Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2943
Revised: April 6, 2010
Accepted: April 13, 2010
Published online: June 21, 2010
AIM: To analyze clinical and pathological characteristics of an aggressive subtype of perianal Paget’s disease (PPD) and explore its rational treatment modalities.
METHODS: PPD patients were retrospectively collected in the institutional colorectal database of the Fudan University Shanghai Cancer Center. Detailed patient histories of past medical condition, diagnosis, treatment, and pathological findings were reviewed. Surgical specimen from diagnosis and surgery were reviewed by two independent pathologists for confirmation of diagnoses. Follow up was accomplished by clinical interview by cellphone.
RESULTS: In total, eight cases of PPD were analyzed. All patients had underlying anorectal adenocarcinoma, including seven with synchronous lesions and one with metachronous lesions. Moreover, all anorectal lesions had a mucin-producing component. The median age at diagnosis was 65 (range 29-81 years), and the male/female ratio was 7:1. The Median follow-up time of all patients was 61.5 mo (range 10-204 mo). One patient treated with abdominoperineal resection (APR) died from lung metastases 10 mo after the APR operation. The other patients are still free of disease at the time of this analysis.
CONCLUSION: PPD is a rare malignancy and is easily misdiagnosed. Underlying anorectal cancer was not unusual and was a significant prognostic factor. Rational treatment of both anorectal cancer and PPD lesion is essential for long-term survival.
- Citation: Lian P, Gu WL, Zhang Z, Cai GX, Wang MH, Xu Y, Sheng WQ, Cai SJ. Retrospective analysis of perianal Paget’s disease with underlying anorectal carcinoma. World J Gastroenterol 2010; 16(23): 2943-2948
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2943.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2943
Perianal Paget’s disease (PPD) is a rare malignancy and less than 200 cases have been reported in literature. The prognosis of simple PPD is relatively favorable, both overall and disease-free survival are approximately 60% at 5-year[1-3].
However, one subtype of this malignancy, PPD with synchronous or metachronous carcinoma of the anorectum, has a poor prognosis, based on limited reports on this entity[1-5]. Due to the rarity of this condition, most of the published literature presented limited numbers of case reports. The natural course of the disease and its optional management is still unknown.
The current report aims to increase the current knowledge of PPD with underlying adenocarcinoma of the anorectum by retrospectively reviewing the natural history and treatment outcome of eight consecutive and non-selected patients diagnosed with this rare condition.
All cases of PPD diagnosed and treated between January 1985 and December 2009 at the colorectal surgical service of Fudan University Shanghai Cancer Center were reviewed retrospectively. According to the institutional colorectal database, patients with histological confirmation of Paget’s disease originating from the perianal area, as well as adenocarcinoma of the rectum and/or anal canal, were included in this analysis.
Detailed patient histories of past medical conditions, diagnosis, treatment, and pathological findings were reviewed.
Preoperative workup included fiberoptic full colonoscopy, computed tomography and/or magnetic resonance imaging of the abdomen and pelvis, and carcinoembryonic antigen tumor marker analysis to confirm the lesions of the perianal area and anorectum and to exclude lesions in other part of the colon.
Surgical specimens from diagnosis and surgery were reviewed by two independent pathologists for confirmation of diagnoses of both Paget’s disease of the perianal area and anorectal adenocarcinoma. Only when the preoperative colonoscopy and postoperative pathological examination confirmed that there was no continuity between the two lesions through mucosa spread and interstitial invasion, were the patients included in the study.
Follow up was accomplished according to the NCCN guideline V2.2009 for rectal cancer and survival information was achieved by clinical and/or cellphone interview.
