Published online May 21, 2010. doi: 10.3748/wjg.v16.i19.2388
Revised: January 15, 2010
Accepted: January 22, 2010
Published online: May 21, 2010
AIM: To evaluate quality of life (QoL) after curative liver resection and identify variables associated with decreased QoL.
METHODS: From October 2001 to July 2004, 323 patients underwent liver resection. At 3-36 mo after discharge, 188 patients were disease free. QoL was assessed using the Short Form (SF)-12 Health Survey with mental and physical component scales (SF-12 MCS and PCS), supplemented with generic questions concerning pain and liver-specific items.
RESULTS: Sixty-eight percent (128/188) returned the questionnaire, which was completed in 75% (96/128) of cases. Median SF-12 PCS and MCS were 46.7 (interquartile range: 34.2-53.9) and 54.1 (42.8-58.2). Fifty percent were pain free with a median symptom score of 1.75 (1.38-2.13). PCS was higher after major hepatectomy [57% (55/96)] compared to minor resection (P = 0.0049), which represented an improved QoL. QoL was not affected by sex but by age compared to the general German population. MCS was higher after liver surgery for metastatic disease [55.9 (47.5-58.8)] compared to primary carcinoma [49.6 (36.5-55.1)] and benign disease [49.2 (37.7-56.3)] (P = 0.0317). There was no correlation between length of postoperative period and QoL. Pain, deficiencies in everyday life and a high symptom score significantly decreased MCS and PCS.
CONCLUSION: Most patients were only marginally affected even after major liver resection; however, minor complications were associated with decreased SF-12 MCS and PCS and need careful attention.
- Citation: Bruns H, Krätschmer K, Hinz U, Brechtel A, Keller M, Büchler MW, Schemmer P. Quality of life after curative liver resection: A single center analysis. World J Gastroenterol 2010; 16(19): 2388-2395
- URL: https://www.wjgnet.com/1007-9327/full/v16/i19/2388.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i19.2388
Over the past few decades, the multimodal concept of quality of life (QoL) has been appreciated in many areas of medical practice[1]. Social, physical and mental factors contribute to QoL[2,3]. QoL assessment has proven to be a valuable parameter for patients and surgeons and may be helpful in determining the optimal treatment. As an outcome parameter, QoL is considered as important as disease-free and overall survival[4]. For many different procedures in surgery, the effect on QoL has been assessed, including liver resection for primary and secondary cancer, organ transplantation and gastrectomy[5-13].
In various benign and malignant liver diseases, resection is a common procedure with curative intention. While major and minor hepatectomy are both safe procedures, little is known about postoperative QoL in these patients[14-16]. Erim et al[17,18] have investigated QoL of live donors who have undergone liver resection for living related liver transplantation. Anxiety and depression increased in live donors, and improved 3 mo after surgery. Furthermore, Kaneko et al[19] have published a study on laparoscopic vs open liver resection in patients with hepatocellular carcinoma. QoL improved earlier after laparoscopic surgery. Their finding is similar to other studies that have compared various laparoscopic with open surgical procedures[19,20]. Recently, it has been published that QoL returns to baseline after liver resection for malignancies in most cases[21,22]. Martin et al[21] have shown in a prospective study of 36 patients that QoL returns to baseline at about 3 mo after liver resection for cancer.
Thus, the present study was designed to assess postoperative QoL of patients who underwent curative liver resection. Curative resection was defined as the absence of recurrent disease at the time of assessment. QoL was compared with that of the general German population, with a special focus on demographics (e.g. age and sex) and medical history (e.g. indication, type of resection, complications and impairment).
Although several studies on QoL after liver resection have been performed, to the best of our knowledge, this is the largest study on QoL of patients who have undergone curative liver resection[19,21,23,24]. In our opinion, this study’s value is the exclusion of any bias caused by the palliative situation of the study population. It seems plausible that patients in a palliative situation might be impaired in their physical and especially mental QoL scores. Therefore, we decided to exclude any patients with signs of recurrence or active disease.
