Published online May 21, 2010. doi: 10.3748/wjg.v16.i19.2371
Revised: February 1, 2010
Accepted: February 8, 2010
Published online: May 21, 2010
AIM: To study the effect of 5-Aza-2’-deoxycytidine (5-Aza-CdR) on heat shock protein 70 (HSP70), human leucocyte antigen-I (HLA-I) and NY-ESO-1 proteins in exosomes produced by hepatoma cells, HepG2 and Hep3B.
METHODS: Exosomes derived from HepG2 and Hep3B cells treated with or without 5-aza-CdR were isolated and purified by ultrafiltration centrifugation and sucrose gradient ultracentrifugation. The number of exosomes was counted under electron microscope. Concentration of proteins in exosomes was measured by bicinchoninic acid protein assay. Expression of HSP70, HLA-I and NY-ESO-1 proteins in exosomes was detected by Western blotting and immunoelectron microscopy. mRNA expression of p53 gene was detected by reverse transcription polymerase chain reaction.
RESULTS: The mRNA expression of p53 gene was increased in both hepatoma cell lines after treatment with 5-Aza-CdR. The number of exosomes and the concentration of total proteins in exosomes were increased significantly after treatment with 5-aza-CdR (P < 0.05). After treatment with 5-Aza-CdR, immunoelectron microscopy and Western blotting showed that the HSP70, HLA-I and NY-ESO-1 proteins were increased in exosomes produced by both hepatoma cell lines.
CONCLUSION: 5-aza-CdR, an inhibitor of DNA methyltransferase, can increase exosomes produced by hepatoma cells and immune-associated protein component of exosomes, which may be mediated by p53 gene up-regulation and 5-Aza-CdR demethylation.
- Citation: Xiao WH, Sanren GW, Zhu JH, Li QW, Kang HR, Wang RL, Song LP, Ye M. Effect of 5-Aza-2’-deoxycytidine on immune-associated proteins in exosomes from hepatoma. World J Gastroenterol 2010; 16(19): 2371-2377
- URL: https://www.wjgnet.com/1007-9327/full/v16/i19/2371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i19.2371
Human hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, with an annual incidence of over half a million. Despite the improvements in surveillance, imaging technology, surgical techniques, and perioperative care, its mortality rate still increases, accounting for 53% of all liver cancer deaths worldwide in China[1]. The most effective treatment modalities available for HCC to date are surgical resection, liver transplantation, and ablative therapy[2,3]. However, they are not indicated for patients who have relapse or are at advanced stage of the disease[4]. Moreover, the most commonly used treatment methods for various cancers, such as radiotherapy and chemotherapy, are often excluded in treatment of HCC due to their intolerable toxicity and insensitivity[5]. It is therefore necessary to develop a novel strategy against the progression and recurrence of HCC.
Exosomes secreted by tumor cells have become a recent hot spot in research of tumor immunology, raising intriguing attention to their immune-stimulating function in vitro and tumor model experiments[6,7]. However, acquiring a sufficient number of exosomes with a high quality for more powerful immune-stimulating effects has remained a great challenge for tumor immunotherapy[8-10]. Apart from the p53-dependent pathway, the mechanisms by which tumors secrete exosomes have not been well understood[11].
5-Aza-2’-deoxycytidine (5-Aza-CdR), a DNA methyltransferase inhibitor and a demethylation promoter in CpG regions of many genes, including the p53 gene, can significantly restore or increase their expression[12,13], including the expression of p53 by damaging DNA[14]. It has been shown that 5-Aza-CdR can significantly increase the expression of immune molecules necessary for anti-tumor cellular immunity by demethylating DNA, such as human leucocyte antigen (HLA)-I, and HLA-II, and significantly enhance the therapeutic effect of anti-tumor immunity in vitro and in animal experiments[15-17]. However, few reports are available on the effects of 5-Aza-CdR on the secretion of exosomes and the protein level in exosomes. This study was to explore the effect of 5-Aza-CdR on the secretion of exosomes, tumor-associated antigens and immune molecules in exosomes, and its mechanisms by which hepatocellular carcinoma cell lines secrete exosomes, in an attempt to provide preliminary experimental evidence for 5-Aza-CdR-modified exosomes-based anti-hepatoma immunotherapy.
