Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2901
Revised: March 26, 2010
Accepted: April 2, 2010
Published online: June 21, 2010
AIM: To study whether high-sensitivity C-reactive protein (hs-CRP) measurement can aid the assessment of disease activity and glucocorticoid treatment in paediatric inflammatory bowel disease (IBD).
METHODS: CRP levels were measured in 39 children with IBD undergoing colonoscopy [median age 12.8 years, Crohn’s disease (CD) n = 20], in 22 other children with IBD followed for acute response to glucocorticoids, and in 33 paediatric non-IBD patients. When standard CRP level was below detection limit (< 5 mg/L), hs-CRP was analyzed.
RESULTS: Sixty-four percent (25/39) of the children with IBD undergoing colonoscopy displayed undetectable (< 5 mg/L) standard CRP levels. Of these, the hs-CRP measurement could not differentiate between active (median, 0.2 mg/L, range, 0.007-1.37, n = 17) or quiescent (0.1 mg/L, 0.01-1.89, n = 8, P = NS) disease. Patients with ileocolonic CD had higher CRP levels (14 mg/L, 0.06-45, n = 13) than patients with no ileal involvement (0.18 mg/L, 0.01-9, n = 7, P < 0.01) or ulcerative colitis (UC) (0.13 mg/L, 0.007-23, P < 0.05). In children with active IBD treated with systemic glucocorticoids, the standard CRP was undetectable in 59% of the patients. The hs-CRP levels did not differ between patients that responded to steroid therapy and in non-responders.
CONCLUSION: The measurement of hs-CRP did not prove useful in the assessment of disease activity or glucocorticoid treatment in paediatric IBD patients that had undetectable standard CRP.
- Citation: Sidoroff M, Karikoski R, Raivio T, Savilahti E, Kolho KL. High-sensitivity C-reactive protein in paediatric inflammatory bowel disease. World J Gastroenterol 2010; 16(23): 2901-2906
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2901
C-reactive protein (CRP) is produced mainly in hepatocytes in response to acute phase stimuli such as inflammation[1]. Its production is driven by circulating cytokines interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) originating from the inflamed tissues[2]. Genetic variation, as well as age and sex affect the CRP levels[3]. As an acute phase response, CRP concentration may increase rapidly up to 1000-fold[4].
CRP is commonly used to screen the activity of chronic inflammatory diseases including inflammatory bowel disease (IBD). A study comparing CRP with clinical, endoscopic and radiologic findings in adults with IBD found an association between CRP, clinical disease activity, endoscopic inflammation and active histological inflammation[5]. Very recently however, a Norwegian group reported that only 29% of adult patients with ulcerative colitis (UC) and 75% of patients with Crohn’s disease (CD) had elevated CRP levels at diagnosis[6]. In general, CD patients have higher CRP levels than patients with UC[7]. Still, also in CD a subgroup of patients with clinically active disease present with low CRP. In two large clinical studies investigating new biological agents (CDP870 and natalizumab, respectively) in active CD, 60% of the patients had CRP levels below 10 mg/L and 25% below 3 mg/L[8,9].
In children, the data on CRP levels in active IBD is sparse. In a report from St Bartholomew’s Hospital, all paediatric patients with active CD (n = 26) had CRP values higher than 5 mg/L; of patients with UC (n = 13), only 60% reached this cut-off level[10].
High-sensitivity CRP (hs-CRP) assays measure CRP levels that were previously thought to be under the detection limit. In adult IBD patients, hs-CRP correlates with clinical disease activity in both UC and CD[11]. In children, this question has not been addressed. Therefore, in this pilot study we investigated the association between hs-CRP and clinical and histological activity in paediatric IBD patients, and evaluated the effect of glucocorticoid treatment on the hs-CRP levels.