Patients who underwent curative partial hepatectomy from October 2001 to July 2004 were recruited for this single-center, observational study on QoL after liver resection. While all patients underwent liver resection with curative intention, curative treatment in the context of this study was defined as the absence of recurrent disease at the time of assessment. The study protocol was approved by the ethical committee and all patients provided informed consent.
A total of 323 patients underwent partial hepatectomy in our hospital from October 2001 to July 2004. One hundred and eighty-eight patients were disease-free at the time of assessment and were eligible to participate in the study, and questionnaires were mailed to these patients. A return rate of 68% (128 patients) for the questionnaires, of which in 75% were filled out completely, further decreased the final study population to 96 patients. Thus, the final population consisted of almost 50% of the eligible study population.
QoL was assessed by the Short-Form (SF)-12 Health Survey, which is the abbreviated version of the widely used generic profile SF-36[25,26]. Both questionnaires have been validated for numerous populations and quantify the overall physical and mental aspects of QoL[27-34]. The SF-12 provides a subset of 12 items from which two summary measures of physical and mental health status (SF-12 PCS and SF-12 MCS) can be derived from subscales for physical function, physical role, pain, general health, vitality, social function, emotional role, and mental health with high validity, reliability and sensitivity[35].
The demographic and postoperative data, and results of the SF-12 Health Survey, a well-established QoL questionnaire, were analyzed, together with a pain assessment on a 0-10 scale and relevant liver-specific items.
Additional illness-specific items were used to assess liver-specific issues, for example, fever, dyspepsia, heartburn, lack of appetite, nausea, vomiting, night sweat and exhaustion. Patients indicated on a scale of 1-5 (1, never; 2, rarely; 3, sometimes; 4, often; 5, very often) the frequency of the individual symptom postoperatively. The calculated mean was used as a symptom score that ranged from 1 to 5. Impairment in everyday activities was measured on a scale of 1-5 (1, none; 2, light; 3, moderate; 4, heavy; and 5, strongest) and patient autonomy was measured as a variable indicating “independent” and “help needed”. Pain was assessed using a 0-10 scale.
As described by Belghiti et al[36], major hepatectomy was defined as resection of three or more segments and minor hepatic resection as resection of two or fewer segments. Thus, our patients were divided into a major and minor hepatectomy group.
Postoperative complications were stratified into surgical (e.g. bile leak or biloma, pneumothorax, wound infection, liver abscess, bleeding, and surgical dehiscence) and medical (e.g. pleural effusion, renal failure, hepatic failure, pneumonia, cardiac insufficiency, and cholangitis), and were assessed from patient records. Complications were defined as described elsewhere[16].
QoL was assessed at least 3 mo after discharge from hospital (range: 3-36 mo). Medical data were collected from follow-up examinations as proof of patient eligibility (e.g. no signs of recurrence). In patients with malignant diseases, staging was performed according to the guidelines of the Association of the Scientific Medical Societies in Germany. Only patients without signs of recurrence were eligible for the study. Subsequently, questionnaires were sent to the patients by mail. Furthermore, patients were contacted by telephone to increase the return rate of questionnaires. Clinical data such as indication for liver resection, type of liver resection, medical and surgical postoperative complications were collected and documented in a database in a prospective manner.
SAS software (version 9.1; SAS Institute, Cary, NC, USA) was used for statistical analysis. The quantitative parameters SF-12 PCS, SF-12 MCS, pain, symptom score, and age are presented as median with interquartile range. Mann-Whitney U test and Kruskal-Wallis one-way analysis of variance were chosen to compare the quantitative parameters between subgroups of patients. Patients were stratified for age, sex, type of resection and indication for surgery. The relationships between the quantitative parameters were analysed using Spearman’s rank correlation coefficient r and its corresponding P value. Two-sided P values were always calculated; P < 0.05 was considered statistically significant, and P < 0.001 was considered highly significant.
