Published online Jan 14, 2010. doi: 10.3748/wjg.v16.i2.237
Revised: October 15, 2009
Accepted: October 22, 2009
Published online: January 14, 2010
AIM: To assess the diagnostic ability of endoscopic ultrasonography (EUS) for evaluating causes of distal biliary strictures shown on endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), even without identifiable mass on computed tomography (CT).
METHODS: The diagnostic ability of EUS was retrospectively analyzed and compared with that of routine cytology (RC) and tumor markers in 34 patients with distal biliary strictures detected by ERCP or MRCP at Dokkyo Medical School Hospital from December 2005 to December 2008, without any adjacent mass or eccentric thickening of the bile duct on CT that could cause biliary strictures. Findings considered as benign strictures on EUS included preservation of the normal sonographic layers of the bile duct wall, irrespective of the presence of a mass lesion. Other strictures were considered malignant. Final diagnosis of underlying diseases was made by pathological examination in 18 cases after surgical removal of the samples, and by clinical follow-up for > 10 mo in 16 cases.
RESULTS: Seventeen patients (50%) were finally diagnosed with benign conditions, including 6 “normal” subjects, while 17 patients (50%) were diagnosed with malignant disease. In terms of diagnostic ability, EUS showed 94.1% sensitivity, 82.3% specificity, 84.2% positive predictive value, 93.3% negative predictive value (NPV) and 88.2% accuracy for identifying malignant and benign strictures. EUS was more sensitive than RC (94.1% vs 62.5%, P = 0.039). NPV was also better for EUS than for RC (93.3% vs 57.5%, P = 0.035). In addition, EUS provided significantly higher sensitivity than tumor markers using 100 U/mL as the cutoff level of carbohydrate antigen 19-9 (94.1% vs 53%, P = 0.017). On EUS, biliary stricture that was finally diagnosed as malignant showed as a hypoechoic, irregular mass, with obstruction of the biliary duct and invasion to surrounding tissues.
CONCLUSION: EUS can diagnose biliary strictures caused by malignant tumors that are undetectable on CT. Earlier detection by EUS would provide more therapeutic options for patients with early-stage pancreaticobiliary cancer.
- Citation: Saifuku Y, Yamagata M, Koike T, Hitomi G, Kanke K, Watanabe H, Murohisa T, Tamano M, Iijima M, Kubota K, Hiraishi H. Endoscopic ultrasonography can diagnose distal biliary strictures without a mass on computed tomography. World J Gastroenterol 2010; 16(2): 237-244
- URL: https://www.wjgnet.com/1007-9327/full/v16/i2/237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i2.237
Determining the etiology of a distal biliary stricture is crucial to the provision of appropriate therapy. However, such determination can be particularly problematic in cases of biliary strictures without an identifiable mass that could cause the stricture on computed tomography (CT) or magnetic resonance imaging (MRI). Empiric resection may be necessary to differentiate benign and malignant strictures, and the decision to operate has traditionally been made on the basis of clinical history and cholangiographic appearance of the stricture[1]. However, determining the cause of a stricture on the basis of morphologic features and brush cytology is unreliable[2].
Intraductal ultrasonography (IDUS) with wire-guided, thin-caliber, high-frequency probes is promising for the diagnosis of biliary stricture[3,4]. This technique is easily performed as an adjunct to endoscopic retrograde cholangiopancreatography (ERCP) without significant lengthening of the procedure. For the diagnosis of biliary stricture without an identifiable mass, however, data from IDUS are insufficient[4-6]. In addition, the number of patients for whom IDUS can be performed has decreased, since magnetic resonance cholangiopancreatography (MRCP) has been replacing the role of diagnostic ERCP. Moreover, if patients with an identifiable mass on CT or MRI are excluded, the proportion of benign strictures is about 30%-50%[4,5,7,8]. This relatively high percentage of benign strictures complicates the decision to perform IDUS in all patients with biliary strictures of indeterminate etiology, as IDUS sometimes causes complications such as ERCP-induced pancreatitis and cholangitis.
The utility of endoscopic ultrasonography (EUS) for diagnosing unexplained biliary strictures thus warrants urgent discussion, since EUS is now the first choice in screening for small pancreatic tumors that cannot be detected by other imaging modalities and is not associated with ERCP-related complications[9]. However, only 1 paper by Lee et al[1] has mentioned the utility of EUS features in patients with unexplained biliary stricture. According to Lee et al[1], sonographic features of EUS, including the presence of a pancreatic mass, an irregular bile duct wall, or bile duct thickness > 3 mm, could improve the diagnosis of unexplained stricture.
The aim of this study was to assess the ability of EUS to diagnose distal biliary strictures for which cross-sectional imaging modalities such as CT and MRI could not detect a causative mass or bile duct thickness. Moreover, we report the sonographic appearance of malignant and benign strictures using EUS, as the lack of easily recognizable morphologic criteria has partly caused the limited availability of EUS for diagnosing biliary strictures of indeterminate etiology.