Study group I: Paediatric IBD patients studied at the time of colonoscopy: The first group was stratified from the consecutive paediatric IBD patients who underwent colonoscopies and agreed to participate in a follow-up study of IBD patients at the Hospital for Children and Adolescents, Helsinki, Finland. Inclusion criteria to the study were (1) endoscopy-based diagnosis of either UC or CD[12], and (2) age ≤ 18 years. Exclusion criteria were (1) previous surgery, and (2) any signs of infection during the preceding week.
The clinical characteristics of the 39 patients are shown in Table 1. 20 patients were newly diagnosed. The other 19 patients received the following medication: 7 patients were on 5-ASA, 3 patients on 5-ASA and glucocorticoid, 3 patients were on 5-ASA, glucocorticoid and azathioprine, 1 patient on 5-ASA and antibiotics, 1 patient on glucocorticoid and azathioprine, and 1 patient on glucocorticoid, azathioprine, antibiotics and infliximab. Two patients had no medication due to quiescent disease and 1 patient had no medication due to non-compliance.
| UC | 19 |
| M/F | 10/9 |
| Pancolitis | 16 |
| Left-sided disease | 3 |
| CD | 20 |
| M/F | 14/6 |
| Ileocolitis | 13 |
| Colitis | 7 |
| Age (median, range, yr) | 12.8 (1.9-18) |
| BMI (median, range, kg/m2) | 18.40 (14.2-25) |
| Fresh diagnosis | 20/39 |
The patients who underwent colonoscopy due to fresh disease, aggravation of the symptoms or lack of response to conventional medication were defined as having clinically active disease according to Physicians Global Assessment (PGA). The patients who underwent colonoscopy in clinical remission according to PGA were categorized as having quiescent disease.
Study group II: Paediatric IBD patients followed up for acute response to glucocorticoids: The second group was a prospectively collected group of 22 children and adolescents (median age 13.1 years, age range 3.5-18 years) with active IBD who were introduced to systemic glucocorticoid treatment due to active disease (Table 2) as described in detail[13]. Six patients in this study were recruited to the prospective extension of the previous study[13]. Other inclusion criteria to the study were similar to group I. The maintenance medication of the patients at the onset of the steroid was: 5-ASA (n = 12), 5-ASA and azathioprine (n = 2), antibiotics (n = 4), no medication (n = 4).
| UC | 14 |
| M/F | 9/5 |
| Pancolitis | 11 |
| Left-sided disease | 3 |
| CD | 7 |
| M/F | 5/2 |
| Ileocolitis | 2 |
| Colitis | 5 |
| IC | 1 male |
| Age (median, range, yr) | 13.1 (3.5-18) |
| BMI (median, range, kg/m2) | 16.9 (13.2-22.6) |
| Fresh diagnosis | 7/22 |
The glucocorticoid treatment was started either with peroral prednisolone (1 mg/kg per day, Prednisolone®, Leiras, Finland, n = 20) or budesonide (9 mg/d, Entocort®, AstraZeneca AB, Sweden, n = 2). The venous blood samples were drawn before the treatment was started and at the clinical follow-up visit at 2-4 wk after starting the glucocorticoid.
Clinician’s judgement (PGA) of clinical improvement (decrease in stool rate and the amount of blood in the stools, abating diarrhea) and decrease in the inflammatory markers [erythrocyte sedimentation rate (ESR) and faecal calprotectin] by the time of the follow-up visit (see above) was defined as therapeutic response. When these patients were recruited, no paediatric activity index for UC yet existed[14]. Acute (appearing during the first month of the treatment) glucocorticoid-related side effects (weight gain, development of moon face, striae or acne) were registered[13]. The therapeutic response and the development of side-effects were both graded with a two-grade scale (good/poor response, yes/no side effects)[15,16].