One hundred and eighty-eight patients underwent partial hepatectomy for benign and malignant hepatic lesions and were recurrence-free at the time of assessment. While 128 patients (68%) returned their questionnaire, it was fully completed in only 96 cases (52% of recurrence-free patients), of which 50% were male. Thus the final study population comprised 96 patients (Table 1). No significant difference could be found in demographics, indications and type of resection between patients with fully and partial filled questionnaires.
| n = 96 | 100% | |
| Median age (yr) | 63.4 | IQR: 54.5-70.5 |
| Male/female | 48/48 | 50/50 |
| Primary malignant | 21 | 21.9 |
| Hepatocellular carcinoma | 12 | 12.5 |
| Cholangiocellular carcinoma | 8 | 8.3 |
| Gallbladder carcinoma | 1 | 1.0 |
| Metastatic | 55 | 57.3 |
| Colorectal | 38 | 39.6 |
| Other | 17 | 17.7 |
| Benign | 20 | 20.8 |
| Adenoma | 4 | 4.2 |
| Focal nodular hyperplasia | 6 | 6.3 |
| Cysts | 1 | 1.0 |
| Echinococcus | 3 | 3.1 |
| Haemangioma | 2 | 2.1 |
| Other | 4 | 4.2 |
| Major hepatectomy | 55 | 57.3 |
| Right hemi-hepatectomy | 35 | 36.5 |
| Extended right hemi-hepatectomy | 3 | 3.1 |
| Left hemi-hepatectomy | 8 | 8.3 |
| Extended left hemi-hepatectomy | 3 | 3.1 |
| Segmentectomy (n > 2) | 6 | 6.3 |
| Minor hepatectomy | 41 | 42.7 |
| Segmentectomy (n = 1) | 23 | 24.0 |
| Segmentectomy (n = 2) | 18 | 18.8 |
| Concomitant extrahepatic resection | 10 | 10.4 |
The diagnoses are listed in Table 1; almost 21% of patients resected had benign lesions and > 79% had malignant liver tumors. Of the latter, > 72% were metastatic. More than 69% of metastases had spread from a colorectal tumor.
The procedures performed are listed in Table 1. There were 55 (57%) major and 41 minor hepatic resections. Patients underwent hemi-hepatectomy or extended hemi-hepatectomy in 45% and > 6% of the cases, respectively. Furthermore, six patients (6%) underwent resection of ≥ 3 segments other than (extended) hemi-hepatectomy. Minor resection was performed in 43% of patients. These patients underwent resection of one segment (24%), or segmental resection that consisted of en bloc resection of two segments or resection of two discontinuous segments (20%).
At least one of both medical and surgical postoperative complications occurred in about 30 (31%) patients (Table 2). Pleural effusion was the most frequent medical complication and occurred in about 9% of all cases. The most frequent surgical complication was bile leakage or biloma, which occurred in 6% of all cases (Table 2). Type of surgery (i.e. major or minor hepatectomy) was not significantly associated with frequency of surgical and medical complications.
| Complication | Total | Major hepatectomy | Minor hepatectomy | |||
| n = 96 | 100% | n = 55 | 100% | n = 41 | 100% | |
| Total morbidity | 30 | 31.2 | 20 | 36.4 | 10 | 24.4 |
| Surgical complications | 19 | 19.8 | 14 | 25.5 | 5 | 12.2 |
| Bile leak/biloma | 6 | 6.3 | 5 | 9.1 | 1 | 2.4 |
| Pneumothorax | 3 | 3.1 | 2 | 3.6 | 1 | 2.4 |
| Wound infection | 2 | 2.1 | 2 | 3.6 | - | - |
| Abscess (liver) | 1 | 1.0 | 1 | 1.8 | - | - |
| Bleeding | 1 | 1.0 | 1 | 1.8 | - | - |
| Surgical dehiscence | 1 | 1.0 | 1 | 1.8 | - | - |
| Other | 5 | 5.2 | 2 | 3.6 | 3 | 7.3 |
| Medical complications | 19 | 19.8 | 11 | 20.0 | 8 | 19.5 |
| Pleural effusion | 9 | 9.4 | 7 | 12.7 | 2 | 4.9 |
| Renal failure | 3 | 3.1 | 2 | 3.6 | 1 | 2.4 |
| Hepatic failure | 2 | 2.1 | 2 | 3.6 | - | - |
| Pneumonia | 2 | 2.1 | - | - | 2 | 4.9 |
| Cardiac insufficiency | 1 | 1.0 | - | - | 1 | 2.4 |
| Cholangitis | 1 | 1.0 | 1 | 1.8 | - | - |
| Other | 3 | 3.1 | 3 | 5.5 | - | - |
| Revision laparotomy | 3 | 3.1 | 2 | 3.6 | 1 | 2.4 |
Table 3 shows the SF-12 PCS and SF-12 MCS median scores stratified for demographic items, type of resection, indication and overall morbidity.