This retrospective analysis was performed on 34 patients who underwent EUS at Dokkyo Medical School Hospital from December 2005 to December 2008 for evaluation of strictures in the biliary tract that were detected by ERCP or MRCP and who didnot have an identifiable mass lesion causing the stricture on cross-sectional CT or MRI. Patients with strictures at the proximal bile duct were excluded from this study, since EUS is already known to be less accurate for strictures of that portion[10]. All patients provided written consent for the procedure performed.
EUS and ERCP were performed with the patient under conscious sedation by 1 of 2 operators (S.Y. and Y.M.). EUS was performed using a 7.5-MHz US probe (UM-200; Olympus, Tokyo, Japan) connected to a standard EUS processor (EU-30; Olympus). This probe provides radial scanning perpendicular to its axis. For the purposes of this study, EUS images were reviewed to identify extrinsic compression at the stricture site without knowledge of the final diagnosis. Evaluation points were: (1) presence of a mass that could create extrinsic compression at the site of the stricture; (2) disruption of the normal 2 or 3 sonographic layers of the bile duct wall[11]; and (3) continuation of a mass into adjacent structures, referring to previously defined criteria for evaluation of biliary strictures on IDUS[3,10,12]. Findings considered as a benign stricture on EUS included preservation of the normal sonographic layers of the bile duct wall, irrespective of the presence of a mass lesion. Other strictures were considered malignant.
Thirty patients underwent dynamic CT with a liver protocol using the method reported by Yanaga et al[13]. All scans were performed on a multidetector helical CT scanner (SOMATOM Sensation 64; Siemens, Tokyo, Japan). A multiphasic scanning technique was used and 3-phase contrast-enhanced CT was performed during the hepatic arterial phase, portal phase and equilibrium phase. For the remaining 4 patients who could not use contrast medium due to renal dysfunction, only plain CT was performed. Images were reconstructed at an 8-mm slice thickness, and interpreted by CT radiologists who specialize in body imaging.
In 25 patients, MRCP was performed using 1.5-T MRI (MAGNETOM Symphony; Siemens) or 3-T MRI (MAGNETOM Trio, A Tim System; Siemens). For ERCP, the procedure was performed in a standard fashion with a side-viewing therapeutic duodenoscope (Olympus JF 260V; Olympus) using standard iodinated contrast medium. Brushings of the biliary stricture were obtained during ERCP. The remainder of the ERCP procedure was then performed according to the individual needs of each patient. When a drainage tube was placed for palliative treatment in patients suffering from obstructive jaundice, bile pooled for 24 h was collected for cytopathological analysis 3 times. For routine cytology (RC), specimens obtained from brushings and bile collected from a drainage tube were reviewed by dedicated gastrointestinal cytopathologists.
As tumor markers, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were examined at admission, and CEA > 5.0 ng/mL or CA19-9 > 37 U/mL were considered to indicate malignancy.
The final diagnosis was based on definitive cytologic studies diagnostic for malignancy or surgical removal followed by pathological examination in 18 patients and clinical follow-up for > 10 mo in 16 patients. Repeat ERCP, biliary brushing and bile collection were performed as clinically indicated. Clinical follow-up included imaging with abdominal CT, EUS and MRCP. Follow-up information was also obtained from hospital medical records, by contacting primary care physicians.
Continuous variables are expressed as median (range). Each test is expressed in proportions in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. Analysis was performed using StatView software (Hulinks, Inc., Tokyo). Comparisons between continuous variables were performed using non-parametric tests. Comparisons between qualitative variables were performed using a χ2 test for independence or Fisher’s exact probability test. Values of P < 0.05 were considered statistically significant.
Thirty-four patients (17 men, 17 women) met the inclusion criteria. The median age of the patients was 71 years (range, 23-90 years). Jaundice (total bilirubin > 2 mg/dL) was evident at presentation in 13 patients. In the 21 patients without jaundice, abnormal liver blood tests were found in 8 patients, and 6 patients complained of abdominal pain. Abnormal hepatobiliary images on transabdominal US at medical check-up were detected in 7 patients, who had neither abnormalities in blood data nor clinical symptoms. No significant difference in clinical characteristics was seen between patients with benign stricture, normal cases and those with malignant strictures (Table 1).
