Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2926
Revised: February 10, 2010
Accepted: February 17, 2010
Published online: June 21, 2010
AIM: To evaluate the influence of multiple samplings during esophagogastroduodenoscopy (EGD) on the accuracy of the rapid urease test, and the validity of newly developed rapid urease tests, HelicotecUT plus test and HelicotecUT test, CLO test and ProntoDry test.
METHODS: A total of 355 patients undergoing EGD for dyspepsia were included. Their Helicobacter pylori (H. pylori) treatment status was either naïve or eradicated. Six biopsy specimens from antrum and gastric body, respectively, were obtained during EGD. Single antral specimens and dual (antrum + body) specimens were compared. Infection status of H. pylori was evaluated by three different tests: culture, histology, and four different commercially available rapid urease tests (RUTs)-including the newly developed HelicotecUT plus test and HelicotecUT test, and established CLO test and ProntoDry test. H. pylori status was defined as positive when the culture was positive or if there were concordant positive results among histology, CLO test and ProntoDry test.
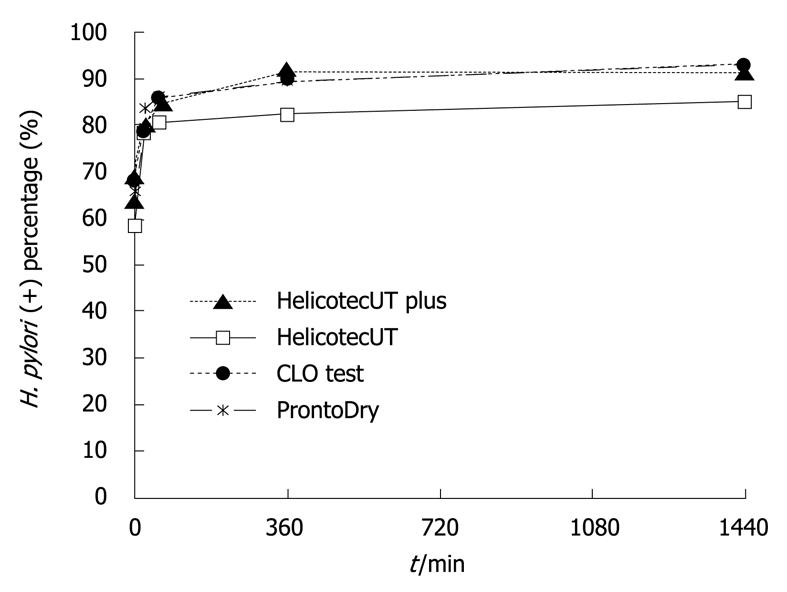
RESULTS: When dual specimens were applied, sensitivity was enhanced and RUT reaction time was significantly reduced, regardless of their treatment status. Thirty minutes were enough to achieve an agreeable positive rate in all the RUTs. Both newly developed RUTs showed comparable sensitivity, specificity and accuracy to the established RUTs, regardless of patient treatment status, RUT reaction duration, and EGD biopsy sites.
CONCLUSION: Combination of antrum and body biopsy specimens greatly enhances the sensitivity of rapid urease test and reduces the reaction duration to 30 min.
- Citation: Hsu WH, Wang SS, Kuo CH, Chen CY, Chang CW, Hu HM, Wang JY, Yang YC, Lin YC, Wang WM, Wu DC, Wu MT, Kuo FC. Dual specimens increase the diagnostic accuracy and reduce the reaction duration of rapid urease test. World J Gastroenterol 2010; 16(23): 2926-2930
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2926
Since the discovery of Helicobacter pylori (H. pylori) by Marshall and Warren in 1983[1], overwhelming evidence confirms that H. pylori infection plays a significant role in the development of chronic active gastritis, peptic ulcer, and even gastric adenocarcinoma[2-4]. H. pylori infection is very common throughout the world, occurring in 40%-50% of the population in developed countries, 80%-90% in developing regions[5], and 54.4% in Taiwan[6]. The treatment of gastrointestinal (GI) disorders resulting from H. pylori infection has become the leading issue in clinical practice.