Controls: Thirty-three children and adolescents (median age 12.1 years, range 1.8-17.2 years) visiting the Outpatient Clinic of the Hospital for Children and Adolescents, Helsinki, Finland, due to gastrointestinal disorder (coeliac disease n = 5, loose stools n = 4, liver dysfunction n = 3, pancreatic dysfunction n = 3, abdominal pain n = 2, Helicobacter gastritis n = 1, gastro-oesophageal reflux disease n = 1), growth follow-up n = 4, derangement in laboratory tests (hypercholesterolemia n = 3, leukopenia n = 1, sideropenia n = 1) or other reasons (obesity n = 1, fatigue n = 2, talassemia n = 1, and toe pain n = 1) served as non-IBD controls. Patients with acute infection, fever or trauma were excluded.
During the colonoscopies, routine biopsies were taken from the ileum, caecum, colon ascendens, transversum, descendens, sigma and rectum. The histological findings were graded by an experienced paediatric pathologist (R.K.) with a modified histology score originally developed for CD (Table 3)[17,18]. The score was calculated: (1) separately for each segment (max 15 points) and (2) adding the scores of the caecum, colon ascendens, transversum, descendens, sigma and rectum to obtain the total score for the colon (max 90 points). Eosinophils were taken into account when analysing the quantity of the polymorphonuclear cells. Instead, lymphoid hyperplasia was omitted from the count, since it represents a normal variant. The presence of granulomas was registered, but not calculated in the score.
| Epithelial damage |
| 0 Normal |
| 1 Focal pathology |
| 2 Extensive pathology |
| Architectural changes |
| 0 Normal |
| 1 Moderately (< 50%) disturbed |
| 2 Severely (> 50%) disturbed |
| Infiltration of mononuclear cells in the lamina propria |
| 0 Normal |
| 1 Moderate increase |
| 2 Severe increase |
| Infiltration of polymorphonuclear cells in the lamina propria |
| 0 Normal |
| 1 Moderate increase |
| 2 Severe increase |
| Infiltration of polymorphonuclear cells in the epithelium |
| 0 Normal |
| 1 If in surface epithelium |
| 2 If cryptitis |
| 3 If a crypt abscess present |
| Presence of erosion or ulcers |
| 0 No |
| 1 Yes |
| The number of biopsy specimens affected |
| 0 None |
| 1 < 33% |
| 2 33%-66% |
| 3 > 66% |
The venous blood samples were scheduled between 11 am and 3 pm. Blood count, standard CRP (detection limit 5 mg/L), and ESR were analysed in a clinical laboratory. In patients with standard CRP levels under the detection limit, serum hs-CRP levels were measured. The hs-CRP analyses were performed with a human C-reactive protein Instant ELISA kit (Bender MedSystems GmbH, Vienna, Austria) that has an intra-assay coefficient of variation (C.V.) of 6.9%, interassay C.V. of 13.1% and detection limit of 3 × 10-6 mg/L. Faecal calprotectin was quantified as previously reported[19].
Mann-Whitney’s U test, Kruskal-Wallis test, Wilcoxon’s signed rank sum test, Fisher’s exact test and Spearman’s rank order correlation test were used when investigating associations between non-parametric variables. Statistical analyses were performed using SPSS by SPSS Inc. software. P < 0.05 was accepted to indicate statistical significance. Values are expressed as median (range) in the text.
The families were informed about the study, and a written informed consent was obtained from the patient and their guardians. The study protocol was approved by the Ethics Committee for the Hospital for Children and Adolescents, University of Helsinki.
