Published online May 28, 2010. doi: 10.3748/wjg.v16.i20.2526
Revised: March 18, 2010
Accepted: March 25, 2010
Published online: May 28, 2010
AIM: To evaluate retrospectively the efficacy of rituximab plus chemotherapy in gastric diffuse large B cell lymphoma (DLBCL).
METHODS: Sixty patients (median age: 58 years) with histologically confirmed gastric DLBCL treated at four Italian institutions between 2000 and 2007, were included in this analysis. Patients were selected by stage (I-IV, Lugano staging system), European Cooperative Oncology Group performance status (0-2) and treatment strategies. Treatment strategies were chemotherapy alone (group A, n = 30) [scheduled as cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) and CHOP-like], and chemotherapy combined with rituximab (group B, n = 30). The primary end point of the study was complete response (CR) rate; the secondary end points were disease-free survival (DFS) at 5 years and overall survival (OS).
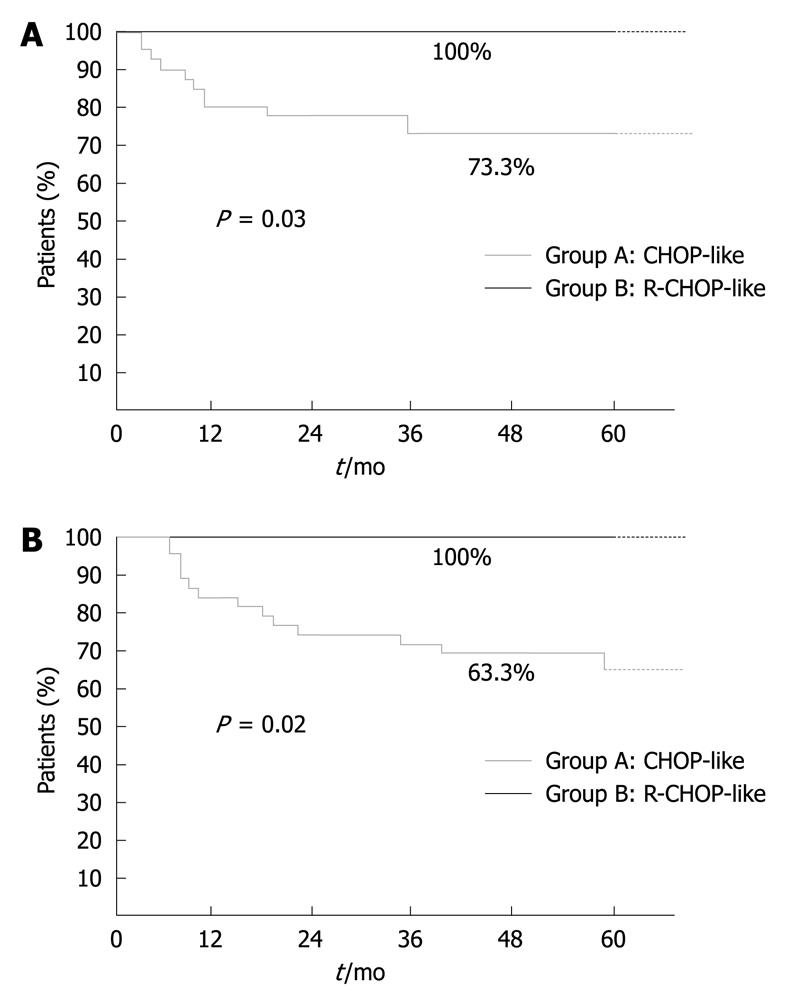
RESULTS: Median follow-up was 62 mo (range: 31-102 mo). We observed a significant difference between the two groups (A vs B) in terms of CR [76.6% (23/30) vs 100%, P = 0.04) and DFS at 5 years [73.3% (22/30) vs 100%, P = 0.03). To date, 19 group A (63.3%) patients are alive and 11 have died, while all group B patients are alive. No significant differences in toxicity were observed between the two groups.
CONCLUSION: Rituximab in combination with chemotherapy improves CR rate, DFS and OS. Further prospective trials are needed to confirm our results.