| Final diagnosis | Benign stricture and normal case (n = 17) | Malignant stricture (n = 17) | Statistical significance |
| Age (yr) | 71.5 (23-90) | 69.0 (56-84) | NS |
| Sex (male/female) | 9/8 | 8/9 | NS |
| Presenting symptoms | NS | ||
| Jaundice | 5 | 8 | |
| Elevated liver enzymes | 2 | 6 | |
| Abdominal pain | 5 | 1 | |
| Abnormal imaging | 5 | 2 |
There were 17 malignant strictures, 11 benign strictures and 6 normal cases. Among 8 cases of peripancreatic cancer (tumor located in the head of the pancreas), the diagnosis was confirmed as pancreatic cancer by pathological examinations using surgical specimens in 6 cases and 2 lesions were considered malignant based on clinical follow-up. Two patients who were clinically diagnosed as peripancreatic cancer died of liver failure 4 mo after EUS examination, accompanied by CA19-9 elevation. Among 7 cases of biliary cancer, the diagnosis was confirmed by pathological examination using surgical specimens in 5 cases and 2 lesions considered malignant on clinical follow-up were located in the middle duct. One patient who was clinically diagnosed with biliary cancer gradually became emaciated and died within 1 year of EUS examination. In another patient, peritoneal dissemination was found at surgery 4 mo after EUS examination. One patient was diagnosed with carcinoma of the papilla of Vater by pathological examination using surgical specimens. In another patient who was clinically diagnosed with carcinoma of the papilla of Vater, cytology taken from the orifice of the papilla was suspicious of adenocarcinoma and the duodenum finally became stenotic 2 years after EUS examination (Table 2).
| n | |
| Benign bile duct stricture | 11 |
| Fibrotic or inflammatory stricture | 4 |
| Dilatation of the bile duct | 3 |
| Chronic pancreatitis | 2 |
| Anomalous arrangement of pancreaticobiliary duct | 1 |
| Congenital choleductal cyst | 1 |
| Malignant bile duct stricture | 17 |
| Peripancreatic cancer | 8 |
| Bile duct cancer | 7 |
| Carcinoma of the papilla of Vater | 2 |
| Normal study | 6 |
The diagnosis of benign strictures was confirmed by surgery in 4 and by clinical follow-up in 7. Patients clinically diagnosed as benign didnot show changes in images or clinical exacerbation. Six cases were considered normal for which strictures detected on ERCP or MRCP were either not reproducible or were due to compression by Oddi’s sphincter (Table 2).
These results suggest that the proportion of malignant strictures was 50% even if lesions which could cause the stricture were not detected on CT and/or MRI. When cases with normal images on EUS were excluded, the proportion of malignancy reached 58.6%.
While EUS is now routinely performed without insertion of apparatus into the biliary tree, few reports have used EUS to evaluate biliary strictures without an identifiable mass on CT. We thus examined the diagnostic ability of EUS, and conducted comparisons with the diagnostic abilities of RC and tumor markers.
Malignancy was correctly diagnosed in 16 of 17 patients. Ultrasonographic findings in patients with malignant strictures correctly judged as malignancy on EUS are summarized in Table 3. A mass accompanied by invasion of surrounding tissues may be suggestive of malignancy. The proportion of invasion of the main pancreatic duct was significantly higher in patients with peripancreatic cancer than in patients with biliary cancer (62.5% vs 0%, P = 0.026), while no significant difference was seen in terms of diagnostic ability, biliary disruption and invasion of adjacent tissues by the tumors. In addition to the sonographic findings in the mass and the bile duct, EUS also detected enlarged lymph nodes > 10 mm in diameter in 50% of patients with malignant strictures. Ascites were observed in 1 patient with peripancreatic cancer. In 1 false-negative case with distal biliary cancer, no mass was detectable and was judged as benign. In this case, the mass may have been hidden by the acoustic shadow formed by impacted stones in the bile duct.
| Final diagnosis | EUS findings | n (%) |
| Peripancreatic cancer (n = 8) | Mass adjacent to the stricture site in the pancreas head | 8 (100) |
| Disruption of the bile duct by the mass | 6 (75) | |
| Continuation into adjacent structures | 6 (75) | |
| Invasion of the main pancreatic duct | 5 (62.5)a | |
| LN swelling | 4 (50) | |
| Ascites | 1 (12.5) | |
| Biliary cancer (n = 6) | Mass adjacent to the stricture site in the pancreas head | 6 (100) |
| Disruption of the bile duct by the mass | 5 (83.3) | |
| Continuation into adjacent structures | 5 (83.3) | |
| Invasion of the main pancreatic duct | 0 (0) | |
| LN swelling | 3 (50) | |
| Cancer of the ampulla of Vater (n = 2) | Mass mainly located on the luminal side | 2 (100) |
| Infiltration of the muscularis propria by the mass | 2 (100) | |
| LN swelling | 1 (50) |
Correct diagnosis of benign disease was made in 14 of 17 patients. Ultrasonographic findings in patients with benign strictures correctly judged as benign on EUS are summarized in Table 4. Smooth tapering of the bile duct, preservation of the normal layered structure of the bile duct wall and no mass adjacent to the stricture site may be suggestive of benign disease. There were 3 false-positive patients with fibrotic strictures, diagnosed as systemic lupus erythematosus in 1 patient and unknown etiology in 2. In these cases considered to have malignancy on EUS, a drainage tube was inserted into the bile duct. In 2 patients, a solid mass was found at the distal end of the duct. The duct wall was thickened, losing the layered structure. In 1 patient, the distal end of the bile duct was filled with an irregular mass that could not be distinguished from the duct wall on EUS. The irregular mass looked extended from the duct, which looked like invasion of the adjacent tissues on imaging. Fibrotic change was ascertained when surgery was performed, while masses could not be confirmed during surgery, probably because the substance that looked like masses was debris.