| n | Physical score | Mental score | |||
| Median | IQR | Median | IQR | ||
| Total | 96 | 46.7 | 34.2-53.9 | 54.1 | 42.8-58.2 |
| Sex | |||||
| Male | 48 | 50.9 | 33.2-54.0 | 55.0 | 44.8-58.9 |
| Female | 48 | 44.6 | 36.3-53.4 | 52.9 | 40.9-57.8 |
| Age (yr) | |||||
| < 41 | 10 | 38.5 | 33.4-50.4 | 41.8 | 32.0-54.1 |
| 41-50 | 7 | 38.6 | 35.4-46.9 | 54.4 | 37.1-62.4 |
| 51-60 | 16 | 47.4 | 33.3-54.9 | 54.6 | 43.6-59.8 |
| 61-70 | 38 | 49.8 | 36.0-54.2 | 55.3 | 43.1-58.8 |
| > 70 | 25 | 50.8 | 37.4-53.8 | 53.4 | 45.0-57.9 |
| Indicationa | |||||
| Benign | 20 | 43.5 | 33.4-54.9 | 49.2 | 37.7-56.3 |
| Prim. malignant | 22 | 41.6 | 32.9-53.1 | 49.6 | 36.5-55.1 |
| Metastatic | 54 | 50.6 | 39.0-54.2 | 55.9 | 47.5-58.8 |
| Type of resectionb | |||||
| Major | 55 | 52.0 | 39.0-54.4 | 54.8 | 43.7-58.5 |
| Minor | 41 | 41.3 | 31.6-51.0 | 52.6 | 38.6-57.9 |
| Morbidity | |||||
| Yes | 30 | 44.2 | 29.7-53.8 | 53.1 | 43.1-57.8 |
| No | 66 | 50.2 | 37.4-54.1 | 54.1 | 42.4-58.8 |
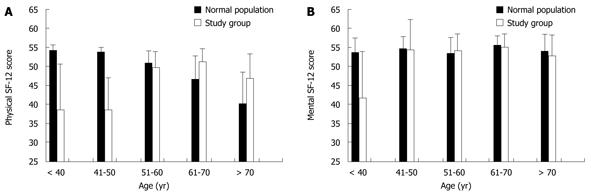
The age-dependent distribution of physical and mental SF-12 scores is shown in Figure 1 for patients vs the population norm. Patients younger than 40-51 years who underwent liver resection showed significantly lower SF-12 PCS (Figure 1A) and SF-12 MCS (Figure 1B) scores compared to their age-matched normal group (P < 0.05).
The mental QoL score was significantly higher (P < 0.05) in patients who underwent liver resection for metastases, with almost 56 (48-59) compared to patients with primary liver malignancies or benign liver disease with scores of about 49 (38-56) and 50 (37-55), respectively. Physical QoL varied according to the type of liver resection. While patients who underwent major resection reached a physical score of 52 (39-54), values after minor resection were about 41 (32-51) (P < 0.005). Sex, age and postoperative morbidity did not have a significant impact on mental or physical QoL.