- Citation: Leopardo D, Lorenzo GD, Renzo AD, Federico P, Luponio S, Buonerba C, Matano E, Merola G, Imbimbo M, Montesarchio E, Rea A, Merola MC, Placido SD, Palmieri G. Efficacy of rituximab in gastric diffuse large B cell lymphoma patients. World J Gastroenterol 2010; 16(20): 2526-2530
- URL: https://www.wjgnet.com/1007-9327/full/v16/i20/2526.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i20.2526
Immunotherapy with rituximab, a chimeric monoclonal antibody against B-cell surface antigen CD20, is widely utilized in non-Hodgkin’s lymphoma[1,2]. Rituximab in addition to cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy represents the gold standard for the treatment of nodal aggressive non-Hodgkin’a lymphoma, since it improves response rate, overall survival (OS) and event-free survival vs chemotherapy alone, with no increase in toxicity. These results were obtained in a phase III randomized study (GELA) that included a population of 399 previously untreated elderly patients with diffuse large B cell lymphoma (DLBCL)[3].
Primary gastric non-Hodgkin’s lymphoma (PGNHL) is the most common extranodal lymphoma, and represents 60%-75% of gastrointestinal lymphoma cases, with an incidence about of 1 per 100 000 in developed countries, which appears to be increasing. PGNHL is more common in men and in individuals aged > 50 years, with a maximum incidence in the seventh decade, but it may also occur in younger patients[4].
Clinical presentation includes a variety of symptoms such as weight loss, anorexia and abdominal pain, whereas gastric bleeding is uncommon. B lymphoma generalized symptoms are less common than in primary nodal lymphoma. All histological lymphoma categories are present, but the main ones are mucosa-associated lymphoid tissue (MALT) lymphoma (low and high grade) and DLBCL[5-7].
The relationship between Helicobacter pylori chronic infection and MALT is well known (about 90% of cases), but its role in gastric DLBCL is controversial[4]. Eradication therapy is an effective option for low-grade MALT lymphoma[8].
The CHOP schedule, as a standard treatment for nodal non-Hodgkin’s lymphoma, has been utilized in several non-randomized studies and represents an effective option[9,10]. Rituximab and CHOP combination is also commonly utilized in the treatment of gastric DLBCL, but it has only been tested in a few studies[11,12].
Therefore, we carried out a retrospective study to evaluate the efficacy of rituximab in combination with chemotherapy in gastric DLBCL. To date, this study represents the largest analysis.
In this retrospective study, we analyzed a group of non-gastrectomized patients with gastric DLBCL, who were treated at our four institutions between 2000 and 2007.
Sixty patients (42 men and 28 women) with a median age of 58 years received only systemic treatment. Performance status according to European Cooperative Oncology Group (ECOG) was 0-2. All patients presented with a histologically confirmed diagnosis of gastric DLBCL. Stages were between I and IV according to the Lugano staging system (Table 1), with primary localization in the stomach at the antrum (46.6%) and antrum-body (25%). B-symptoms were present in only 14% patients. β2 microglobulin was elevated in 60% of patients and lactate dehydrogenase was elevated in 85.0% (Table 2). The primary end point was the complete response (CR) rate. Secondary end points were disease-free survival (DFS) at 5 years and OS.
| Stage I: tumor confined to GI tract |
| Stage II: tumor extending into abdomen from GI site |
| II 1 = local nodal involvement |
| II 2 = distant nodal involvement |
| Stage III: penetration of serosa to involve adjacent organs or tissue |
| Stage IV: disseminated extranodal involvement, or supradiaphragmatic nodal involvement |
| Clinicopathological characteristics | |
| Age (yr) | |
| Median | 58 |
| Range | 19-76 |
| Sex | |
| Male | 42 |
| Female | 28 |
| Performance status | |
| ECOG 0 | 0 (65%) (39/60) |
| ECOG 1 | 1 (28.3%) (17/60) |
| ECOG 2 | 2 (3.3%) (17/60) |
| Primary gastric site | |
| Body | 18.3% (11/60) |
| Antrum | 46.6% (28/60) |
| Fundus | 10% (6/60) |
| Antrum-body | 25% (15/60) |
| Metastatic sites | |
| No | 23.3% (14/60) |
| Node | 55% (33/60) |