A large number of methods have been utilized to diagnose the status of H. pylori infection, and most of them require endoscopic biopsies for tissue sampling. Nonetheless, to date, no single test other than culture is able to confirm H. pylori infection[7-9]. There are inherent advantages and disadvantages in each diagnostic test. For example, rapid urease tests (RUTs) are subject to false-negative results due to sampling errors. After H. pylori eradication, bacterial load may be reduced to patchy colonization, which is likely to result in false negative findings[10]. This prompts us to reappraise the feasibility of current sampling methods. One small piece of gastric mucosa specimen sampled from the antrum is the most popular method for RUT. A strategy for multiple samplings is required to improve the diagnostic yield. In spite of this, RUT is regarded of one of the best tests for diagnosis of H. pylori infection, given its high sensitivity and specificity. In addition, RUT is user friendly and cost effective[11-14]. Whereas some commercial kits may require at least 4 h[15,16], the ideal RUT reaction duration is 30 min because it enables endoscopists to obtain the results before patients leave the endoscopy suite.
The aim of this prospective study is to evaluate the validity of newly developed rapid urease tests, HelicotecUT plus test and HelicotecUT test, and the influence of multiple samplings during esophagogastroduodenoscopy (EGD) on the accuracy of the rapid urease test.
Between December 2008 and August 2009 355 patients in Kaohsiung Medical University Hospital, Kaohsiung, Taiwan were included in this study. They were scheduled to undergo EGD to evaluate dyspepsia and exclude organic lesions because their symptoms failed to improve after medication. All patients signed their informed consent and received complete EGD. Patients were separated according to their H. pylori treatment status: 200 patients (82 men, 118 women; mean age: 55.9 years, range: 17-86 years) in the treatment naïve group and 155 patients (69 men, 86 women; mean age: 58.0 years, range: 26-83 years) in the eradicated group. Patients meeting the following criteria were excluded: antibiotics, bismuth salts, or proton pump inhibitor used during the previous 1 mo; prior gastric surgery; presence of a bleeding peptic ulcer; severe concomitant diseases; and pregnancy or lactation.
Histological examination was done in the pathology core lab, and the gastric specimens were fixed with formalin, embedded in paraffin and stained with hematoxylin and eosin. Culture of H. pylori was carried out on the second set of specimens, which were rubbed on the surface of a Campy-BAP agar plate [Brucella agar (Difco) + IsoVitalex (Gibco) + 10% whole sheep blood], and then incubated at 35°C under microaerobic conditions (5% O2, 10% CO2, and 85% N2) for 4-5 d. H. pylori culture was considered as positive if one or more colonies of gram-negative, oxidase (+), catalase (+), and urease (+) spiral or curved rods were present. The results of RUT were interpreted as positive if the color of the gel changed from yellow to pink or red within 24 h at room temperature, and the reaction duration was recorded in minutes accordingly. A total of four RUTs were examined, including HelicotecUT plus test (Strong Biotech Corp-Taiwan), HelicotecUT test (Strong Biotech Corp-Taiwan), CLO test (Kimberly Clark-USA), and ProntoDry test (Medical Intruments Corp-France). The media contained in the CLO test and HelicotecUT were semi-solid phase (agar gel) and in HelicotecUT plus and ProntoDry test were solid phase (dry plate). Single antral specimens and dual (antrum + body) specimens were compared as well.
Six biopsy specimens from the lesser curvature side of the antrum and the greater curvature side of the gastric body, respectively were obtained to afford culture, histology and 4 different RUTs. The infection status of H. pylori infection was considered positive if the results from either antrum or gastric body met the following criteria: (1) positive culture; or (2) concordant positive results among histology and both CLO and ProntoDry tests. The results of both the CLO test and ProntoDry test were interpreted independently. After we confirmed H. pylori status, the diagnostic accuracy of each RUT was evaluated.