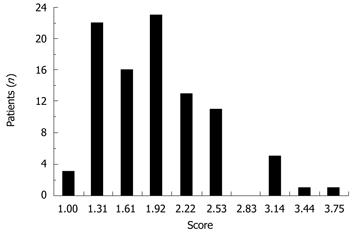
Postoperative symptoms rarely occurred (Figure 2). The median value of symptom scores was 1.75 (1.38-2.13).
Subjective impairment after surgery correlated with lower summary score (P < 0.001) (Table 4). Patients who were not able to manage their living alone without the help of others had lower scores for both SF-12 PCS (P < 0.05) and SF-12 MCS (P < 0.001) (Table 4). The occurrence of medical problems considered to be minor was associated with decreased QoL scores (Table 4). Patients with postoperative wound infections showed significantly lower values for both SF-12 PCS (P < 0.05) and SF-12 MCS (P < 0.05) compared to patients without this complication and population norm.
High symptoms scores, pain and deficiencies had significant negative correlations to physical and mental SF-12 scores. Furthermore, high symptom scores correlated with high levels of pain and deficiencies in day-to-day life experienced by patients. Pain and scores of SF-12 PCS had a significant negative correlation (r = -0.65, P < 0.0001), while high degrees of deficiencies in the patients’ daily routine correlated with low QoL values (r = -0.59 and -0.51 for PCS and MCS, respectively, P < 0.0001). Furthermore, high symptom scores were associated with pain and the feeling of being handicapped in life.
In mail surveys, non-responder bias has to be kept in mind. Our study provided valid data for 68% of our patients, and data for the missing 32% could only be generated by extrapolation. Different approaches exist for the extrapolation of data from mail surveys, which have been discussed previously[37-39]. Due to the relatively high response rate - compared to a mean response rate to mail surveys published in medical journals of 30%-60% - and since there was no systematic difference as far as demographic and medical characteristics were concerned, we assumed that our data were valid in the context in which they were investigated[38].
Postoperative QoL has been assessed for many medical and surgical treatments[40-43]. Most of these studies aimed not only at providing descriptive data, but also to help physicians and surgeons to choose optimal treatment[44]. Our results describe QoL after liver resection for patients who received curative treatment. In contrast to Martin et al[21], we included patients with all common types of indication for liver resection. Furthermore, we ensured that only patients who were disease free at least 3 mo after liver resection were included.
The present study found that patients younger than 50 years of age had lower QoL compared to their age-matched normal population group and to older patients. The impact of surgery on younger patients’ QoL might be stronger for several reasons. Besides medical reasons (i.e. comorbidity and different indications), it has to be taken into account that, in general populations, QoL (especially physical) is usually found to decline with age, and patients might have different expectations in different age groups. Patients older than 70 years of age reported a higher physical QoL than their age-matched controls.
Patients with liver metastases reported a higher physical as well as mental QoL than those who underwent resection for primary malignant or benign liver lesions (Table 3). We assumed that most patients who underwent surgery for metastatic disease already knew from the primary surgical intervention that physical wellbeing would return some time after surgery. Furthermore, we assumed that the fulfilled hope to be cured from the metastases of an already resected primary tumor might have a great impact on QoL[45].
Surprisingly, patients who underwent major liver resection reported a higher physical QoL compared to those who underwent minor resection (Table 3). Patients might compare their QoL to their recovery period, which may be prolonged and more complicated after extensive surgery, thus leading to a subjectively improved QoL.
Neither surgical nor medical complications were more frequent after major compared to minor hepatectomy (Table 2). Furthermore, postoperative complications that are considered to be minor medical problems, such as impaired wound healing, were found to interfere with QoL (Table 4). In all patients, the symptom score, pain and deficiencies in daily routine correlated significantly with QoL.
Data presented here show that 3-36 mo after discharge from hospital, mental and physical scores tended to be close to those of the general population, as long as QoL-decreasing factors (e.g. pain, or impaired wound healing) were absent. Furthermore, we were also able to identify important determinants of QoL.