Eight cases were diagnosed with PPD with synchronous or metachronous anorectal adenocarcinoma. Patients’ characteristics are detailed in Table 1. The median age at diagnosis was 65 years (range 29-81 years), and the male/female ratio was 7:1. The median follow-up time of all patients was 61.5 mo (range 10-204 mo). One patient treated with APR died from lung metastases 10 mo after the APR operation. The other seven patients are still free of disease at the time of this analysis, one of whom had local recurrence of PPD and received salvage wide local excision.
| Patient | Gender | Age (yr) | Disease status | Treatment |
| 1 | Male | 80 | Synchronous | APR |
| 2 | Male | 29 | Synchronous | APR |
| 3 | Female | 68 | Synchronous | APR |
| 4 | Male | 63 | Synchronous | APR |
| 5 | Male | 53 | Synchronous | Excision biopsy of anorectal lesion + radical radiation therapy for both Paget’s disease and anorectal cancer |
| 6 | Male | 61 | Synchronous | APR |
| 7 | Male | 81 | Synchronous | APR |
| 8 | Male | 70 | Metachronous | Radiation therapy; Inguinal node resection and APR for recurrence |
Eczema-like symptoms including eczema-like skin change, itching, and pain were the most common presenting symptoms and occurred in five of eight cases as the only initial finding. Digestive tract symptoms, including hematochezia, increased frequency of defecation without diarrhea, and constipation, respectively, were observed in 3, 2, and 1 cases of the remaining three cases. The median time from symptom onset to diagnosis of Paget’s disease was 27.3 mo (range 6-96 mo).
Six patients were pathologically diagnosed as PPD before surgery or definitive radiation, and pathological confirmation of PPD was obtained from specimens after radical surgery in two additional cases.
All patients received treatment for curative intent including surgery and radiation therapy. Seven patients received radical surgery. Five of the seven patients were treated with abdominoperineal resection (APR), and one had trans-anal excision followed by APR.
One patient was treated with induction radiation therapy to 50.4 Gy delivered over 5.5 wk (1.8 Gy/daily fraction) as the initial treatment for Paget’s disease. He developed local recurrence of Paget’s disease and lymphadenopathy in the left inguinal area one year after the completion of radiation therapy, and was diagnosed with adenocarcinoma of the lower rectum. The patient was treated with APR and adjuvant chemotherapy. One additional patient received excision biopsy of the rectal cancer followed by definitive radical radiation therapy then adjuvant chemotherapy. Radical surgery was not performed for rectal cancer due to his poor general health. The total dose for definitive radiation therapy was 59.4 Gy given at 1.8 Gy/daily fractions. Adjuvant chemotherapy (five cycles of capecitabine) was used in two patients.