In this study, χ2 test was chosen for statistical analysis.
The positive status of H. pylori infection was 33.5% (67/200) in the treatment naïve group and 21.9% (34/155) in the eradicated group. The culture yield in both groups was 67.2% (45/67) (21 men, 24 women; mean age: 57.8 years, range: 27-83 years) in the treatment naïve group and 44.1% (15/34) (6 men, 9 women; mean age: 55.0 years, range: 37-74 years) in the eradicated group. This result suggested the important role of other biopsy-based tests, particularly RUT, in establishing the diagnosis of H. pylori infection.
The diagnostic power including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of the four different RUTs (HelicotecUT plus test, HelicotecUT test, CLO test, ProntoDry test, presenting in the same order afterwards) was shown in Table 1. In the treatment naïve group, all four RUTs showed comparable sensitivities, specificities and accuracy at 30 min (P = 0.988) and at 24 h (P = 0.642). Although the diagnostic yield of H. pylori infection was slightly improved at 24 h, the tests appeared readable and accurate at 30 min (30 min vs 24 h, χ2 test of accuracy, P = 0.156) (Figure 1). Interestingly, the diagnostic accuracy of dual specimens at 30 min was as comparable as single specimens at 24 h (χ2 test of accuracy, P = 0.346).
| Method | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | P1 |
| Sampling over antrum with reaction time: 30 min | ||||||
| HelicotecUT plus | 80.6 | 96.2 | 91.5 | 90.8 | 91.0 | |
| HelicotecUT | 77.6 | 98.5 | 96.3 | 90.0 | 92.0 | |
| CLO | 84.6 | 96.2 | 91.8 | 92.1 | 92.0 | |
| ProntoDry | 83.6 | 95.5 | 90.3 | 92.0 | 91.5 | 0.988 |
| Sampling over antrum with reaction time: 24 h | ||||||
| HelicotecUT plus | 91.0 | 94.7 | 89.7 | 95.5 | 93.5 | |
| HelicotecUT | 85.1 | 94.7 | 89.1 | 92.6 | 91.5 | |
| CLO | 92.5 | 95.5 | 91.2 | 96.2 | 94.5 | |
| ProntoDry | 92.5 | 94.7 | 90.0 | 96.2 | 94.0 | 0.642 |
| Sampling over antrum plus body with reaction time: 30 min | ||||||
| HelicotecUT plus | 98.5 | 92.5 | 86.8 | 99.2 | 94.5 | |
| HelicotecUT | 95.5 | 94.7 | 90.1 | 97.7 | 95.0 | |
| CLO | 97.0 | 94.0 | 89.0 | 98.4 | 95.0 | |
| ProntoDry | 97.0 | 91.7 | 85.5 | 98.4 | 93.5 | 0.902 |
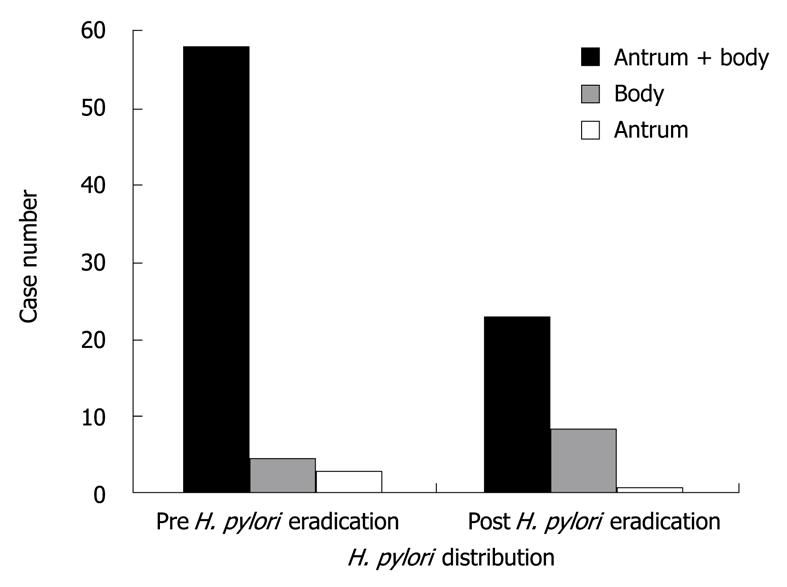
In the eradicated group, the distribution of H. pylori shifted from antrum to gastric body after H. pylori eradication was clearly observed in this study (Figure 2). Because of the lower bacterial loads and patchy distribution, in all 4 RUTs, the diagnostic accuracy of single specimens at 30 min was as low as single specimens at 24 h (χ2 test of accuracy, P = 0.124). Both results were significantly inferior to dual specimens at 30 min (χ2 test of accuracy, P = 0.01) (Table 2).