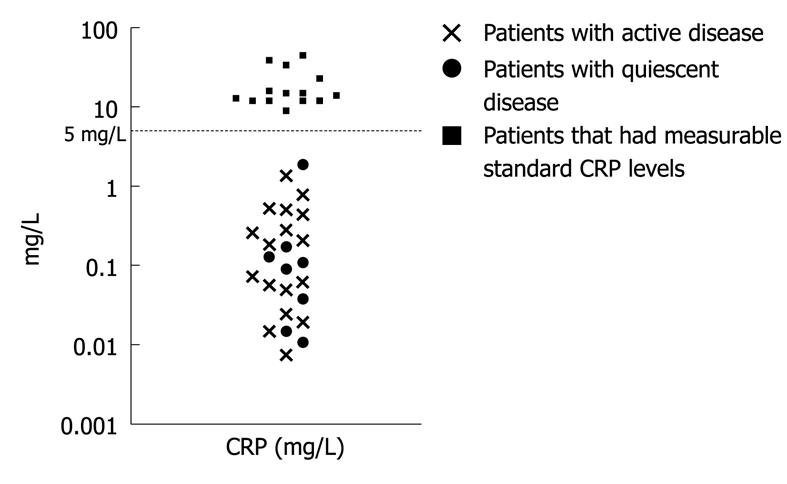
Standard CRP testing was undetectable (< 5 mg/L) in 64% (25/39) paediatric IBD patients investigated at the time of the colonoscopy. Of the patients with standard CRP levels below the detection limit, hs-CRP was measurable in all cases (Figure 1).
The median CRP level in the whole IBD group was 0.4 mg/L (0.007-45 mg/L). In all IBD patients, either with a fresh (0.7 mg/L, 0.02-39) or existing (0.2 mg/L, 0.007-45) diagnosis of colitis, CRP levels were higher than in the control patients (P < 0.01, see below). However, in patients with standard CRP levels under the detection limit of 5 mg/L, the hs-CRP measurement could not stratify the patients according to disease activity (active disease 0.2 mg/L, 0.007-1.37, n = 17 vs quiescent disease 0.1 mg/L, 0.01-1.89, n = 8, P = NS).
In CD patients, standard CRP was undetectable in 45% (9/20) of the patients, in UC in 84% (16/19) of the patients (P < 0.05). Patients with ileocolonic CD had clearly higher CRP levels (14 mg/L, 0.06-45, n = 13) than patients with CD colitis (0.18 mg/L, 0.01-9, n = 7, P < 0.01) whose CRP levels were similar to the patients with UC (0.13 mg/L, 0.007-23, P = NS). The CRP levels in CD patients were also higher in those presenting with granulomas (14 mg/L, 0.5-39, n = 9) than in those without granulomas (0.5 mg/L, 0.01-45, n = 11, P < 0.05). However, patients with granulomas did not have significantly more ileocolonic disease than colitis (P = 0.07).
The correlations between CRP levels, inflammatory markers and histology score, and the median (range) of these variables are presented in Table 4. The CRP levels did not correlate with age or weight; however, boys had higher CRP levels (4.9 mg/L, 0.007-45, n = 24) than girls (0.2 mg/L, 0.01-14, n = 15, P < 0.05).
| Median (range) | Correlation with CRP | |
| Inflammatory markers | ||
| Calprotectin (n = 35) | 690 μg/g (14-4400) | 0.488b |
| ESR (n = 37) | 18 mm/h (2-97) | 0.618d |
| WBC (n = 38) | 7.6 E9/L (3.2-17.4) | -0.011 |
| Histology score | ||
| Ileum (n = 29)1 | 0 (0-15) | 0.475a |
| Caecum (n = 38) | 4 (0-14) | -0.036 |
| Ascendens (n = 34) | 4.5 (0-13) | -0.198 |
| Transversum (n = 36) | 6 (0-15) | -0.158 |
| Descendens (n = 39) | 6 (0-15) | -0.077 |
| Sigma (n = 35) | 5 (0-14) | -0.014 |
| Rectum (n = 38) | 5.5 (0-14) | -0.051 |
| Total colon (n = 32) | 30 (0-74) | -0.083 |
The median CRP level in the 33 non-IBD controls was 0.03 mg/L (0.008-0.7 mg/L), significantly lower that in the above-mentioned group of 39 IBD patients (P < 0.001). In controls, the distribution of age (12.1 years, 1.8-17.2), sex (20 males, 13 females) and weight (37 kg, 12.8-84.6) were comparable to the 39 IBD patients (P = NS). Unlike in the IBD patients, CRP in the control patients correlated with weight (r = 0.460, P < 0.01). The control patients in the highest hs-CRP quartile were admitted due to obesity (n = 1), chronic autoimmune hepatitis (n = 1), leukopenia (n = 1), hypercholesterolemia (n = 1), talassemia minor (n = 1), gastro-oesophageal reflux disease (n = 1), coeliac disease (n = 1) and growth follow-up (n = 1).