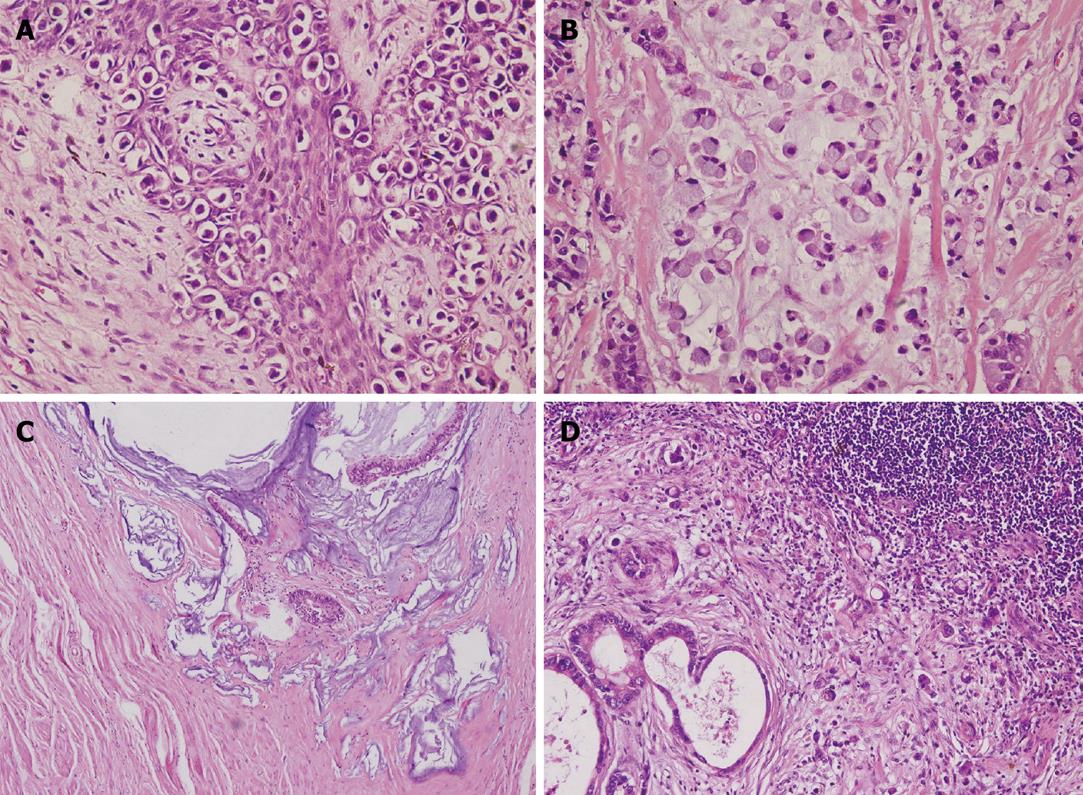
Pathological study of the resected specimens of seven surgically treated patients and excisional biopsy of one case treated with definitive radiation revealed typical characteristics of Paget’s cells as depicted by Marchesa et al[1]: large cytologically malignant cells (Paget cells) that had vesicular nuclei and prominent nucleoli and contained abundant pale or granular, sometimes vacuolated, cytoplasm. Moreover, all cases had Paget’s disease confined in the perianal area with clear margins from their synchronous anorectal adenocarcinoma. For the only case treated with definitive radiation therapy, colonoscopy before surgery showed a clear distance, without evidence of disease, between Paget’s disease and the lower rectal lesion, and a clear margin was observed from the excised specimen from the rectum. Of all cases, the underlying adenocarcinoma originated from the lower rectum and involved the dentate line. All anorectal lesions had a mucin-producing component, which had rarely been reported previously in the published literature. Clinicopathological features of the seven surgically treated cases are detailed in Table 2. Figure 1 illustrates histopathological images of PPD, anorectal adenocarcinoma, and metastases in a lymph node from one typical case.
| Factors | Value |
| Distance to anal edge of anorectal cancer (cm) | 2.69 ± 0.70 |
| Diameter of anorectal cancer (cm) | 3.13 ± 1.76 |
| Diameter of PPD (cm) | 4.81 ± 2.59 |
| Dental line involving (%) | 100 |
| Mucin producing component | 8/8 (100) |
| Mucinous adenocarcinoma | 5/8 (62.5) |
| Signet ring cell cancer | 1/8 (12.5) |
| Mix typed | 2/8 (25) |
| Stages of anorectal cancer | |
| I | 5/8 (62.5) |
| II | 2/8 (25) |
| III | 1/8 (12.5) |
Paget’s disease is an intra-epithelial adenocarcinoma and was first reported in the breast by Sir Paget J in 1874. Since then, Paget’s cells and Paget’s disease have been reported in several extramammary sites, including the axilla, perineum, groin, thigh, scrotum, and vulva[6]. In 1893, Darrier and Coulillaud reported the first case of Paget’s disease in the perianal region.
PPD is often referred to as an intraepithelial adenocarcinoma within 6 cm scope around the anus and below the dentate line. Signet ring cells with pale, vacuolated cytoplasm are present, which are usually positive with a periodic acid-Schiff mucin stain under microscopic examination[7,8].