| Method | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | P1 |
| Sampling over antrum with reaction time: 30 min | ||||||
| HelicotecUT plus | 50.0 | 100.0 | 100.0 | 87.7 | 89.0 | |
| HelicotecUT | 44.1 | 100.0 | 100.0 | 86.4 | 87.7 | |
| CLO | 55.9 | 100.0 | 100.0 | 89.0 | 90.3 | |
| ProntoDry | 50.0 | 100.0 | 100.0 | 87.7 | 89.0 | 0.913 |
| Sampling over antrum with reaction time: 24 h | ||||||
| HelicotecUT plus | 61.8 | 100.0 | 100.0 | 90.3 | 91.6 | |
| HelicotecUT | 58.8 | 100.0 | 100.0 | 90.0 | 91.0 | |
| CLO | 64.7 | 99.2 | 95.7 | 91.0 | 91.6 | |
| ProntoDry | 64.7 | 100.0 | 100.0 | 91.0 | 92.3 | 0.983 |
| Sampling over antrum plus body with reaction time: 30 min | ||||||
| HelicotecUT plus | 88.2 | 99.2 | 96.8 | 96.8 | 96.8 | |
| HelicotecUT | 82.4 | 100.0 | 100.0 | 95.3 | 96.1 | |
| CLO | 88.2 | 98.3 | 93.8 | 96.7 | 96.1 | |
| ProntoDry | 82.4 | 100.0 | 100.0 | 95.3 | 96.1 | 0.987 |
There are many diagnostic tests available for H. pylori infection. Overall, patients prefer non-invasive tests over invasive tests. Consequently, recent studies have been dedicated to non-invasive tests as a result[17]. No statistical difference was reported between these two kinds of tests, given their comparable diagnostic performances[14,18]. However, during the initial diagnosis of H. pylori infection, biopsy-based diagnostic tests are usually considered in terms of costs for patients who receive endoscopic examination. RUT is the method of choice among biopsy-based diagnostic tests given their user friendliness and cost-effectiveness[13,19,20]. Short reaction duration and high diagnostic performance are the two attributes of RUT. Therefore, RUT has gained its popularity among most gastroenterologists.
RUT is made available in many commercial kits. The CLO test has been established for more than a decade and used as the main standard for rapid urease tests. Its good performance, with a sensitivity of 89.6%, a specificity of 100%, a PPV of 100%, and a NPV of 84.1%, has been acclaimed[14]. Compared with the CLO test, the newly developed HelicotecUT plus test and HelicotecUT test are equally competent in terms of their diagnostic yield. Our data showed similar results among all four RUTs, even in the eradicated group. Given their comparable diagnostic performance, the tests of lower cost, like HelicotecUT plus test and HelicotecUT test, are of more advantage.