The median pre-treatment CRP level of the 22 IBD patients starting peroral glucocorticoid therapy was 0.6 mg/L (0.01-39), similar to the level of CRP in the 39 IBD patients undergoing colonoscopy (P = NS). At the onset of the glucocorticoid treatment, the standard CRP levels were below the detection limit of 5 mg/L in 59% (13/22) of the patients. After 2-4 wk of treatment, the median level was 0.08 mg/L (0.004-60, P < 0.05). Of the 13 patients whose hs-CRP was measured (standard CRP undetectable), 7 patients responded well to the glucocorticoids; however the change in their hs-CRP levels during the glucocorticoid did not differ from those patients who had poor response (P = 0.16). The development of glucocorticoid-related side effects was not associated with serum CRP levels (data not shown).
The aim of our study was to analyse whether hs-CRP can improve the assessment of disease activity in paediatric patients with IBD. We measured CRP with standard and high-sensitivity methods in children and adolescents presenting with colitis, and studied the association with other inflammatory markers (ESR, faecal calprotectin, and white blood cell count) and with the histological activity of the inflammation in the gut.
Even though CRP is commonly used as a marker of inflammatory activity in IBD patients, our findings support the concept that a significant number of patients with active IBD present with low CRP levels[6,10]. Here, standard CRP was under the detection limit of 5 mg/L in 60% of the paediatric patients with active colitis. All patients with undetectable standard CRP had measurable hs-CRP levels but there was no cut-off value for low CRP values with which to differentiate active and quiescent disease.
In this study all patients with CD presented with colitis. Thereby, it was somewhat surprising that there was a clear difference in the CRP values between UC and CD, the latter presenting with higher values. However, high CRP values associated with ileocolonic CD and in CD colitis with no ileal involvement the CRP values were similar to UC. This is in contrast to previous studies in adults with IBD showing no difference between disease location and CRP levels in CD patients, or even opposing results - ileal disease associated with low CRP levels[6,20]. However, in our study we investigated also the association between CRP and histological activity of the colonoscopy biopsies, and found that the CRP levels correlated only with the histological score of the ileal biopsies, not with colonic inflammation. Studies in adults with CD have shown that an accumulation of intra-abdominal fat has been observed in CD patients, and that CRP levels correlate with radiological findings of small bowel perienteric inflammation (increased fat density)[21,22]. Since CRP is expressed in human adipose tissue, our findings may reflect higher local CRP production in the inflamed perienteric ileal region[23].
The association between granulomas and CRP levels in our study is a novel observation. In the literature, the evidence on the clinical significance of granulomas in CD patients is conflicting. Some studies associate the presence of granulomas with a more aggressive disease course, whereas others do not report any connection between granulomas and disease severity[24,25]. Recently, a study from the Children’s Hospital of Philadelphia did not find a relationship between CRP levels and granulomas, although the presence of granulomas was associated with a more severe disease course[26]. The discrepancy between the CRP findings in these two studies could be explained by the fact that the patient populations differ, because the US study also included patients presenting granulomas only in the upper GI tract or terminal ileum. In an in vitro study, invasive Escherichia coli strains recovered from CD lesions caused macrophages to secrete TNF-α[27]. It is intriguing to speculate that higher local production of TNF-α in the granulomatous lesions of CD patients could explain the higher CRP levels associating with granulomas in our study. However, as yet the relationship remains elusive.
Only a few groups have studied the hs-CRP levels in IBD, and none of them have concentrated on the treatment-induced changes[11,28,29]. In this study, we additionally analysed a group of children and adolescents with IBD that started peroral glucocorticoid therapy due to the activation of the disease. In our small group of patients however, the measurement of hs-CRP did not provide any additional information as to the treatment of these patients.
