic parenchyma are visible).
A, B: Hematoxylin-eosin staining (A: × 125, B: × 500): atrophic acini surrounded by a fibrous stroma, associated with periductal lymphoplasmocytic infiltration; C, D: Anti-IgG4 immunostaining (C: × 125, D: × 500): the plasma cell infiltration expresses IgG4.
Based on these results, the diagnosis of focal AIP associated with cholangitis was proposed. IgG4 serum level was normal (100 mg/mL, N < 140 mg/mL) and the antinuclear antibody test was negative (< 1/80).
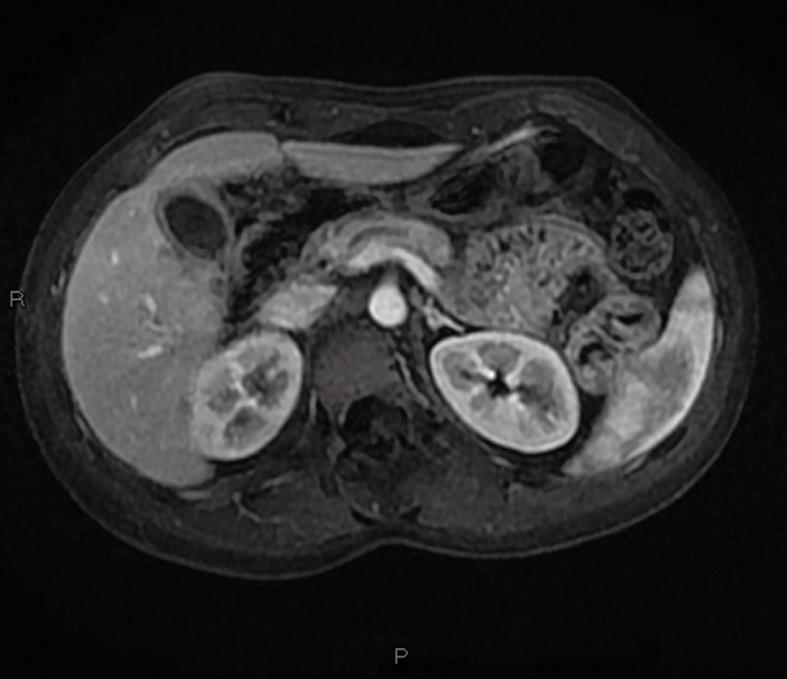
Jaundice decreased spontaneously 1 wk after admission (total bilirubin: 69 μmol/L; conjugated bilirubin: 50 μmol/L). As there was remaining abdominal pain and pruritis, steroid therapy was started at the dose of 40 mg of oral prednisone per day. After 2 wk of treatment, the patient was asymptomatic and hepatic blood tests had returned to normal values. The steroid therapy was continued at the same dose for two additional weeks and progressively decreased and stopped after 3 mo. Bilio-pancreatic MRI at the end of the treatment was normal (Figure 4). Six months after the end of the steroid therapy, the patient remained asymptomatic and the blood tests were normal.
Figure 4 MRI at the end of steroid therapy.
T1-weighted MRI with gadolinium injection: normal main pancreatic duct and gallbladder; the mass in the head of the pancreas has totally disappeared.
DISCUSSION
Most cases of AIP occur in middle-aged patients and about half of the patients are affected when in their sixties[9,12,13]. Cases occurring in childhood or adolescence are exceptional: to our knowledge, only five pediatric cases have been reported (range: 10-16 years), including one in association with a hereditary chronic pancreatitis[14-19].
The most frequent morphological aspect of AIP is the diffuse “duct destructive” form (70% of cases): the pancreas is diffusely enlarged and a loss of lobularity is commonly seen (“sausage-like” appearance); the swollen parenchyma is hypodense on MDCT, hypointense on MRI in T1-weighted sequences and there is a delayed and moderate enhancement after intravenous contrast medium injection due to fibrosis[7,11,20,21]. A typical capsule-like rim appearing as a low-density area on MDCT and a hypointense T2 area on MRI surrounding the pancreas is often present[20,21]. The main pancreatic duct is diffusely narrowed, irregular, or even not visible, because of the pericanalicular inflammatory infiltration[20,21]. Pancreatic atrophy and calcifications are rare but have been reported in some chronic cases[7,11,20,21]. In the focal (pseudo-tumoral) form, the lesion typically appears as a hypovascular mass compared with surrounding pancreatic parenchyma: isodense on MDCT and hypointense on MRI in T1-weighted sequences, with a low enhancement after intravenous contrast medium injection[7,11,21]. This imaging presentation may be difficult to differentiate from a pancreatic adenocarcinoma in a middle-aged patient with pancreatic symptoms[10,11,21,22].
