Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2907
Revised: April 6, 2010
Accepted: April 13, 2010
Published online: June 21, 2010
AIM: To clarify the endoscopic and clinical findings of cytomegalovirus (CMV) gastritis after allogeneic hematopoietic stem cell transplantation (allo-SCT).
METHODS: Between 1999 and 2005, 523 patients underwent allo-SCT at our hospital, and 115 of these patients with gastrointestinal symptoms underwent esophagogastroduodenoscopy.
RESULTS: CMV gastritis was diagnosed pathologically in seven patients (1.3%) with the other 108 patients serving as controls. Six of the seven patients developed positive CMV antigenemia, and five complained of abdominal pain. Development of abdominal pain preceded CMV antigenemia in four of the five patients. Endoscopic examination showed oozing (n = 2), erosion (n = 6), and redness (n = 5) in the seven patients with CMV gastritis, while the control patients showed oozing (n = 3), erosion (n = 24), and redness (n = 100). Erosion and oozing were more frequently documented in patients with CMV gastritis compared with the controls, and the differences were statistically significant (P = 0.0012 and 0.029, respectively). CMV inclusion bodies were documented in 12 of 14 biopsy specimens obtained from erosive lesions, while they were identified in 4 of 15 biopsy specimens obtained from lesions other than erosions (P = 0.0025).
CONCLUSION: This study suggests that erosion and oozing, as well as abdominal pain, are useful indicators in the diagnosis of CMV gastritis following allo-SCT.
- Citation: Kakugawa Y, Kami M, Matsuda T, Saito Y, Kim SW, Fukuda T, Mori SI, Shimoda T, Tanosaki R, Saito D. Endoscopic diagnosis of cytomegalovirus gastritis after allogeneic hematopoietic stem cell transplantation. World J Gastroenterol 2010; 16(23): 2907-2912
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2907
Cytomegalovirus (CMV) disease is a serious complication after allogeneic hematopoietic stem cell transplantation (allo-SCT)[1], which is widely accepted as a curative therapy for advanced hematological malignancies including leukemia and malignant lymphoma. CMV disease can involve many organs and the gastrointestinal (GI) tract is a common target[2].
CMV antigenemia is one of the most widely used methods to detect CMV reactivation in a variety of clinical settings[3]; however, it is of limited value in predicting and diagnosing GI CMV disease[4]. GI CMV disease is usually diagnosed based on pathological examination of endoscopically obtained mucosal biopsy specimens. Few reports have been published regarding endoscopic examination in diagnosing CMV gastritis after allo-SCT[5-7]. This study aimed to investigate endoscopic findings of CMV gastritis after allo-SCT in addition to its clinical features.
Between January 1999 and September 2005, 523 patients underwent allo-SCT at the National Cancer Center Hospital in Tokyo, Japan. Among them, 115 patients with GI symptoms underwent esophagogastroduodenoscopy (EGD). Written informed consent was obtained from all patients before EGD. We retrospectively reviewed records of medical, endoscopic and pathological examination in the 115 EGD patients. CMV gastritis was diagnosed pathologically in seven patients (1.3%) by hematoxylin-eosin staining and immunohistochemical staining with an anti-CMV antibody. The other 108 patients served as controls.
All EGD patients orally received 100 mL of a solution containing 1 g of pronase and 1 g of sodium bicarbonate to remove mucus and bubbles on the gastric mucosa before EGD. Antiperistaltic agents (scopolamine butylbromide 20 mg or glucagon 1 mg) and sedatives (pethidine hydrochloride 17.5-35 mg or midazolam 2-3 mg) were injected intravenously. Conventional endoscopic instruments (GIF Q240; Olympus Co, Ltd, Tokyo, Japan) were used, and biopsy specimens were obtained endoscopically from severely involved areas. When abnormal findings were not found, biopsy specimens were obtained from normal appearing areas.