There is considerable controversy about the origin of PPD. Helwig et al[9] considered perianal and vulval Paget’s disease to be a manifestation of a multicentric effect of an unknown carcinogenic stimulus on apocrine structures, epidermis, and glandular elements of the rectum and the urethra. Otherwise, some researchers observed histochemical similarity between the mucin in the intraepidermal cells and the underlying carcinoma cells, and formed the view that the cells in the epidermis represented metastatic spread[10-14]. In our group, eight anorectal carcinomas also had mucin-producing components, such as mucinous adenocarcinoma, signet ring cell cancer, or mixed type. However, fiberoptic full colonoscopy confirmed disease-free distance between Paget’s disease and the anorectal carcinoma. Moreover, pathologists failed to find continuity between the two lesions. This result reinforced the supposition of “metastatic origination of perianal Paget’s disease” from a much bigger sample size basis. The mucin secretion character of the anorectal lesion and clinical manifestation of mucinous discharges suggested that mucin might play an important role in the histogenesis of PPD. We hypothesized that mucin may function in Paget’s disease formation by two ways: cancer cell produced-mucin might stimulate carcinogenesis of apocrine structures/epidermis in the perianal area directly. On the other hand, mucinous tumor cells might infiltrate through the epidermal barrier and form a cancer nest beneath.
After PPD was first reported by Darrier and Coulilaud in 1893, new cases emerged gradually[4,15-19], but systemic analysis of this disease was scarce. Only in prestigious medical institutes, such as the Memorial Sloan-Kettering Cancer Center, the Mayo Clinic, and the Cleveland Clinic Foundation, was this type of disease collected and analyzed systemically; however, the sample sizes were very small (27, 13, and 14 cases, respectively)[1-3]. Unfortunately, these studies focused on simple PPD, and the aggressive subtype with underlying anorectal carcinoma was still unknown.
Interestingly, all patients with PPD in our group had underlying adenocarcinoma in the anorectum. Therefore, we attempted to summarize the natural history, clinic-pathological characteristics, treatment experiences, and information about survival to boost the knowledge of this rare condition.
Eczema-like symptoms were the most common initial complaints, including eczema-like skin change, itching, and pain, which were easily underestimated by the patients themselves and by physicians. On the other hand, digestive symptoms could be the first presentation, but are usually ignored by the patients themselves. In our group, the median time from symptom onset to diagnosis of Paget’s disease was 27.3 mo. PPD with underlying anorectal carcinoma in has been rarely reported. In some cases, anorectal carcinoma was neglected in the diagnosis and treatment modality after confirmation the diagnosis of Paget’s disease. A total of 155 cases of Paget’s disease have been reported. The median age of onset was 65.3 years and male/female ratio was 1.06. The proportion of underlying anorectal cancer was as high as 41.8%.
A wide range of treatment modalities have been previously reported, including surgery (wide local excision with or without reconstruction and grafting[20-23], abdominoperineal resection[1-3], Mohs micrographic surgery[24]), radiotherapy[25], chemo-radiotherapy[26-28], and photodynamic therapy[29,30].
Surgical methods are still the mainstay in the management of PPD. Table 3 shows the stage and management recommended by Shutze et al[31] in 1990 and followed by most oncologists.
| Stage | Description | Management |
| I | Paget’s cells found in perianal epidermis and adnexae without primary carcinoma | WLE |
| IIA | Cutaneous Paget’s disease with associated adnexal carcinoma | WLE |
| IIB | Cutaneous Paget’s disease with associated anorectal carcinoma | APR |
| III | Paget’s disease in which associated carcinoma has spread to regional nodes | ILND + WLE/APR |
| IV | Paget’s disease with distant metastases of associated carcinoma | CT + RT + LPM |
According to this staging system, seven PPD with synchronous anorectal carcinoma should be grouped as stage IIB and APR should be recommended. For patient 8 in Table 1, he was diagnosed as stage III metachronous anorectal cancer and radiation therapy, inguinal lymph node dissection, and APR were carried out sequentially.