| Final diagnosis | Reason to diagnose as benign stricture | EUS findings |
| Inflammatory stricture clinically diagnosed as acute cholangitis (n = 1) | No exacerbation during follow-up (> 23 mo) | Stenosis of the distal end of the bile duct |
| The normal layered structure of the bile duct wall | ||
| No mass adjacent to the stricture site | ||
| Biliary dilation (n = 3) | No change for > 18 mo | The dilated bile duct gradually tapering at the ampulla of Vater (n = 2) |
| A 1-cm long narrowing portion at the distal end of the duct smoothly continuous from the dilated proximal duct (n = 1) | ||
| Chronic pancreatitis including 1 autoimmune pancreatitis (n = 2) | No exacerbation during follow-up (> 10 mo) | Smooth tapering of the distal end of the bile duct without a mass adjacent to the stricture site (in case of autoimmune pancreatitis) |
| Marked calcification at the stricture site (n = 1) | ||
| Anomalous arrangement of the pancreaticobiliary duct (n = 1) | Confirmed by MRCP | Connection of the pancreatic duct to the biliary duct outside the papilla of Vater |
| Congenital choleductal cyst (n = 1) | Confirmed by surgery | Cystic dilatation at the distal end of the bile duct |
Six strictures identified by ERCP or MRCP were ultrasonographically considered as benign and finally diagnosed as “normal” duct. Four strictures were not reproducible during clinical follow-up. Diagnoses on EUS were calcification on the duct wall in 2 patients, cuneate deformity of the duct wall in 1 patient and normal biliary tree in 1 patient. Two were considered as strictures due to compression by Oddi’s sphincter, accompanied by acute cholangitis which improved and was not recurrent.
To summarize, EUS identified lesions that could cause biliary strictures, irrespective of pathological features, in 32 of 34 patients (sensitivity 90.5%, specificity 100%, PPV 100%, NPV 86.7%, accuracy 94.1%) (Table 5). In terms of pathological features, EUS correctly identified causes in 16 of 17 malignant strictures and in 14 of 17 benign lesions. With regard to the proportion of correct diagnosis, no significant difference was seen between patients with malignant and benign lesions (94.1% vs 82.4%, P = 0.60). The diagnostic abilities of EUS were 94.1% sensitivity, 82.3% specificity, 84.2% PPV, 93.3% NPV and 88.2% accuracy (Table 6). Following RC, adequate samples were obtained in 24 patients (16 malignant, 8 benign strictures), and RC correctly identified the causes in 8 of 14 malignant strictures and in 8 of 16 benign strictures. In terms of the proportion of correct diagnosis, no significance difference was evident between patients with malignant and benign lesions (62.5% vs 100%, P = 0.066). The diagnostic abilities of RC were 62.5% sensitivity, 100% specificity, 100% PPV, 57.2% NPV, and 75% accuracy.
| Diagnostic ability | % |
| Sensitivity | 90.5 |
| Specificity | 100 |
| PPV | 100 |
| NPV | 86.7 |
| Accuracy | 94.1 |
Tumor markers were measured in 34 patients, and correctly identified malignancy in 13 of 17 malignant strictures and correctly identified a benign disease in 12 of 17 benign strictures. In terms of the proportion of correct diagnosis, no significant difference was seen between patients with malignant and benign lesions (76.5% vs 70.6%, P > 0.999). The diagnostic abilities of tumor markers were 76.5% sensitivity, 70.6% specificity, 72.2% PPV, 75% NPV and 73.5% accuracy.
The diagnostic ability of CA19-9 has been reported to be improved when the cutoff level is increased to 100 U/mL[14], for the level of CA19-9 increases in patients with cholestasis[15]. Analysis of tumor markers was thus performed using 100 U/mL as the cutoff level for CA19-9. The results were 53% sensitivity, 82.4% specificity, 75% PPV, 63.6% NPV and 67.7% accuracy. With regard to the proportion of correct diagnoses using 100 U/mL as the cutoff level of CA19-9, no significant difference was seen between patients with malignant and benign lesions (53.0% vs 82.4%, P = 0.14).
Diagnostic sensitivity and NPV were superior for EUS than for RC (P = 0.039 and 0.035, respectively). Compared with tumor markers using 100 U/mL as the cutoff level for CA19-9, EUS showed significantly higher sensitivity (P = 0.017).