Biopsy specimens were fixed immediately in a 10% buffered formalin solution and subsequently stained with hematoxylin-eosin. All tissues were examined by expert pathologists. Diagnosis of CMV gastritis was based on histological identification of CMV inclusion bodies by hematoxylin-eosin staining and immunohistochemical staining with an anti-CMV antibody. Diagnosis of graft-versus-host disease (GVHD) was determined in accordance with a report published previously[8].
All patients were monitored at least once a week for CMV reactivation by CMV antigenemia assay using monoclonal antibody against C7-HRP (Teijin, Tokyo, Japan) after engraftment.
A patient was considered to be infected with CMV when CMV antigenemia assay detected CMV in the blood. A patient was considered to have CMV disease when CMV was demonstrated in biopsy specimens by hematoxylin-eosin staining and immunohistochemical analysis. Ganciclovir was initiated when either more than 10 cells per 50 000 cells were positive according to the CMV antigenemia assay in patients transplanted from related donors, a single cell per 50 000 cells was positive in patients transplanted from unrelated donors, or a patient was diagnosed as having CMV disease[9].
Acute GVHD was graded according to the consensus criteria[10,11] and all patients with grades II-IV acute GVHD were treated with 0.5-2.0 mg/kg per day of methylprednisolone.
Univariate analysis using Fisher’s exact test was performed to compare differences in patient characteristics, clinical features, and endoscopic findings between the seven patients with CMV gastritis and the other 108 patients who had GI symptoms, but did not have CMV gastritis. Values of P < 0.05 were considered significant.
Patient characteristics are shown in Table 1. There was a significant difference in the number of patients given tacrolimus with methotrexate as GVHD prophylaxis between the two groups (P = 0.018).
| Variables | Patients with CMV gastritis (n = 7) | Patients without CMV gastritis (n = 108) | |
| Median age (range) | 47 (26-62) | 45 (18-69) | |
| Gender | Male/female | 5/2 | 65/43 |
| Underlying diseases | Acute leukemia | 1 | 41 |
| Chronic leukemia | 2 | 15 | |
| Malignant lymphoma | 3 | 21 | |
| Myelodysplastic syndrome | 1 | 22 | |
| Others | 0 | 9 | |
| Preparative regimens | Myeloablative/reduced-intensity | 2/5 | 48/60 |
| Stem cell sources | Marrow/peripheral blood/cord blood | 3/3/1 | 39/64/5 |
| GVHD prophylaxis | CSP alone/CSP + MTX/CSP + MMF/ | 2/3/0/2a/0 | 36/64/2/2a/4 |
| FK506 + MTX/FK506 Alone |
Five of the seven patients with CMV gastritis complained of abdominal pain, while 31 of the 108 control patients complained of abdominal pain (P = 0.030) (Table 2). The pain was localized in the upper abdomen in all four patients with CMV gastritis whose medical reports provided the specific location of their pain (Table 3). Three patients required significant analgesia (morphine hydrochloride for one and pentazocine hydrochloride for the other two). Abdominal pain improved with ganciclovir in four of the five patients with abdominal pain, and the remaining patient (Case 1) died of bacterial pneumonia without any improvement in CMV gastritis.