Brown et al[25] reported six cases of PPD treated by radiotherapy as adjuvant or radical modality. They found that PPD had a high recurrence rate after radiotherapy. However, only three patients had an associated underlying anal carcinoma: one 9 years pre-diagnosis of PPD, one at diagnosis (both adeno-and squamous carcinoma), and one 10 years after diagnosis of PPD. Reports of chemo-radiation are also infrequent. Although promising, there have not been many subsequent reports following these initial cases[26-28]. In our study, two patients received radical radiotherapy and adjuvant chemotherapy with capecitabine. One patient had recurrence and inguinal lymph metastasis, and an APR operation confirmed the existence of underlying anorectal cancer. Subsequently, he had local recurrence in the perineum and received wide local excision and is presently tumor-free. The other received transanal excision of an anorectal lesion and followed by radical radiation therapy for both Paget’s disease and rectal cancer, and five cycles of adjuvant chemotherapy with capecitabine. No evidence of recurrence has been found at the latest follow. The results of this research are promising; however, further research is required.
Photodynamic therapy has also been successfully used in early perianal skin tumors, such as Bowen’s disease and PPD[29,30]. However, because of limitation of conditions, this technique could not be used in our patients.
Survival of simple PPD was favorable. McCarter et al[3] collected 27 cases of PPD in the Memorial Sloan-Kettering Cancer Center, and most patients (74%) were treated with wide excision. At a median follow-up of 67 mo, 56 percent (15/27) had no evidence of disease, and two patients had died of metastatic disease. The overall and disease-free survival at 5 years was 59% and 64%, respectively, which decreased to 33% and 39%, respectively, by 10 years. Sarmiento et al[2] reported 13 PPD patients in the Mayo Clinic over a 25-year history. Mean follow-up was 6.7 years. Eleven patients underwent local resection without adjuvant therapy. Almost all recurrences were treated by wider local excision. The 5-year recurrence rate was 61%. Overall 5-year and 10-year survival was 67%. Marchesa et al[1] reviewed their experience in the Cleveland Clinic Foundation: ten patients with a mean follow-up longer than 5 years were studied to determine the outcome after surgical treatment. Two patients underwent local excision (LE) with macroscopic clearance of the surgical margins; the remaining eight patients underwent wide local excision (WLE), i.e. > 1 cm microscopic clearance of the surgical margins. The actuarial 8-year recurrence rate for patients treated with LE and WLE was 100% and 50%, respectively. Progression to invasive carcinoma occurred after a median time of 56 mo (range 23-72 mo) in two patients treated with LE, and in one of eight patients treated with WLE. All four patients with recurrence after WLE were successfully treated (no further recurrence) with a second WLE. Actuarial 8-year survival was 0% in the LE group and 40% (SE = 21.9) in the WLE group.
However, as shown above, most of the reports were limited in patient number and to the simple form of PPD. For patients with underlying anorectal cancer, the information was scarce and required documentation. In this study, eight patients with the aggressive subtype were presented, which is the largest group studied to date. One patient died from lung metastases of rectal cancer 10 mo after operation. The other seven cases are tumor-free. This result verified that underlying anorectal cancer was the most important prognostic factor of PPD. Successful management of this lesion is essential for survival.
Until now, less than 200 cases of perianal Paget’s disease (PPD) had been reported in the literature, most of which were case reports. Unfortunately, frequent misdiagnoses and/or neglect of underlying cancer occurred in the clinical practice. Thus it was necessary to comprehensively document this disease to summarize its clinical and pathological characteristics, to analyze its carcinogenesis, and to explore rational treatment modalities.
In this study, the authors non-selectively and consecutively documented PPD treated in their institution. All of the cases were exclusively the aggressive subtype with underlying cancer. These clinical experiences provided strong reinforcement of the accumulated knowledge of this disease.
This study well delineated the clinical characteristics of this unusual disease and determined the fundamental modality of preoperative workup, surgery, and postoperative surveillance. In particular, the study demonstrated the mucin-producing character of underlying anorectal cancer and suggested its relationship with carcinogenesis of Paget’s disease.