Sampling error is the major drawback of RUT. The diagnostic yield of biopsy-based methods is usually compromised in older patients who are more likely to have extensive gastric mucosa atrophy and intestinal metaplasia. The latter, if happening in the antrum, prevents H. pylori colonization and consequently promotes the migration of H. pylori to the gastric body. Several mechanisms have been suggested, including loss of a H. pylori-specific receptor-like structure on the epitherlium and active secrection of secretory IgA for metaplastic mucosa[21-23]. In addition, after H. pylori eradication, the suppression of the density and urease activity of H. pylori by antibiotics may reduce the sensitivity of RUT. Despite this, Rollán et al[24] reported good sensitivity in the CLO test (91.7%) in their study of 12 H. pylori-positive patients and 47 H. pylori-negative patients with duodenal ulcers after eradication treatment. They took 3 biopsy specimens from different sites and put them in one test vial. This suggested that more specimens may prevent the false negative results due to sampling error. In this study, we also found that dual specimens improved the overall sensitivity of RUT, even after eradication. Furthermore, dual specimens achieved the highest performance even at 30 min. Our results further portray that more specimens for diagnosis can amend the inherent error of biopsy-based tests.
The major limitation of this study is a lack of global evaluation tests, such as urea breathing test or stool H. pylori antigen test (HpsA), which have been established as reliable tests to detect the presence of H. pylori in the hosts. Sampling bias may exist within our biopsy-based diagnostic tests, including culture, histology and RUT. In addition, the prevalence of H. pylori infection was low in our study cohort. It may be that our patients were mainly from urban area (Kaohsiung City) and might have a lower H. pylori infection rate[6].
In conclusion, a combination of antrum and body biopsy specimens for rapid urease test greatly enhances sensitivity and reduces the reaction duration to 30 min. The newly developed HelicotecUT plus test and HelicotecUT test are as effective as the established CLO test and ProntoDry test.
Helicobacter pylori (H. pylori) infection plays a significant role in the development of chronic active gastritis, peptic ulcer, and even gastric adenocarcinoma. H. pylori infection has become the leading issue in clinical practice. A large number of methods have been utilized to diagnose H. pylori infection. Rapid urease test (RUT) is regarded of one of the best tests for diagnosis of H. pylori infection, given its high sensitivity and specificity. However, sampling bias and reaction were clinical issues in clinical practice of RUT.
This study aimed to evaluate the validity of newly developed RUTs, HelicotecUT plus test and HelicotecUT test, and the influence of multiple samplings during esophagogastroduodenoscopy (EGD) on the accuracy of the RUT.
The newly developed HelicotecUT plus test and HelicotecUT test are as effective as the established CLO test and ProntoDry test.
Combination of antrum and body biopsy specimens for rapid urease test greatly enhances sensitivity and reduces the reaction duration to 30 min.
In this manuscript, the authors compared the diagnostic value of two new rapid urease test kits for detection of H. pylori and they compared the diagnostic accuracy when analysing only one antral sample or when analysing a antral and corpal samples. The golden standard used to confirm H. pylori status was either a positive culture or concordant result between two to three other tests.