Limitations of our study are the relatively small sample size, especially when the patients are divided into subgroups of UC and CD. In addition, apart from inflammation, minor trauma, acute infection, obesity and the use of NSAIDs, and genetic heterogeneity in the basal expression of CRP may affect the hs-CRP levels[30]. However, all patients undergoing colonoscopy had normal BMI and did not have any clinical signs of acute infection. As the subjects are children, we cannot rule out the possibility of minor bumps and bruises that might have affected the hs-CRP levels. Also, the possible intake of NSAIDs was unfortunately not recorded.
In conclusion, our study reinforces the concept that a significant number of paediatric patients with active IBD may present with CRP levels that are under the detection limit. Hs-CRP instead, was detectable in all the patients. Unfortunately, in this pilot study the measurement of hs-CRP levels in the patients that had undetectable standard CRP levels could not stratify the patients according to disease activity or response to treatment.
C-reactive protein (CRP) is used to assess disease activity in diverse inflammatory disorders including inflammatory bowel disease (IBD). However, in IBD a significant number of patients present with low CRP levels despite clinically active disease. In paediatric patients with IBD the performance of CRP is an understudied area.
High-sensitivity CRP (hs-CRP) measures CRP levels that were previously thought to be under the detection limit. In paediatric IBD, this kind of highly sensitive marker is needed for the detection of the presence of inflammation. Thus, the authors studied the performance of hs-CRP in paediatric IBD to see whether it improves the assessment of inflammation in these patients and/or associates with the response to glucocorticoid therapy.
Hs-CRP is a sensitive marker of inflammation that is used in various conditions (asthma, cardiovascular disease). In paediatric IBD, the performance of the hs-CRP has not been studied previously. The authors show here that standard CRP test is negative in a considerable number of paediatric patients with active IBD and the routine measurement of CRP is thus not informative enough. Hs-CRP detects low levels of CRP but disappointingly it does not help to distinguish children with active intestinal inflammation from those with quiescent disease or those responding to glucocorticoid treatment from non-responders. Interestingly, the levels of hs-CRP correlated with the presence of ileal inflammation.
The determination of hs-CRP seems to be of minor aid in assessing the presence of inflammation in paediatric IBD. In ileal disease, however, hs-CRP correlates with the presence of histological inflammation, a finding warranting further studies.
IBD comprising Crohn’s disease (CD) and ulcerative colitis is a chronic, relapsing and remitting disease of the gastrointestinal tract characterized by diarrhea, abdominal pain and weight loss. CRP is an acute phase protein produced by liver that is used in the diagnosis of inflammatory disorders.
This clinical study has originality, is well designed and executed; the results are well presented and interpreted. Although this study shows a negative result, it has still provided valuable information on the CRP measurement in children IBD patients. One of the weaknesses is the small sample size of subgroup patients particularly CD patients in Table 2. It would strengthen the conclusion if more patients were included in this study.