In this article, the clinical characteristics of PPD and its close relationship with anorectal cancer have been demonstrated. The data is a major contribution to the documentation of this disease and will help clinicians to make correct diagnoses and to choose rational treatment modalities.
Paget’s disease is a type of cancer with low invasiveness and often occurs in areas rich in sweat gland structures, such as the nipple, axilla, groin, scrotum, and vulva. However, Paget’s disease in the perianal region usually has a feature of underlying anal or rectal cancer, is particularly aggressive, and deserves more attention.
The authors have to be commended for this very large series of patients with perianal Paget's disease. This manuscript is very interesting and well written.
Peer reviewers: Fernando Azpiroz, MD, Digestive System Research Unit, University Hospital Vall d’Hebron, Paseo Vall d’Hebron, 119-129, Barcelona 08035, Spain; Alessandro Fichera, MD, FACS, FASCRS, Assistant Professor, Department of Surgery - University of Chicago, 5841 S. Maryland Ave, MC 5031, Chicago, IL 60637, United States
S- Editor Wang YR L- Editor Stewart GJ E- Editor Lin YP
| 1. | Marchesa P, Fazio VW, Oliart S, Goldblum JR, Lavery IC, Milsom JW. Long-term outcome of patients with perianal Paget's disease. Ann Surg Oncol. 1997;4:475-480. |
| 2. | Sarmiento JM, Wolff BG, Burgart LJ, Frizelle FA, Ilstrup DM. Paget's disease of the perianal region--an aggressive disease? Dis Colon Rectum. 1997;40:1187-1194. |
| 3. | McCarter MD, Quan SH, Busam K, Paty PP, Wong D, Guillem JG. Long-term outcome of perianal Paget's disease. Dis Colon Rectum. 2003;46:612-616. |
| 4. | Goldman S, Ihre T, Lagerstedt U, Svensson C. Perianal Paget's disease: report of five cases. Int J Colorectal Dis. 1992;7:167-169. |
| 5. | Williams SL, Rogers LW, Quan SH. Perianal Paget's disease: report of seven cases. Dis Colon Rectum. 1976;19:30-40. |
| 6. | Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget's disease. A critical reexamination. Am J Dermatopathol. 1979;1:101-132. |
| 7. | Arminski TC, Pollard RJ. Paget's disease of the anus secondary to a malignant papillary adenoma of the rectum. Dis Colon Rectum. 1973;16:46-55. |
| 8. | Armitage NC, Jass JR, Richman PI, Thomson JP, Phillips RK. Paget's disease of the anus: a clinicopathological study. Br J Surg. 1989;76:60-63. |
| 9. | Helwig EB, Graham JH. Anogenital (extramammary) Paget's disease. A clinicopathological study. Cancer. 1963;16:387-403. |
| 10. | Lertprasertsuke N, Tsutsumi Y. Latent perianal Paget's disease associated with mucin-producing rectal adenocarcinoma. Report of two cases. Acta Pathol Jpn. 1991;41:386-393. |
| 11. | Ishida-Yamamoto A, Sato K, Wada T, Takahashi H, Toyota N, Shibaki T, Yamazaki K, Tokusashi Y, Miyokawa N, Iizuka H. Fibroepithelioma-like changes occurring in perianal Paget's disease with rectal mucinous carcinoma: case report and review of 49 cases of extramammary Paget's disease. J Cutan Pathol. 2002;29:185-189. |
| 12. | Goldblum JR, Hart WR. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179. |
| 13. | Sasaki M, Terada T, Nakanuma Y, Kono N, Kasahara Y, Watanabe K. Anorectal mucinous adenocarcinoma associated with latent perianal Paget's disease. Am J Gastroenterol. 1990;85:199-202. |