Atypical imaging in patients with AIP is not uncommon; diverse imaging features have been reported. However, this report describes enhancement suggestive of a vascular lesion which has not been described before in AIP and which was suggestive of an endocrine tumor[23]. This could be due to the diagnosis being performed at an early stage thus the pancreatic parenchyma mostly contained acute inflammatory changes and minimal fibrosis. Another noticeable feature was major enlargement of the main pancreatic duct, which was suggestive of an upstream expansion due to a malignant tumor[21]. An upstream pancreatic duct dilation was reported in up to 57% of patients with focal AIP in an earlier study[24]. Similar main pancreatic duct abnormalities have recently been reported in a 14-year-old girl with AIP[19]. Again, this may have resulted from the association of an early stage diagnosis of the AIP and its focal location: pericanalicular fibro-inflammatory infiltration may not have been present at this stage and the pancreatic duct would have been free to enlarge in its distal part upstream from the stenosis due to the extrinsic compression exerted by the mass located in the head of the pancreas. The vascular narrowing in our case was also evocative of malignancy, but this has already been reported in AIP[11,21]. In contrast, the features of cholangitis supported the diagnosis of AIP since IgG4-associated cholangitis is a common extrapancreatic lesion associated with AIP[8,9,21]. Due to the difficulty of differential diagnosis, we preferred performing a pancreatic biopsy rather than a somatostatin-receptor scintigraphy.
Hence, our report underlines the helpfulness of performing pancreatic biopsy for the diagnosis of a pancreatic mass in the case of negative IgG4 serology and atypical imaging findings according to the HISORt criteria, to avoid an unjustified pancreatic resection[25,26]. From our observations, the histologic features were characteristic of lymphoplasmocytic sclerosing pancreatitis[2,5,9]. The positivity of the IgG4 immunostaining also reinforced the suspicion of AIP, despite the fact that the sensitivity of this technique for the diagnosis of AIP is only 21% to 47% in the literature[27,28]. The absence of malignant cells was also helpful in the diagnostic discussion[22]. Several studies have shown the usefulness of the EUS controlled fine-needle biopsy for the diagnosis of AIP[29-32].
Another original element in this observation was the demonstration of IgG4 plasma cell expression by immunostaining in an extrapancreatic location, i.e. duodenal site. Deheragoda et al[33] reported that 85% of biopsies in any organs (liver, stomach, duodenum, colon, kidney, bone marrow, salivary gland) from AIP patients exhibited positive IgG4 immunostaining compared with 0.6% of specimens from controls, a result which was independent from the IgG4 serum concentration. In our observation, the presence of IgG4 positive plasma cells outside of the pancreas (periampulla duodenal mucosa) was contributive to the diagnosis, as previously reported[34]. Altogether, these findings support the hypothesis that AIP is likely be a part of a multisystemic disease.
Whereas the common differential diagnosis of focal AIP in adults is pancreatic adenocarcinoma, the originality of our report is that the mass was evocative of endocrine tumor because of its hypervascular aspect on imaging[10,11,22,23]. Pediatric cases of endocrine tumors have been described: the Surveillance, Epidemiology, and End Results (SEER) register of the National Cancer Institute registered 19 cases between 1973 and 2004 in North America[35]. Jaundice is very uncommon in endocrine tumors (< 5% of cases), whereas about one third (up to 70%-80%) of AIP patients have jaundice[13,36]. The pancreatic biopsy examination and the favorable evolution before and after steroid therapy allowed us to rule out other differential diagnoses such as a solid-pseudopapillary tumor, a pancreatoblastoma (10 cases each reported in the SEER register) or other malignant pancreatic tumors (canalar adenocarcinoma, sarcoma and acinar adenocarcinoma: 7, 4 and 5 cases, respectively in the SEER register)[3,6,35]. Overall, pancreatic biopsies may be useful to avoid misdiagnosing and delaying the treatment of a malignant pancreatic lesion in pediatric cases[10,11,22,37].
The particular problem in pediatric cases of AIP is the risk of pancreatic insufficiency due to recurrence and pancreatic atrophy, as well as extrapancreatic involvement with occurrence of other autoimmune disorders, such as chronic inflammatory bowel disease[7,9,11,38-40]. Consequently, it is important to carefully follow up these young patients in order to diagnose early and treat these complications, paying particular attention to steroid therapy side effects.