Peer reviewer: Zhang-Xu Liu, MD, PhD, Assistant Professor, Research Center for Liver diseases, Keck School of Medicine, University of Southern California, 2011 Zonal Avenue, HMR101, Los Angeles, CA 90033, United States
S- Editor Wang YR L- Editor O'Neill M E- Editor Lin YP
| 1. | Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104-111. |
| 2. | Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661-665. |
| 3. | Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999-2000. Clin Chem. 2003;49:1353-1357. |
| 4. | Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487-48490. |
| 5. | Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707-712. |
| 6. | Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, Moum B. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518-1523. |
| 7. | Fagan EA, Dyck RF, Maton PN, Hodgson HJ, Chadwick VS, Petrie A, Pepys MB. Serum levels of C-reactive protein in Crohn's disease and ulcerative colitis. Eur J Clin Invest. 1982;12:351-359. |
| 8. | Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807-818. |
| 9. | Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912-1925. |
| 10. | Beattie RM, Walker-Smith JA, Murch SH. Indications for investigation of chronic gastrointestinal symptoms. Arch Dis Child. 1995;73:354-355. |
| 11. | Zilberman L, Maharshak N, Arbel Y, Rogowski O, Rozenblat M, Shapira I, Berliner S, Arber N, Dotan I. Correlated expression of high-sensitivity C-reactive protein in relation to disease activity in inflammatory bowel disease: lack of differences between Crohn's disease and ulcerative colitis. Digestion. 2006;73:205-209. |
| 12. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. |
| 13. | Vihinen MK, Raivio T, Verkasalo M, Jänne OA, Kolho KL. Circulating glucocorticoid bioactivity during peroral glucocorticoid treatment in children and adolescents with inflammatory bowel disease. J Clin Gastroenterol. 2008;42:1017-1024. |
| 14. | Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, Walters TD, Zachos M, Mamula P, Beaton DE. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423-432. |
| 15. | Vihinen MK, Kolho KL, Jänne OA, Andersson S, Raivio T. Circulating adiponectin as a marker for glucocorticoid-related side effects in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:504-506. |
| 16. | Vihinen MK, Kolho KL, Ashorn M, Verkasalo M, Raivio T. Bone turnover and metabolism in paediatric patients with inflammatory bowel disease treated with systemic glucocorticoids. Eur J Endocrinol. 2008;159:693-698. |
| 17. | D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262-267. |
| 18. | Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221-1229. |
| 19. | Kolho KL, Raivio T, Lindahl H, Savilahti E. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41:720-725. |
| 20. | Florin TH, Paterson EW, Fowler EV, Radford-Smith GL. Clinically active Crohn's disease in the presence of a low C-reactive protein. Scand J Gastroenterol. 2006;41:306-311. |
| 21. | Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Müller-Alouf H, Hafraoui S, Emilie D, Ectors N, Peuchmaur M. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73-81. |
| 22. | Colombel JF, Solem CA, Sandborn WJ, Booya F, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Bodily KD, Fletcher JG. Quantitative measurement and visual assessment of ileal Crohn's disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561-1567. |
| 23. | Peyrin-Biroulet L, Chamaillard M, Gonzalez F, Beclin E, Decourcelle C, Antunes L, Gay J, Neut C, Colombel JF, Desreumaux P. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577-583. |
| 24. | Heresbach D, Alexandre JL, Branger B, Bretagne JF, Cruchant E, Dabadie A, Dartois-Hoguin M, Girardot PM, Jouanolle H, Kerneis J. Frequency and significance of granulomas in a cohort of incident cases of Crohn's disease. Gut. 2005;54:215-222. |
| 25. | Rubio CA, Orrego A, Nesi G, Finkel Y. Frequency of epithelioid granulomas in colonoscopic biopsy specimens from paediatric and adult patients with Crohn's colitis. J Clin Pathol. 2007;60:1268-1272. |
| 26. | De Matos V, Russo PA, Cohen AB, Mamula P, Baldassano RN, Piccoli DA. Frequency and clinical correlations of granulomas in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2008;46:392-398. |
| 27. | Glasser AL, Boudeau J, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect Immun. 2001;69:5529-5537. |
| 28. | Poullis AP, Zar S, Sundaram KK, Moodie SJ, Risley P, Theodossi A, Mendall MA. A new, highly sensitive assay for C-reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur J Gastroenterol Hepatol. 2002;14:409-412. |
| 29. | Maharshak N, Arbel Y, Gal-Oz A, Rogowski O, Shapira I, Berliner S, Vered Y, Canaani J, Dotan I. Comparative analysis of Bayer wide-range C-reactive protein (wr-CRP) and the Dade-Behring high sensitivity C-reactive protein (hs-CRP) in patients with inflammatory bowel disease. J Dig Dis. 2008;9:140-143. |