| Variables | Patients with CMV gastritis (n = 7) | Patients without CMV gastritis (n = 108) | ||
| Gastrointestinal symptoms at EGD | Nausea | 2 | 60 | |
| Vomiting | 1 | 26 | ||
| Abdominal pain | 5a | 31a | ||
| Abdominal discomfort | 2 | 23 | ||
| Hematemesis | 1 | 2 | ||
| Tarry stool | 2 | 6 | ||
| Watery diarrhea | 4 | 32 | ||
| Appetite loss | 0 | 40 | ||
| CMV | Median onset of CMV gastritis | Days (range) | 63 (33-167) | NA |
| CMV antigenemia (C7-HRP) at EGD | Positive/negative/not done | 6b/1/0 | 28b/71/9 | |
| Median number of positive cells per 50 000 (range) | 8d (0-143) | 0d (0-167) | ||
| Involved organs of CMV diseases | Esophagitis/duodenitis/enterocolitis/ | 1/2/1/0/0 | 0/0/4/0/0 | |
| pneumonitis/retinitis | ||||
| GVHD | Positive (clinical grade: I/II/III/IV) | 7c (2/2/3/0) | 65c (45/10/9/1) | |
| Demographics | Gastrointestinal symptoms | CMV antigenemia assay | ||||
| Age (yr), gender, diagnosis | Any symptoms | Abdominal pain | Onset (d) | Level at EGD (cells per 50 000) | ||
| Onset (d) | Localization in abdomen | |||||
| Case 1 | 34, male, CML | Abdominal pain, tarry stool | 81 | Upper abdomen | 88 | 8 |
| Case 2 | 43, female, MDS | Nausea, abdominal pain, tarry stool, hematemesis, watery diarrhea | 53 | No description | 69 | 2 |
| Case 3 | 60, male, AML | Abdominal pain, watery diarrhea | 62 | Upper abdomen | 73 | 143 |
| Case 4 | 48, female, ML | Abdominal pain, watery diarrhea | 36 | Upper abdomen | 31 | 10 |
| Case 5 | 47, male, ML | Abdominal pain, watery diarrhea | 30 | Upper abdomen | 32 | 4 |
| Case 6 | 62, male, CML | Abdominal discomfort | NA | NA | 47 | 32 |
| Case 7 | 26, male, ML | Nausea, vomiting, abdominal discomfort | NA | NA | NA | 01 |
Watery diarrhea was found in four of the seven patients with CMV gastritis, and was complicated by intestinal GVHD in three of these four patients. Watery diarrhea improved with ganciclovir in a patient with CMV gastritis who had no evidence of intestinal GVHD.
All seven patients with CMV gastritis had GVHD, while 65 of the 108 control patients had GVHD (P = 0.044) (Table 2). Five of the seven patients with CMV gastritis had grade II-IV GVHD that was being treated by corticosteroids.
Six of the seven patients with CMV gastritis and 28 of the 108 controls showed positive CMV antigenemia (P = 0.0026) (Table 2). The median number of positive cells in the CMV antigenemia test among the seven patients with CMV gastritis was 8 cells per 50 000 cells (range, 0-143) at the time of EGD.
Development of abdominal pain preceded the CMV antigenemia in four of the five patients who complained of it, and the median interval between onset of abdominal pain and the first positive CMV antigenemia was 7 d (range, -5 to 16 d) (Table 3).
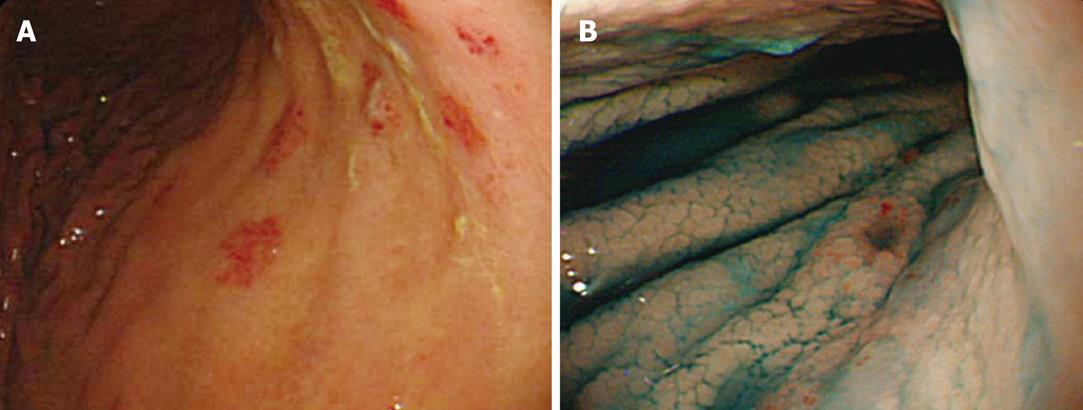
Erosion was observed in six of the seven patients with CMV gastritis and in 24 of the 108 control patients (P = 0.0012) (Table 4). The erosive lesions were located in the antrum (n = 2), body (n = 2), and antrum-body (n = 2) of the stomach. Two of the six patients had a solitary erosion, and the other four patients had multiple erosions of various sizes. Erosions were flat in four patients and raised in the other two. A representative example of erosion is shown in the accompanying figure; multiple erosions are clearly shown in the gastric body (Figure 1).