Hypoechoic masses with irregular margins, tumor size > 10 mm and mixed echogenicity reportedly imply malignancy on IDUS[3,10,12]. Thus, (1) the presence of a mass, (2) tumor size > 10 mm, (3) the margin and internal echo of a mass; (4) disruption of the normal sonographic layers of the bile duct wall, and (5) continuation of a mass into adjacent structures were analyzed to study the relationship between EUS findings and final diagnosis (Table 7).
| Benign stricture (n = 11) | Malignant stricture (n = 17) | Statistical significance (P) | |
| Mass | 0.0069 | ||
| + | 5 | 16 | |
| - | 6 | 1 | |
| Size of mass (mm) | NS | ||
| ≤ 10 | 3 | 3 | |
| > 10 | 2 | 13 | |
| Shape | 0.025 | ||
| Round | 4 | 3 | |
| Irregular | 1 | 13 | |
| Internal echo | 0.004 | ||
| Hyperechoic | 4 | 1 | |
| Hypo or mixedechoic | 1 | 15 | |
| Disruption of the common bile duct | 0.0013 | ||
| + | 1 | 13 | |
| - | 10 | 4 | |
| Invasion to surrounding tissue | < 0.001 | ||
| + | 1 | 16 | |
| - | 10 | 1 | |
The proportion of patients with malignancy was significantly higher when a mass was detected (P = 0.0069), especially with an irregular margin (P = 0.025) and hypoechoic or mixed echoic pattern (P = 0.004), the bile duct was accompanied by disruption (P = 0.0013) and a case showed invasion to surrounding tissues (P < 0.001). There was no significant difference between malignant and benign strictures in terms of tumor size.
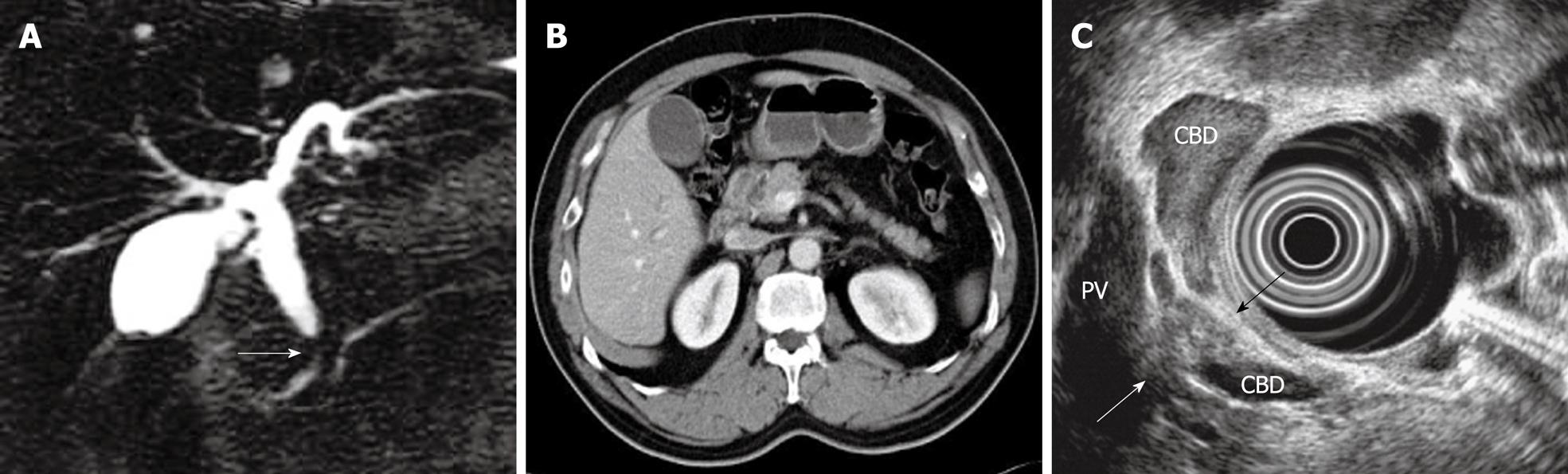
To summarize, the data suggest that biliary stricture can be diagnosed as malignancy on EUS when a mass is adjacent to the stricture, has an irregular margin, a hypoechoic or mixed echoic pattern, disruption of the bile duct, and invasion to surrounding tissue. A typical case is shown in Figure 1.