| 14. | Merot Y, Mazoujian G, Pinkus G, Momtaz-T K, Murphy GF. Extramammary Paget's disease of the perianal and perineal regions. Evidence of apocrine derivation. Arch Dermatol. 1985;121:750-752. |
| 15. | Delaunoit T, Neczyporenko F, Duttmann R, Deprez C, da Costa PM, de Koster E. Perianal Paget's disease: case report and review of the literature. Acta Gastroenterol Belg. 2004;67:228-231. |
| 16. | Tulchinsky H, Zmora O, Brazowski E, Goldman G, Rabau M. Extramammary Paget's disease of the perianal region. Colorectal Dis. 2004;6:206-209. |
| 17. | Tarng SJ, Fan HA, Lin SE, Lai CR, Tang RP, You YD, Chen JS, Chang-Chieh CR, Wang JY, Lin JC. Perianal Paget's disease--report of 4 cases. Changgeng Yixue Zazhi. 1990;13:314-321. |
| 18. | Tjandra J. Perianal Paget's disease. Report of three cases. Dis Colon Rectum. 1988;31:462-466. |
| 19. | Beck DE, Fazio VW. Perianal Paget's disease. Dis Colon Rectum. 1987;30:263-266. |
| 20. | Archer CB, Louback JB, MacDonald DM. Spontaneous regression of perianal extramammary Paget's disease after partial surgical excision. Arch Dermatol. 1987;123:379-382. |
| 21. | Murakami K, Tanimura H, Ishimoto K, Yamaue H, Yamade N, Shimamoto T. Reconstruction with bilateral gluteus maximus myocutaneous rotation flap after wide local excision for perianal extramammary Paget's disease. Report of two cases. Dis Colon Rectum. 1996;39:227-231. |
| 22. | Lam DT, Batista O, Weiss EG, Nogueras JJ, Wexner SD. Staged excision and split-thickness skin graft for circumferential perianal Paget's disease. Dis Colon Rectum. 2001;44:868-870. |
| 23. | Araki Y, Noake T, Hata H, Momosaki K, Shirouzu K. Perianal Paget's disease treated with a wide excision and gluteal fold flap reconstruction guided by photodynamic diagnosis: report of a case. Dis Colon Rectum. 2003;46:1563-1565. |
| 24. | Mohs FE, Blanchard L. Microscopically controlled surgery for extramammary Paget's disease. Arch Dermatol. 1979;115:706-708. |
| 25. | Brown RS, Lankester KJ, McCormack M, Power DA, Spittle MF. Radiotherapy for perianal Paget's disease. Clin Oncol (R Coll Radiol). 2002;14:272-284. |
| 26. | Thirlby RC, Hammer CJ Jr, Galagan KA, Travaglini JJ, Picozzi VJ Jr. Perianal Paget's disease: successful treatment with combined chemoradiotherapy. Report of a case. Dis Colon Rectum. 1990;33:150-152. |
| 27. | Secco GB, Lapertosa G, Sertoli MR, Scarpati D, Riboli EB. Perianal Paget's disease: case report of an elderly patient treated with polychemotherapy and radiotherapy. Tumori. 1984;70:381-383. |
| 28. | Balducci L, Athar M, Smith GF, Khansur T, McKenzie D, Crawford ED. Metastatic extramammary Paget's disease: dramatic response to combined modality treatment. J Surg Oncol. 1988;38:38-44. |
| 29. | Runfola MA, Weber TK, Rodriguez-Bigas MA, Dougherty TJ, Petrelli NJ. Photodynamic therapy for residual neoplasms of the perianal skin. Dis Colon Rectum. 2000;43:499-502. |
| 30. | Vereecken P, Awada A, Ghanem G, Marques da Costa C, Larsimont D, Simoens C, Mendes da Costa P, Hendlisz A. A therapeutic approach to perianal extramammary Paget's disease: topical imiquimod can be useful to prevent or defer surgery. Med Sci Monit. 2007;13:CS75-CS77. |
| 31. | Shutze WP, Gleysteen JJ. Perianal Paget's disease. Classification and review of management: report of two cases. Dis Colon Rectum. 1990;33:502-507. |