Oozing was observed in two of the seven patients with CMV gastritis and in three of the 108 control patients (P = 0.029). Oozing was located in the antrum with erosion (Case 3), and in the antrum-body with mucosal sloughing (Case 2).
Detailed information regarding pathological findings is shown in Table 5. CMV inclusion bodies were documented in 12 of 14 biopsy specimens obtained from erosive lesions, while they were identified in 4 of 15 biopsy specimens obtained from lesions other than erosions (P = 0.0025) (Table 5).
| Positive specimens with CMV inclusion bodies/total specimens in EGD biopsy | Response to ganciclovir | Outcome | ||||||
| Total | Erosions | Mucosal sloughing | Other findings or normal mucosa | Abdominal pain | CMV antigenemia assay | Outcome | Cause of death | |
| Case 1 | 1/2 | 1/2 | None | 0 | Continued | Continued | Death | Bacterial pneumonia |
| Case 2 | 2/2 | None | 2/21 | 0 | Improved | Turned negative | Death | Recurrence of primary disease |
| Case 3 | 2/3 | 2/31 | None | 0 | Improved | Turned negative | Death | Renal failure |
| Case 4 | 5/7 | 4/4 | None | 12/3 | Improved | Turned negative | Alive | NA |
| Case 5 | 1/5 | 1/1 | None | 0/4 | Improved | Turned negative | Alive | NA |
| Case 6 | 4/7 | 3/3 | None | 12/4 | NA | Turned negative | Alive | NA |
| Case 7 | 1/3 | 1/1 | None | 0/2 | NA3 | NA4 | Death | Recurrence of primary disease |
| Case 7 | 26, male, ML | Nausea, vomiting, abdominal discomfort | NA | NA | NA | 01 | ||
Four patients died, and CMV disease was not the primary cause of death in any of them (Table 5). Two died from recurrences of their primary diseases, one died of bacterial pneumonia and one died of renal failure.
The present study clarifies the endoscopic findings of CMV gastritis following allo-SCT in addition to its clinical features. CMV gastritis was diagnosed pathologically in seven patients (1.3%) among 523 patients who underwent allo-SCT at our facility. The incidence is comparable to a previous study (1.7%)[12]. None of the seven patients died of CMV gastritis, while three patients complained of significant abdominal pain requiring analgesia which impaired their quality of life. CMV gastritis was a clinically important complication after allo-SCT.
No detailed information on clinical features of CMV gastritis has been previously reported. In the present study, abdominal pain was a common symptom of CMV gastritis. The pain was localized in the upper abdomen in all four patients whose medical reports provided the specific location of their pain. Ganciclovir administration improved abdominal pain in these four patients, supporting the likelihood that this symptom was attributable to CMV gastritis. Clinicians should pay particular attention to upper abdominal pain following allo-SCT as a possible symptom of CMV gastritis.
The association between watery diarrhea and CMV gastritis may be minimal as it remained unclear whether such diarrhea was due to CMV gastritis or overlapping intestinal GVHD. In the present study, ganciclovir improved symptoms in only one of four patients with diarrhea. In contrast, CMV gastritis was complicated by intestinal GVHD in three of those four patients. Our observations suggested that watery diarrhea in patients with CMV gastritis was more likely due to intestinal GVHD rather than the CMV gastritis itself.