Consensus has yet to be reached on the management of biliary strictures with unknown etiology. Even after excluding biliary strictures with an identifiable mass on cross-sectional imaging, our data indicate that the risk of malignant stricture is 50%, consistent with previous data[4,5,7,8]. The frequency of malignancy is not negligible, allowing surgical exploration as a pre-emptive approach. In particular, when accompanied by obstructive jaundice, the majority of biliary strictures are believed to be malignant and surgery is routinely performed[7]. However, the remaining 50% of biliary strictures are benign or are eventually diagnosed as normal when no identifiable mass is seen on CT/MRI. In addition, our data show that 38.5% of biliary strictures are benign, even if accompanied with jaundice. Since the frequency of benign strictures is quite high and these strictures can be managed by endoscopic therapies, recommending exploratory surgery in all patients may not be justifiable. To differentiate patients with a low risk of malignancy from those with a high risk of malignancy who would benefit from surgical exploration, a new diagnostic approach should be provided for the management decision.
IDUS has recently seen clinical use at the time of ERCP for patients with painless jaundice and no identifiable mass on CT/MRI[5,16]. In previous studies, IDUS has proven quite accurate in distinguishing benign and malignant strictures, with reported accuracies of 76%-90%[3,6,17]. In addition, for indeterminate biliary strictures of unknown etiology, IDUS has provided approved diagnostic ability[5,8]. According to Stravropoulos, the sensitivity and specificity of IDUS are both 83%. When IDUS is used with ERCP, IDUS increases the diagnostic accuracy of ERCP from 58% to 90%[5]. According to Domagk, IDUS provides correct preoperative diagnosis in 83% of cases[8].
For indeterminate biliary strictures, our study shows that the diagnostic abilities of EUS were 94.1% sensitivity, 82.3% specificity, 84.2% PPV, 93.3% NPV and 88.2% accuracy, comparable to the abilities of IDUS. In addition, compared with RC and tumor markers, EUS showed significantly higher sensitivity and NPV to distinguish benign and malignant strictures. These results suggest that EUS offers high diagnostic ability for distal biliary strictures even without a mass on CT, detecting pancreaticobiliary cancers at a very early stage.
Despite a long search, the only previously published paper on the diagnostic ability of EUS for biliary strictures with unknown etiology was that by Lee et al[1]. Their diagnostic criteria for malignancy included the finding of a pancreatic head mass and/or irregular bile duct wall, similar to the criteria in our study. According to Lee et al[1], EUS for malignancy offers 88% sensitivity, 100% specificity, 100% PPV, and 84% NPV. Compared with our results, Lee et al[1] achieved much higher specificity. Unlike our patients, however, almost all patients in their study had a stent in place at the time of referral for EUS, possibly making the bile duct wall thicker and/or irregular even in patients with benign stricture. Irregularity of the duct wall might thus have been diagnosed as malignant when the wall was extremely irregular, which would have made the diagnostic criteria for malignancy stricter than expected.
Patients with a final diagnosis of “normal” reaped the greatest benefits from this study, in which the definition of benign strictures was strict. In “normal” cases, EUS could exclude the possibility of malignancy, avoiding unnecessary follow-up and invasive procedures such as surgical exploration.
In our institution, patients with operable lesions judged as malignant based upon our EUS “criteria” and no known contraindication underwent surgery. Patients with lesions judged as benign on EUS and low clinical suspicion for malignancy were treated endoscopically or clinically followed at the outpatient clinic. When clinical data were suggestive of malignancy, further diagnostic procedures such as surgical exploration were performed, irrespective of EUS results. EUS plus MRCP could replace ERCP with IDUS as the first choice to direct management decisions for indeterminate biliary strictures with no identifiable mass on cross-sectional imaging, especially in cases showing low clinical suspicion for malignancy.
Three false-positive patients showed biliary stricture caused by benign fibrosis. When these patients were referred for EUS, endoscopic biliary drainage tubes had been inserted. Biliary drainage tubes could have caused recurrent cholangitis due to tube occlusion by biliary sludge, leading to thickening of the bile duct wall. In 1 case of benign stricture caused by fibrosis, a hypoechoic mass was detected at the end of the bile duct on EUS, but was not found during surgery. This case indicates that biliary sludge should be considered as a possible diagnosis when a drainage tube is inserted[18,19]. The introduction of contrast-enhanced EUS to search for vascular images inside a mass may lead to differentiation of biliary sludge from tumor.
Our study didnot perform EUS-FNA, which could have increased the diagnostic ability. According to Lee et al[1], however, EUS-FNA shows 47% sensitivity, 100% specificity, 100% PPV, and 50% NPV, suggesting that sonographic features of biliary strictures are more accurate for diagnosis than the results of EUS-FNA cytology. While some authors have reported EUS-FNA as highly diagnostic for biliary strictures[20,21], the lesions that could explain strictures in those papers were large enough to be detected on CT/MRI, possibly increasing the diagnostic ability of cytology.
RC examination could have compensated for false-negative results in 1 patient with bile duct cancer. In this patient, we could not detect the tumor on EUS, as the tumor was behind impacted stones in the bile duct. This case supports previous reports that malignancy is frequently accompanied by biliary stones[22], recommending cytology examination to prevent false-negative diagnosis, particularly in cases with stones in the biliary tree.