QoL is always subject to individual judgement and compared by the patients to their own experience. Thus, elderly patients, who generally experience decreased physical QoL in comparison to younger patients, might find the effect of surgery less invasive. While this is clearly speculative, this might be an explanation for the higher physical QoL scores after surgery in elderly patients.
Moreover, individual judgement of QoL offers an explanation for the counter-intuitive finding of higher QoL scores after major hepatectomy in comparison to minor resection. Patients undergoing major resection most likely experience a higher impact of the more invasive surgery. Thus, after recovery, they might experience a higher change in their physical scores.
In contemporary surgery, QoL is considered as important as disease-free survival and morbidity. Thus, we investigated important factors that potentially determine QoL in patients undergoing curative hepatic resection. This study clearly demonstrates that even after a short time following liver resection, the vast majority of patients score equal or even higher in SF-12 compared to that of the population norm.
However, there is a small number of patients whose QoL might be affected by pain, impaired wound healing and subjectively perceived deficiencies in their daily routine following liver resection. This implies that appropriate pain and wound management is needed and coping with deficiencies in daily routine needs to be addressed postoperatively.
Furthermore, clinicians should be aware that QoL might be appraised differently depending upon age and underlying disease, in order to discuss the expectations of the surgical procedure.
Quality of life (QoL) has been appreciated in many areas of medical practice and has proven to be helpful in determining the optimal treatment. As an outcome parameter, QoL is considered as important as disease-free and overall survival. Although major and minor hepatectomy are both safe procedures, little is known about their postoperative QoL. Thus, this study was designed to assess the postoperative QoL of patients who underwent curative liver resection and to compare their QoL to that of the general German population, with a special focus on demographics and medical history.
Although several studies on QoL after liver resection have been performed, to the best knowledge of the authors of this article, this is the largest study on QoL of patients who underwent curative liver resection. In the authors’ opinion, this study’s value is the exclusion of any bias caused by the palliative situation of the study population. It seems plausible that patients in a palliative situation might be impaired in their physical and especially mental QoL scores. Therefore, the authors decided to exclude any patients with signs of recurrence or active disease.
In contrast to other studies, the authors eliminated the palliative bias and thus were able to evaluate the effect of surgery itself on QoL. While interpretation of our findings needs to be done with due care and, as in other studies on this subject, is speculative, they were able to identify contributors to QoL, and more importantly, did not identify a negative effect of curative hepatectomy on QoL.
The authors think that their findings are helpful in defining the optimal treatment for patients who require liver resection. Thus, their data justify the indication for surgery and can help surgeons to recommend major and minor hepatectomy to patients who need this type of surgery.
QoL is a multimodal concept and can be measured with standardized questionnaires such as the Short-Form Health Survey (SF). Both versions (SF-12 and SF-36) have been validated and provide information on both physical and mental QoL.
This is a large study of QoL in 323 patients undergoing liver resection for various indications. QoL was assessed using standard verification protocols.