Endoscopic findings characteristic of CMV gastritis after allo-SCT have not been fully investigated, but the present study indicates that erosion and oozing might be useful markers for early diagnosis of CMV gastritis. Vascular endothelium infected with CMV narrows vessels and induces local ischemia[13] eventually resulting in erosions and oozing. In fact, most CMV inclusion bodies were obtained from erosion sites. Erosions from CMV gastritis developed in all stomach sites and varied in size. Endoscopists should suspect CMV gastritis and obtain multiple biopsies whenever erosions are found in any stomach site.
In contrast, none of the seven patients with CMV gastritis had punched out ulcers which had previously been considered characteristic of GI CMV disease[14-16]. In the present study, early EGD might have enabled early diagnosis of CMV gastritis before progression to ulcers. In two patients (Cases 4 and 6), CMV inclusion bodies were identified pathologically from normal mucosa as well as erosions. This result demonstrates the necessity of biopsy even if only normal findings are identified when EGD is performed.
CMV antigenemia reflects the severity of CMV reactivation[3,17], but the clinical significance of CMV antigenemia remains unknown in the diagnosis of GI CMV disease because of the wide variation in positive findings, ranging from a low of 21%[4] to a high of 73%[18]. In this study, CMV antigenemia was positive in six of the seven patients with CMV gastritis. This result supports the usefulness of CMV antigenemia in the diagnosis of CMV gastritis. It should be noted that abdominal pain preceded CMV antigenemia in four of the five patients with positive CMV antigenemia and abdominal pain. Our observations suggest that elaboration of physical and endoscopic examinations is even more important than detection of CMV antigenemia in the early diagnosis of CMV gastritis.
Patients with GVHD, and patients given corticosteroids for treatment of GVHD, carry a high risk of CMV disease[19]. In this study, such increased risk was confirmed as all seven patients with CMV gastritis also had GVHD and five of them had grade II-IV GVHD that was being treated by corticosteroids. GVHD, by itself and also accompanied by corticosteroid administration, are exacerbating factors in the existence of CMV gastritis.
The present investigation was a retrospective study based on our examination of medical records as well as endoscopic and pathological findings. The small size of the study does not exclude the possibility of unrecognized bias. Since EGD was not conducted in all allo-SCT recipients, underestimation of the frequency of CMV gastritis is a possibility. Consequently, further prospective evaluation is warranted to clarify the endoscopic findings for early diagnosis of CMV gastritis.
The results of this study suggest that endoscopic and clinical findings are useful indicators in the diagnosis of CMV gastritis following allo-SCT. Use of EGD is warranted for the establishment of an early diagnosis of CMV gastritis following allo-SCT.
Cytomegalovirus (CMV) disease is a serious complication after allogeneic hematopoietic stem cell transplantation (allo-SCT), which is widely accepted as a curative therapy for advanced hematological malignancies including leukemia and malignant lymphoma. CMV disease can involve many organs and stomach is a common target.
Few reports have been published regarding endoscopic examination in diagnosing CMV gastritis after allo-SCT. In this study, the authors demonstrate the endoscopic findings of CMV gastritis after allo-SCT in addition to its clinical features.
The present study indicated that erosion and oozing might be useful markers for early diagnosis of CMV gastritis.
Endoscopists should suspect CMV gastritis and obtain multiple biopsies whenever erosions are found in any stomach site when performing esophagogastroduodenoscopy in patients after allo-SCT.
This is an interesting manuscript on an important topic. The study presented here is a retrospective one with a small number of affected patients, but it offers some deeper insights into this complex problem. Limitations of this study and difficulties in diagnosis and interpretation are addressed by the authors in the discussion section.