| Pulmonary | 18.3% (11/60) |
| Bone | 0% (0/60) |
| Liver | 1.6% (1/60) |
| Spleen | 0% (0/60) |
| Pleural effusion | 1.6% (1/60) |
| Bio-humoral parameters | |
| Lactate dehydrogenase (high level) | 85.0% (51/60) |
| β2 microglobulin (high level) | 60% (36/60) |
| Lugano staging system | |
| I | 28.3% (17/60) |
| II | 46.6% (28/60) |
| III | 16.6% (10/60) |
| IV | 8.3% (5/60) |
| B symptoms | 15% (9/60) |
We selected 60 cases from an archive of patients that had received chemotherapy plus immunotherapy or chemotherapy alone from 2000 to 2007. Thirty of these patients (group A) received only chemotherapy according to CHOP or CHOP-like (MACOP-B) schedules. The CHOP schedule consisted of cyclophosphamide at 750 mg/m2 on day 1, doxorubicin 50 mg/m2 on day 1, vincristine 1.4 mg/m2 up to a maximal dose of 2 mg on day 1, and prednisone 100 mg/d for 5 d, every 21 d. MACOP-B schedule consisted of methotrexate 100 mg/m2 on day 1, adriamycin 35 mg/m2 on day 1, cyclophosphamide 350 mg/m2 on day 1, vincristine 1.4 mg/m2 up to a maximal dose of 2 mg on day 1, prednisone 40 mg/m2 on days 1-5, and bleomycin 10 mg/m2, every 21 d. A group of 30 patients (group B) received rituximab (375 mg/m2 administrated on day 1 of each cycle of CHOP or CHOP-like MACOP-B) (Table 3). Rituximab infusion was interrupted in the event of fever, chills, edema, congestion of the head and neck mucosa, hypotension or any other serious adverse event, and it was resumed when the event resolved. If the absolute neutrophil (granulocyte) count was < 1500/μL or the platelet count was 100 000/μL, chemotherapy was halted for up to 1 wk, and treatment was stopped. The dose of rituximab was not modified, and rituximab was continued when CHOP was stopped. Treatment was stopped, however, if lymphoma progressed, if the patient declined to continue, or at the discretion of the physicians in cases of concurrent illness or adverse events.
| Chemo-immunotherapy treatment | |
| No. of patients | |
| CHOP-like | 30 |
| CHOP-like and rituximab | 30 |
| Second or other lines | 7.50% |
| No. of cycles | |
| Median | 6 (3-24) |
| mean | 8.3 (3-24) |
| Results | |
| Median follow-up | 77 mo |
| CHOP-like | CR = 76.6%, DFS at 5 yr = 73.3%, OS = 63.3% |
| CHOP-like and rituximab | CR = 100%, DFS at 5 yr = 100%, OS = 100% |
Response to treatment was classified as CR, partial response (PR) or progressive disease (PD) according to the International Workshop criteria[13]. CR was defined as the disappearance of all lesions and radiological or biological abnormalities observed at diagnosis, and the absence of new lesions. PR was defined as regression of all measurable lesions by > 50%, disappearance of non-measurable lesions, and absence of new lesions. PD was defined as the appearance of new lesions, any growth of the initial lesions by > 25%, or growth of any measurable lesion that had regressed during treatment by > 50% from its smallest dimensions. All adverse events reported by the patient or observed by the investigator were collected and were graded according to the NCI toxicity criteria (CTCAE v3.0).
Quantitative data were described by the median (range), and qualitative data were expressed as counts and percentages. Duration of the disease-free interval and OS were estimated according to the Kaplan-Meier method. Fisher’s exact test was used to assess the significance of all correlations. Statistical significance was achieved if P was < 0.05. All reported P values were two-sided.
Median follow-up was 62 mo (range: 31-102 mo). No difference between the two groups was observed in term of stage, ECOG performance status and metastatic sites. Rituximab plus chemotherapy vs chemotherapy alone yielded a statistically significant advantage in terms of CR (100% vs 76.6%, P = 0.004), DFS at 5 years (100% vs 73.3%, P = 0.03) (Figure 1A) and OS (100% vs 63.3%, P = 0.02) (Figure 1B). We observed in group A, five patients with PR and two with PD. Only 7.5% of patients underwent a second or additional line of therapy (Table 3). To date, 19 group A patients are alive and 11 have died [nine due to lymphoma-related causes, one to brain metastatic melanoma, and one to hepatocarcinoma (hepatitis C virus-related)]. All patients in group B are alive.