Peer reviewers: Kazuhiro Hanazaki, MD, Professor and Chairman, Department of Surgery, Kochi Medical School, Kochi University, Kohasu, Okohcho, Nankoku, Kochi 783-8505, Japan; Tomoharu Yoshizumi, MD, PhD, Department of Surgery, Saiseikai Fukuoka General Hospital, 1-3-46, Tenjin, Chuou-ku, Fukuoka 810-0001, Japan; Christopher Christophi, Professor and Head of The University of Melbourne, Department of Surgery, Austin Hospital. Melbourne, 145 Studley Road, Victoria 3084, Australia
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 2. | Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology. 2007;16:691-706. |
| 3. | Keller M, Sommerfeldt S, Fischer C, Knight L, Riesbeck M, Lowe B, Herfarth C, Lehnert T. Recognition of distress and psychiatric morbidity in cancer patients: a multi-method approach. Ann Oncol. 2004;15:1243-1249. |
| 5. | Scurtu R, Groza N, Otel O, Goia A, Funariu G. Quality of life in patients with esophagojejunal anastomosis after total gastrectomy for cancer. Rom J Gastroenterol. 2005;14:367-372. |
| 6. | Perez DJ, McGee R, Campbell AV, Christensen EA, Williams S. A comparison of time trade-off and quality of life measures in patients with advanced cancer. Qual Life Res. 1997;6:133-138. |
| 7. | Lee LJ, Chen CH, Yao G, Chung CW, Sheu JC, Lee PH, Tsai YJ, Wang JD. Quality of life in patients with hepatocellular carcinoma received surgical resection. J Surg Oncol. 2007;95:34-39. |
| 8. | Kondo Y, Yoshida H, Tateishi R, Shiina S, Mine N, Yamashiki N, Sato S, Kato N, Kanai F, Yanase M. Health-related quality of life of chronic liver disease patients with and without hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:197-203. |
| 9. | Hjermstad MJ, Hollender A, Warloe T, Karlsen KO, Ikonomo I, Kvaloy S, Nome O, Holte H. Quality of life after total or partial gastrectomy for primary gastric lymphoma. Acta Oncol. 2006;45:202-209. |
| 10. | Banz VM, Inderbitzin D, Fankhauser R, Studer P, Candinas D. Long-term quality of life after hepatic resection: health is not simply the absence of disease. World J Surg. 2009;33:1473-1480. |
| 11. | Redaelli CA, Wagner M, Krahenbuhl L, Gloor B, Schilling MK, Dufour JF, Buchler MW. Liver surgery in the era of tissue-preserving resections: early and late outcome in patients with primary and secondary hepatic tumors. World J Surg. 2002;26:1126-1132. |
| 12. | Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Dodd MJ, Painter PL. Objective measures of health-related quality of life over 24 months post-liver transplantation. Clin Transplant. 2005;19:1-9. |
| 13. | Chen L, Liu Y, Li GG, Tao SF, Xu Y, Tian H. Quality of life in patients with liver cancer after operation: a 2-year follow-up study. Hepatobiliary Pancreat Dis Int. 2004;3:530-533. |
| 14. | Schemmer P, Bruns H, Weitz J, Schmidt J, Buchler MW. Liver transection using vascular stapler: a review. HPB (Oxford). 2008;10:249-252. |
| 15. | Schemmer P, Friess H, Dervenis C, Schmidt J, Weitz J, Uhl W, Buchler MW. The use of endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg. 2007;24:300-305. |
| 16. | Schemmer P, Friess H, Hinz U, Mehrabi A, Kraus TW, Z’graggen K, Schmidt J, Uhl W, Buchler MW. Stapler hepatectomy is a safe dissection technique: analysis of 300 patients. World J Surg. 2006;30:419-430. |
| 17. | Erim Y, Beckmann M, Kroencke S, Valentin-Gamazo C, Malago M, Broering D, Rogiers X, Frilling A, Broelsch CE, Schulz KH. Psychological strain in urgent indications for living donor liver transplantation. Liver Transpl. 2007;13:886-895. |
| 18. | Erim Y, Beckmann M, Valentin-Gamazo C, Malago M, Frilling A, Schlaak JF, Gerken G, Broelsch CE, Senf W. Quality of life and psychiatric complications after adult living donor liver transplantation. Liver Transpl. 2006;12:1782-1790. |
| 19. | Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190-194. |
| 20. | Korolija D, Sauerland S, Wood-Dauphinee S, Abbou CC, Eypasch E, Caballero MG, Lumsden MA, Millat B, Monson JR, Nilsson G. Evaluation of quality of life after laparoscopic surgery: evidence-based guidelines of the European Association for Endoscopic Surgery. Surg Endosc. 2004;18:879-897. |