Our results indicate that tumor markers using 100 U/mL as the cutoff level for CA19-9 are less diagnostic than imaging features of biliary strictures detected on EUS, when 100 U/mL is considered as malignancy[14]. This is compatible with other studies[15,23] noting that tumor markers specific to cancers along the biliary tract are unavailable. When 37 U/mL was used as the cutoff level for CA19-9, no significant difference was seen between the diagnostic abilities of EUS and tumor markers. This is probably because the CA19-9 level increases in cases of cholestasis[15], leading to false-positive cases.
Although preliminary, this study may be the first report to suggest criteria for EUS to classify biliary strictures as benign or malignant. Limited information is available on the criteria for EUS, partly due to the limited availability of EUS[7], while definite criteria for IDUS to classify biliary strictures as benign or malignant have almost been established. According to Menzel, hypoechoic masses with irregular margins and inhomogeneous echo-poor areas invading surrounding tissue on IDUS are suggestive of malignancy[10]. Tamada et al[3] reported that the presence of a hypoechoic mass with irregular margins, or infiltration of surrounding tissues, size > 10 mm, and disruption of normal sonographic layers of the bile duct wall, is predictive of malignancy. These data using IDUS are compatible with our results on EUS, suggesting that the presence of a mass adjacent to the biliary stricture, particularly with irregular margins, hypo- or mixed-echoic pattern, disruption of the bile duct, and invasion to surrounding tissue imply malignancy. In our study, mass size was not correlated with the pathological diagnosis of strictures. In the study by Tamada et al[3], malignant masses may have been larger than benign masses, as lesion size was not among the inclusion criteria for subjects in that study. Since our objectives were biliary strictures for which CT could not identify a mass, the lesions should have been much smaller than those studied in other investigations. Malignant and benign lesions may thus have shown no significant difference in size in the present study.
A major limitation in this study was that dynamic CT was performed according to the routine protocol in all cases. If thinner section thickness (e.g. 1-2 mm instead of 8 mm) was used or 3-dimensional analysis instead of horizontal analysis alone was performed, the causative mass could have been detected. If the dynamic study had been adjusted to the location of each biliary stricture, more detailed information could also have been obtained[24]. However, no imaging modalities have shown detectability of small pancreatic cancer superior to EUS[9]. In extrahepatic bile duct carcinoma, particularly without thickening of biliary walls, identification of local extension or depth using CT alone is known to be difficult[25]. In the diagnosis of cancer of the papilla of Vater, depicting tumors by CT is accepted as difficult[14]. These reports indicate that detectability of lesions that can cause biliary stricture remains insufficient using CT alone.
In conclusion, sonographic appearance on EUS offers high diagnostic ability for extrahepatic biliary strictures, even if cross-sectional imaging modalities cannot depict the causative lesion. Particularly in cases of benign stricture where the final diagnosis is “normal”, invasive procedures such as surgical exploration or excessive follow-up investigations may be avoided. Furthermore, earlier diagnosis of malignant strictures by EUS would provide more therapeutic options for patients with very early-stage pancreaticobiliary cancer.
There is no consensus regarding how to manage distal biliary stricture without an identifiable mass on cross-sectional imaging, since benign and malignant strictures cannot be definitely distinguished. For indeterminate biliary strictures, intraductal ultrasonography (IDUS) has recently yielded a diagnostic accuracy of around 80%-90%. However, the ability of endoscopic ultrasonography (EUS) to diagnose unexplained strictures has not been fully examined.
The authors demonstrated that EUS can diagnose biliary strictures caused by malignant tumors that are undetectable on computed tomography scan. They also gave a summary of the sonographic features of malignant strictures, which could increase the diagnostic sensitivity of EUS.
There is only one previously published study on the diagnostic utility of EUS for biliary strictures with an unknown etiology. In that study, however, almost all patients had a stent in place, possibly making the bile duct wall thicker and irregular even in patients with benign strictures. Since this clinical study included many patients without a stent, sonographic features between benign and malignant strictures could be compared, providing characteristic EUS images for malignancy.
EUS plus magnetic resonance cholangiopancreatography could be a first-line, noninvasive examination for the diagnosis of distal biliary stricture, followed by IDUS/endoscopic retrograde cholangiopancreatography examinations or histological confirmation, when the latter are required, especially in cases showing low clinical suspicion for malignancy.
This is an interesting retrospective study of clinical relevance. In spite of some limitations, this paper adds to the core knowledge related to diagnostic challenges in patients with a bile duct stricture.