Peer reviewer: Dr. Herwig R Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, A-8036 Graz, Austria
S- Editor Wang YR L- Editor Logan S E- Editor Ma WH
| 1. | Stocchi R, Ward KN, Fanin R, Baccarani M, Apperley JF. Management of human cytomegalovirus infection and disease after allogeneic bone marrow transplantation. Haematologica. 1999;84:71-79. |
| 2. | Spencer GD, Hackman RC, McDonald GB, Amos DE, Cunningham BA, Meyers JD, Thomas ED. A prospective study of unexplained nausea and vomiting after marrow transplantation. Transplantation. 1986;42:602-607. |
| 3. | Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063-4071. |
| 4. | Mori T, Mori S, Kanda Y, Yakushiji K, Mineishi S, Takaue Y, Gondo H, Harada M, Sakamaki H, Yajima T. Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:431-434. |
| 5. | Fujishima N, Hirokawa M, Fujishima M, Saitoh H, Odashima M, Nanjo H, Sawada K. Cytomegalovirus-associated granulomatous gastritis after cord blood transplantation for acute myeloid leukemia. Int J Hematol. 2007;85:362-363. |
| 6. | Minami H, Matsushita T, Sugihara T, Kodera Y, Sakai S, Shimokata K. Cytomegalovirus-induced gastritis in a bone marrow transplant patient. Jpn J Med. 1990;29:433-435. |
| 7. | Strayer DS, Phillips GB, Barker KH, Winokur T, DeSchryver-Kecskemeti K. Gastric cytomegalovirus infection in bone marrow transplant patients: an indication of generalized disease. Cancer. 1981;48:1478-1483. |
| 8. | Sale GE, Shulman HM, McDonald GB, Thomas ED. Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol. 1979;3:291-299. |
| 9. | Kanda Y, Mineishi S, Saito T, Saito A, Ohnishi M, Niiya H, Chizuka A, Nakai K, Takeuchi T, Matsubara H. Response-oriented preemptive therapy against cytomegalovirus disease with low-dose ganciclovir: a prospective evaluation. Transplantation. 2002;73:568-572. |
| 10. | Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825-828. |
| 11. | Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, Erickson K, Flowers M, Hansen J, Loughran T. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250-259. |
| 12. | van Burik JA, Lawatsch EJ, DeFor TE, Weisdorf DJ. Cytomegalovirus enteritis among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2001;7:674-679. |
| 13. | Roberts WH, Sneddon JM, Waldman J, Stephens RE. Cytomegalovirus infection of gastrointestinal endothelium demonstrated by simultaneous nucleic acid hybridization and immunohistochemistry. Arch Pathol Lab Med. 1989;113:461-464. |
| 14. | Wilcox CM, Chalasani N, Lazenby A, Schwartz DA. Cytomegalovirus colitis in acquired immunodeficiency syndrome: a clinical and endoscopic study. Gastrointest Endosc. 1998;48:39-43. |
| 15. | Hinnant KL, Rotterdam HZ, Bell ET, Tapper ML. Cytomegalovirus infection of the alimentary tract: a clinicopathological correlation. Am J Gastroenterol. 1986;81:944-950. |
| 16. | Iwasaki T. Alimentary tract lesions in cytomegalovirus infection. Acta Pathol Jpn. 1987;37:549-565. |
| 17. | Gondo H, Minematsu T, Harada M, Akashi K, Hayashi S, Taniguchi S, Yamasaki K, Shibuya T, Takamatsu Y, Teshima T. Cytomegalovirus (CMV) antigenaemia for rapid diagnosis and monitoring of CMV-associated disease after bone marrow transplantation. Br J Haematol. 1994;86:130-137. |
| 18. | Halme L, Höckerstedt K, Salmela K, Lautenschlager I. Cytomegalovirus detected in the upper gastrointestinal tract parallel with CMV-antigenemia in liver transplant patients. Transplant Proc. 1999;31:487. |
| 19. | Winston DJ, Ho WG, Champlin RE. Cytomegalovirus infections after allogeneic bone marrow transplantation. Rev Infect Dis. 1990;12 Suppl 7:S776-S792. |