The most common toxicities were grade 2/3 and 4 granulocytopenia (76.6% in group B and 73.3% in group A, P > 0.05) and recurrent infection (all grades) (43.2% in group B and 36.6% in group A, P > 0.05) (Table 4).
| CHOP-like | CHOP-like and rituximab | |||||
| G1 | G2/3 | G4 | G1 | G2/3 | G4 | |
| Hematological | ||||||
| Hemoglobin level | 6 (20) | 6 (20) | 1 (3.3) | 7 (23.3) | 6 (20) | 1 (3.3) |
| Neutrophil count | 8 (26.6) | 12 (40) | 10 (33.3) | 9 (30) | 12 (40) | 11 (36.6) |
| Platelet count | 4 (13.3) | 3 (10) | 2 (6.6) | 3 (10) | 2 (6.6) | 1 (3.3) |
| Gastrointestinal | ||||||
| Nausea/vomiting | 10 (33.3) | 4 (13.3) | 0 (0) | 10 (33.3) | 4 (13.3) | 1 (3.3) |
| Diarrhea | 3 (10) | 2 (6.6) | 0 (0) | 3 (10) | 1 (3.3) | 0 (0) |
| Stomatitis | 6 (20) | 2 (6.6) | 0 (0) | 6 (20) | 3 (10) | 0 (0) |
| Constipation | 3 (10) | 1 (3.3) | 0 (0) | 3 (10) | 1 (3.3) | 0 (0) |
| Dysphagia | 1 (3.3) | 2 (6.6) | 0 (0) | 1 (3.3) | 1 (3.3) | 0 (0) |
| Pyrosis | 5 (16.6) | 4 (13.3) | 0 (0) | 6 (20) | 3 (10) | 0 (0) |
| Other | ||||||
| Fatigue | 8 (26.6) | 6 (20) | 1 (3.3) | 9 (30) | 6 (20) | 1 (3.3) |
| Alopecia | 3 (10) | 5 (16.6) | 14 (46.6) | 4 (13.3) | 6 (20) | 17 (56.6) |
| Fever | 3 (10) | 4 (13.3) | 1 (3.3) | 4 (13.3) | 5 (16.6) | 1 (3.3) |
| Allergic reaction | 1 (3.3) | 1 (3.3) | 0 (0) | 2 (6.6) | 1 (3.3) | 0 (0) |
| Pharyngitis rythema | 0 (0) | 0 (0) | 0 (0) | 1 (3.3) | 0 (0) | 0 (0) |
| Paresthesia | 9 (30) | 2 (6.6) | 0 (0) | 7 (23.3) | 3 (10) | 1 (3.3) |
| Arthromyalgia | 1 (3.3) | 1 (3.3) | 0 (0) | 1 (3.3) | 1 (3.3) | 0 (0) |
| Recurrent infections | 6 (20) | 4 (13.3) | 1 (3.3) | 7 (23.3) | 5 (16.6) | 1 (3.3) |
| Thrombosis | 2 (6.6) | 1 (3.3) | 0 (0) | 2 (6.6) | 1 (3.3) | 0 (0) |
| Cough | 2 (6.6) | 2 (6.6) | 1 (3.3) | 3 (10) | 2 (6.6) | 0 (0) |
| Pain | 1 (3.3) | 1 (3.3) | 0 (0) | 2 (6.6) | 1 (3.3) | 0 (0) |
Our analysis is believed to be the first study to compare directly chemo-immunotherapy and chemotherapy alone in gastric non-Hodgkin’s lymphoma. We demonstrated a statistically significant improvement with chemo-immunotherapy in terms of CR, DFS at 5 years and OS, without a significantly greater toxicity.
Rituximab was introduced as a treatment for prior nodal lymphoma at the end of the 1990s. A randomized phase III trial (GELA group) has underlined the effectiveness of rituximab in prior nodal B cell lymphoma. Three hundred and ninety-nine previously untreated elderly patients (≥ 60 years) with stage II-IV DLBCL were randomized to receive either standard CHOP or rituximab plus CHOP for eight cycles. CR rate was significantly higher in the group that received rituximab plus CHOP than in the group that received CHOP alone (76% vs 63%, P = 0.005), with a median follow-up of 2 years, DFS and OS times were significantly longer in the rituximab plus CHOP group (P < 0.001 and P = 0.007, respectively). Clinically relevant toxicity was not significantly greater with rituximab plus CHOP[3].
However, as far as we are aware, no previous studies have compared immunotherapy-chemotherapy with chemotherapy alone in gastric DLBCL.