Peer reviewers: Tarkan Karakan, Associate Professor, Department of Gastroenterology, Gazi University, Ankara, 06500, Turkey; Dr. Ching-Chung Lin, Gastroenterology Division, Mackay Memorial Hospital, 5F, No 28, Lane 286, Shidong Road, Taipei 11154, Taiwan, China; Jon Arne Soreide, Professor, Department of Surgery, Stavanger University Hospital, Stavanger, N-4068, Norway
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
| 1. | Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069-1073. |
| 2. | Fogel EL, deBellis M, McHenry L, Watkins JL, Chappo J, Cramer H, Schmidt S, Lazzell-Pannell L, Sherman S, Lehman GA. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc. 2006;63:71-77. |
| 3. | Tamada K, Ueno N, Tomiyama T, Oohashi A, Wada S, Nishizono T, Tano S, Aizawa T, Ido K, Kimura K. Characterization of biliary strictures using intraductal ultrasonography: comparison with percutaneous cholangioscopic biopsy. Gastrointest Endosc. 1998;47:341-349. |
| 4. | Farrell RJ, Agarwal B, Brandwein SL, Underhill J, Chuttani R, Pleskow DK. Intraductal US is a useful adjunct to ERCP for distinguishing malignant from benign biliary strictures. Gastrointest Endosc. 2002;56:681-687. |
| 5. | Stavropoulos S, Larghi A, Verna E, Battezzati P, Stevens P. Intraductal ultrasound for the evaluation of patients with biliary strictures and no abdominal mass on computed tomography. Endoscopy. 2005;37:715-721. |
| 6. | Vazquez-Sequeiros E, Baron TH, Clain JE, Gostout CJ, Norton ID, Petersen BT, Levy MJ, Jondal ML, Wiersema MJ. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest Endosc. 2002;56:372-379. |
| 7. | Krishna NB, Saripalli S, Safdar R, Agarwal B. Intraductal US in evaluation of biliary strictures without a mass lesion on CT scan or magnetic resonance imaging: significance of focal wall thickening and extrinsic compression at the stricture site. Gastrointest Endosc. 2007;66:90-96. |
| 8. | Domagk D, Poremba C, Dietl KH, Senninger N, Heinecke A, Domschke W, Menzel J. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut. 2002;51:240-244. |
| 9. | Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460-1469. |
| 10. | Menzel J, Poremba C, Dietl KH, Domschke W. Preoperative diagnosis of bile duct strictures--comparison of intraductal ultrasonography with conventional endosonography. Scand J Gastroenterol. 2000;35:77-82. |
| 11. | Mesenas S, Vu C, Doig L, Meenan J. Duodenal EUS to identify thickening of the extrahepatic biliary tree wall in primary sclerosing cholangitis. Gastrointest Endosc. 2006;63:403-408. |
| 12. | Tamada K, Tomiyama T, Wada S, Ohashi A, Satoh Y, Ido K, Sugano K. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut. 2002;50:326-331. |
| 13. | Yanaga Y, Awai K, Nakaura T, Namimoto T, Oda S, Funama Y, Yamashita Y. Optimal contrast dose for depiction of hypervascular hepatocellular carcinoma at dynamic CT using 64-MDCT. AJR Am J Roentgenol. 2008;190:1003-1009. |
| 14. | Cwik G, Wallner G, Skoczylas T, Ciechanski A, Zinkiewicz K. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg. 2006;141:968-973; discussion 974. |
| 15. | Tsukada K, Takada T, Miyazaki M, Miyakawa S, Nagino M, Kondo S, Furuse J, Saito H, Tsuyuguchi T, Kimura F. Diagnosis of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:31-40. |
| 16. | Strasberg SM. ERCP and surgical intervention in pancreatic and biliary malignancies. Gastrointest Endosc. 2002;56:S213-S217. |
| 17. | Menzel J, Domschke W. Gastrointestinal miniprobe sonography: the current status. Am J Gastroenterol. 2000;95:605-616. |
| 18. | Leung JW, Ling TK, Kung JL, Vallance-Owen J. The role of bacteria in the blockage of biliary stents. Gastrointest Endosc. 1988;34:19-22. |
| 19. | Donelli G, Guaglianone E, Di Rosa R, Fiocca F, Basoli A. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clin Med Res. 2007;5:53-60. |
| 20. | Fritscher-Ravens A, Broering DC, Sriram PV, Topalidis T, Jaeckle S, Thonke F, Soehendra N. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc. 2000;52:534-540. |
| 21. | Erickson RA, Garza AA. EUS with EUS-guided fine-needle aspiration as the first endoscopic test for the evaluation of obstructive jaundice. Gastrointest Endosc. 2001;53:475-484. |
| 22. | Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577-1582. |
| 23. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. |
| 24. | Michl P, Pauls S, Gress TM. Evidence-based diagnosis and staging of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:227-251. |
| 25. | Miyakawa S, Ishihara S, Takada T, Miyazaki M, Tsukada K, Nagino M, Kondo S, Furuse J, Saito H, Tsuyuguchi T. Flowcharts for the management of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:7-14. |