| 21. | Martin RC, Eid S, Scoggins CR, McMasters KM. Health-related quality of life: return to baseline after major and minor liver resection. Surgery. 2007;142:676-684. |
| 22. | Dasgupta D, Smith AB, Hamilton-Burke W, Prasad KR, Toogood GJ, Velikova G, Lodge JP. Quality of life after liver resection for hepatobiliary malignancies. Br J Surg. 2008;95:845-854. |
| 23. | Eid S, Stromberg AJ, Ames S, Ellis S, McMasters KM, Martin RC. Assessment of symptom experience in patients undergoing hepatic resection or ablation. Cancer. 2006;107:2715-2722. |
| 24. | Poon RT, Fan ST, Yu WC, Lam BK, Chan FY, Wong J. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg. 2001;136:693-699. |
| 25. | Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220-233. |
| 26. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. |
| 27. | Sanderson K, Andrews G. The SF-12 in the Australian population: cross-validation of item selection. Aust N Z J Public Health. 2002;26:343-345. |
| 28. | Pezzilli R, Morselli-Labate AM, Frulloni L, Cavestro GM, Ferri B, Comparato G, Gullo L, Corinaldesi R. The quality of life in patients with chronic pancreatitis evaluated using the SF-12 questionnaire: a comparative study with the SF-36 questionnaire. Dig Liver Dis. 2006;38:109-115. |
| 29. | Lam CL, Tse EY, Gandek B. Is the standard SF-12 health survey valid and equivalent for a Chinese population? Qual Life Res. 2005;14:539-547. |
| 30. | Kiely JM, Brasel KJ, Guse CE, Weigelt JA. Correlation of SF-12 and SF-36 in a trauma population. J Surg Res. 2006;132:214-218. |
| 31. | Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: a structured review of generic self-assessed health instruments. Qual Life Res. 2005;14:1651-1668. |
| 32. | Globe DR, Levin S, Chang TS, Mackenzie PJ, Azen S. Validity of the SF-12 quality of life instrument in patients with retinal diseases. Ophthalmology. 2002;109:1793-1798. |
| 33. | Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1171-1178. |
| 34. | Côté I, Grégoire JP, Moisan J, Chabot I. Quality of life in hypertension: the SF-12 compared to the SF-36. Can J Clin Pharmacol. 2004;11:e232-e238. |
| 35. | Resnick B, Nahm ES. Reliability and validity testing of the revised 12-item Short-Form Health Survey in older adults. J Nurs Meas. 2001;9:151-161. |
| 36. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. |
| 37. | Locker D, Wiggins R, Sittampalam Y, Patrick DL. Estimating the prevalence of disability in the community: the influence of sample design and response bias. J Epidemiol Community Health. 1981;35:208-212. |
| 38. | Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129-1136. |
| 39. | Armstrong JS, Overton TS. Estimating nonresponse bias in mail surveys. J Mark Res. 1977;14:396-402. |
| 40. | Montgomery M, Håkanson B, Ljungqvist O, Ahlman B, Thorell A. Twelve months’ follow-up after treatment with the EndoCinch endoscopic technique for gastro-oesophageal reflux disease: a randomized, placebo-controlled study. Scand J Gastroenterol. 2006;41:1382-1389. |
| 41. | McQuellon RP, Loggie BW, Fleming RA, Russell GB, Lehman AB, Rambo TD. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65-73. |
| 42. | Mahmood Z, McMahon BP, Arfin Q, Byrne PJ, Reynolds JV, Murphy EM, Weir DG. Endocinch therapy for gastro-oesophageal reflux disease: a one year prospective follow up. Gut. 2003;52:34-39. |
| 43. | Dymek MP, le Grange D, Neven K, Alverdy J. Quality of life and psychosocial adjustment in patients after Roux-en-Y gastric bypass: a brief report. Obes Surg. 2001;11:32-39. |
| 44. | Wight JP, Edwards L, Brazier J, Walters S, Payne JN, Brown CB. The SF36 as an outcome measure of services for end stage renal failure. Qual Health Care. 1998;7:209-221. |
| 45. | Knox CD, Feurer ID, Wise PE, Lamps LW, Kelly Wright J, Chari RS, Lee Gorden D, Wright Pinson C. Survival and functional quality of life after resection for hepatic carcinoid metastasis. J Gastrointest Surg. 2004;8:653-659. |