A few studies have evaluated immuno-chemotherapy in gastric DLBCL. In one single-arm study, 15 patients were treated with rituximab plus chemotherapy. CR rate was 87% and PR rate was 13%. The CR rate was high in this study, as in our analysis, but there was no direct comparison with chemotherapy alone[11]. A phase II trial of 42 patients with early-stage disease did not show any advantage of rituximab plus CHOP chemotherapy. A CR was obtained in 95% of cases, and relapses were observed in two[12]. The treatment did not achieve a statistically significant effect in terms of DFS at 5 years and OS at 5 years in comparison with the historical control[14].
Our results are in agreement with this previous study in term of CR, and confirmed that the chemotherapy-immunotherapy was more active than chemotherapy alone.
Our study had several limitations. First, it was a retrospective study with potential bias in the patients and methods. Second, chemotherapy was not the same in each group (the first patients were treated mainly with the MACOP-B schedule, while the more recent patients were treated mainly with the CHOP schedule).
In conclusion, our results are promising in terms of CR, DFS and safety. However, prospective randomized trials are needed to confirm these preliminary results.
Rituximab in addition to cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy represents the gold standard for the treatment of nodal aggressive non-Hodgkin’s lymphoma.
Rituximab and CHOP combination is also commonly utilized in the treatment of gastric diffuse large B cell lymphoma (DLBCL), but it has only been tested in a few studies. Therefore, the authors carried out a retrospective study to evaluate the efficacy of rituximab in combination with chemotherapy in gastric DLBCL.
To date, this study represents the largest analysis. The analysis represents the first study to compare directly chemo-immunotherapy and chemotherapy alone in gastric non-Hodgkin’s lymphoma. The results are promising in terms of CR, DFS and safety. However, prospective randomized trials are needed to confirm these preliminary results.
Rituximab is a chimeric monoclonal antibody against B-cell surface antigen CD20 and it is widely utilized in non-Hodgkin’s lymphoma therapy. Primary gastric non-Hodgkin’s lymphoma is the most common extranodal lymphoma, and represents 60%-75% of gastrointestinal lymphoma cases. The main histological types are mucosa-associated lymphoid tissue lymphoma (low and high grade) and DLBCL. The CHOP schedule consists of cyclophosphamide, doxorubicin, vincristine and prednisone.
The study is interesting as it is the first to compare the two types of therapy in these gastric tumors.
Peer reviewer: Guida Portela-Gomes, MD, PhD, Professor, Faculty of Medicine, University of Lisbon, Rua Domingos Sequeira-128, Estoril 2765-525, Portugal
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Ann Oncol. 2003;14:520-535. |
| 2. | Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63:803-843. |
| 3. | Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-242. |
| 4. | Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008;19:1992-1999. |
| 5. | Isaacson PG. Gastrointestinal lymphoma. Hum Pathol. 1994;25:1020-1029. |
| 7. | Bayerdörffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591-1594. |
| 8. | Neubauer A, Thiede C, Morgner A, Alpen B, Ritter M, Neubauer B, Wündisch T, Ehninger G, Stolte M, Bayerdörffer E. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997;89:1350-1355. |
| 9. | Raderer M, Valencak J, Osterreicher C, Drach J, Hejna M, Kornek G, Scheithauer W, Brodowicz T, Chott A, Dragosics B. Chemotherapy for the treatment of patients with primary high grade gastric B-cell lymphoma of modified Ann Arbor Stages IE and IIE. Cancer. 2000;88:1979-1985. |
| 10. | Raderer M, Chott A, Drach J, Montalban C, Dragosics B, Jäger U, Püspök A, Osterreicher C, Zielinski CC. Chemotherapy for management of localised high-grade gastric B-cell lymphoma: how much is necessary? Ann Oncol. 2002;13:1094-1098. |
| 11. | Wöhrer S, Püspök A, Drach J, Hejna M, Chott A, Raderer M. Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) for treatment of early-stage gastric diffuse large B-cell lymphoma. Ann Oncol. 2004;15:1086-1090. |
| 12. | Avilés A, Castañeda C, Cleto S, Neri N, Huerta-Guzmán J, Gonzalez M, Nambo MJ. Rituximab and chemotherapy in primary gastric lymphoma. Cancer Biother Radiopharm. 2009;24:25-28. |
| 13. | Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. |