Peer reviewer: Kristin Verbeke, PhD, Professor, Laboratory Digestion and Absorption, University Hospital Leuven, E462, Herestraat 49, B-3000 Leuven, Belgium
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH
| 1. | Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. |
| 2. | Goodwin CS, Armstrong JA, Marshall BJ. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986;39:353-365. |
| 3. | Wyatt JI, Rathbone BJ, Dixon MF, Heatley RV. Campylobacter pyloridis and acid induced gastric metaplasia in the pathogenesis of duodenitis. J Clin Pathol. 1987;40:841-848. |
| 4. | Hsu CT, Yeh C, Cheng HH. Helicobacter pylori, gastritis and duodenitis in the healing process of duodenal ulcer. J Formos Med Assoc. 1992;91:81-84. |
| 5. | Vaira D, Miglioli M, Mulè P, Holton J, Menegatti M, Vergura M, Biasco G, Conte R, Logan RP, Barbara L. Prevalence of peptic ulcer in Helicobacter pylori positive blood donors. Gut. 1994;35:309-312. |
| 6. | Lin JT, Wang JT, Wang TH, Wu MS, Lee TK, Chen CJ. Helicobacter pylori infection in a randomly selected population, healthy volunteers, and patients with gastric ulcer and gastric adenocarcinoma. A seroprevalence study in Taiwan. Scand J Gastroenterol. 1993;28:1067-1072. |
| 7. | Dill S, Payne-James JJ, Misiewicz JJ, Grimble GK, McSwiggan D, Pathak K, Wood AJ, Scrimgeour CM, Rennie MJ. Evaluation of 13C-urea breath test in the detection of Helicobacter pylori and in monitoring the effect of tripotassium dicitratobismuthate in non-ulcer dyspepsia. Gut. 1990;31:1237-1241. |
| 8. | Kolts BE, Joseph B, Achem SR, Bianchi T, Monteiro C. Helicobacter pylori detection: a quality and cost analysis. Am J Gastroenterol. 1993;88:650-655. |
| 9. | Whitehead R, Truelove SC, Gear MW. The histological diagnosis of chronic gastritis in fibreoptic gastroscope biopsy specimens. J Clin Pathol. 1972;25:1-11. |
| 10. | van Zwet AA, Thijs JC, Roosendaal R, Kuipers EJ, Peña S, de Graaff J. Practical diagnosis of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1996;8:501-507. |
| 11. | el-Zimaity HM, al-Assi MT, Genta RM, Graham DY. Confirmation of successful therapy of Helicobacter pylori infection: number and site of biopsies or a rapid urease test. Am J Gastroenterol. 1995;90:1962-1964. |
| 12. | Marshall BJ, Warren JR, Francis GJ, Langton SR, Goodwin CS, Blincow ED. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gastroenterol. 1987;82:200-210. |
| 13. | Cohen H, Laine L. Endoscopic methods for the diagnosis of Helicobacter pylori. Aliment Pharmacol Ther. 1997;11 Suppl 1:3-9. |
| 14. | Cutler AF, Havstad S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136-141. |
| 15. | Katsuragi K, Noda A, Tachikawa T, Azuma A, Mukai F, Murakami K, Fujioka T, Kato M, Asaka M. Highly sensitive urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori. Helicobacter. 1998;3:289-295. |
| 16. | Peura DA. Helicobacter pylori: a diagnostic dilemma and a dilemma of diagnosis. Gastroenterology. 1995;109:313-315. |
| 17. | Wu DC, Kuo CH, Lu CY, Su YC, Yu FJ, Lee YC, Lin SR, Liu CS, Jan CM, Wang WM. Evaluation of an office-based urine test for detecting Helicobacter pylori: a Prospective Pilot Study. Hepatogastroenterology. 2001;48:614-617. |
| 18. | Lin SK, Lambert JR, Schembri M, Nicholson L, Finlay M, Wong C, Coulepis A. A comparison of diagnostic tests to determine Helicobacter pylori infection. J Gastroenterol Hepatol. 1992;7:203-209. |
| 19. | Hunt RH, Malfertheiner P, Yeomans ND, Hawkey CJ, Howden CW. Critical issues in the pathophysiology and management of peptic ulcer disease. Eur J Gastroenterol Hepatol. 1995;7:685-699. |
| 20. | Elitsur Y, Hill I, Lichtman SN, Rosenberg AJ. Prospective comparison of rapid urease tests (PyloriTek, CLO test) for the diagnosis of Helicobacter pylori infection in symptomatic children: a pediatric multicenter study. Am J Gastroenterol. 1998;93:217-219. |
| 21. | Atherton JC. Non-endoscopic tests in the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11 Suppl 1:11-20. |
| 22. | Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342-345. |
| 23. | Craanen ME, Dekker W, Blok P, Ferwerda J, Tytgat GN. Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut. 1992;33:16-20. |
| 24. | Rollán A, Giancaspero R, Arrese M, Figueroa C, Vollrath V, Schultz M, Duarte I, Vial P. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection after antibiotic treatment. Am J Gastroenterol. 1997;92:1268-1274. |