HepG2 cell line was generously provided by Professor You-Yong Lu, Beijing Cancer Institute. Hep3B cell line was purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (CAS).
5-Aza-CdR, heavy water, cane sugar (analytically pure) and protein A colloidal gold (SPA) were purchased from Sigma Company (Santa Clara, CA, USA). FBS and DMEM culture media were purchased from GIBCo Company (Carlsbad, CA, USA). Western blotting reagents used in this study included rabbit anti-human heat shock protein 70 (HSP70) polyclonal antibody from Abcam (Cambridge, UK), mouse anti-human HLA-I monoclonal antibody from Chemicon (Los Angeles, CA, USA), mouse anti-human NY-ESO-1 monoclonal antibody from ZyMed (San Diego Diego, South CA, USA). Western blotting kit was obtained from Pierce (Rockford, IL, USA), and BCA protein assay kit was from Puli Lai Gene Technology Co., Ltd (Beijing, China).
Instruments used in this study included a Himac-CP70G low-temperature ultra-high speed centrifuge and a Hitachi TEM H-7500 transmission electron microscope (Hitachi Corporation, Tokyo, Japan). Electrophoresis devices used in this study included an electrophoresis tank and a trans-membrane tank (Beijing 61 Instrument Factory, China), a GelDoc2000 gel imager (Bio-Rad Corporation, Chicago, IL, USA), a 100 ku MWCO Centriplus centrifugal ultrafiltration tube and a 100 ku MWCO Millipore Amicon high recovery-high-flow tangential flow ultrafiltration centrifuge tube (Millipore Corporation, Bedford, MA, USA).
Human hepatoma cell lines, HepG2 and Hep3B, were maintained at 37°C in 10% DMEM containing 10% FCS (Gibco Corporation, Carlsbad, CA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma Corporation, Santa Clara, CA, USA). HepG2 and Hep3B cells were divided into 3 control groups and 3 experimental groups, respectively, for regular culture. Cell viability was 95% as determined by trypan blue exclusion. Twenty-four hours after inoculation, cells in experimental groups were treated with 5-aza-CdR at a concentration of 1 × 10-6 mol/L and 150 mL of culture supernatant was collected 72 h later from each group, while cells in control groups were cultured without any drug and 150 mL of culture supernatant was collected from each group as controls.
Exosomes were isolated as previously described[18]. In brief, 150 mL of a medium from confluent cultures (5-7 d) was harvested and centrifuged twice (2000 g and 10 000 g) to remove cells and debris. Clarified supernatant was then ultrafiltrated using 100 ku MWCO Centriplus centrifugal ultrafiltration tubes to remove big-molecule compounds and reduce the volume of samples before ultracentrifugation on a 30% sucrose/D2O cushion, which was collected and diluted in PBS. Ultrafiltration was then performed using a 100 ku MWCO Millipore Amicon high recovery- high-flow tangential flow ultrafiltration centrifuge tube. Finally, 5 mL of exosomes was obtained and stored at 4°C for no more than 48 h before use.
Total RNA was extracted from HepG2 and Hep3B cells using a Promega’s total RNA extraction kit 72 h before and after 5-Aza-Cdr treatment. The sequences of p53 upstream and downstream primers used in this study are 5'-ACCCAGGTCCAGATGAAG-3' and 5'-CACTCGGATAAGATGCTGA-3', respectively. The length of amplified fragments was 422 bp. The sequences of β-actin upstream and downstream primers used in this study are 5'-CGGGAAATCGTGCGTGACATT-3' and 5'-GGAGTTGAAGGTAGTTTCGTGG-3', respectively. The length of amplified fragments was 150 bp. RT reaction system contained 2 μg total RNA, 150.5 μg oligo (dT), 5 × PCR buffer, 1 mmol/L dNTP, 20U RNasin, 200 U M-MLV reverse transcriptase. PCR system contained 2 μL reverse transcriptase reaction products, 500 pmol/L of each upstream and downstream primer, 200 μmol/L of each dNTP, 1 U Taq DNA synthesis enzyme, and 10 × PCR buffer. The total reaction volume was 20 μL. Thirty cycles of PCR amplification were performed under the following conditions: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 5 min. Finally, 6 uL of PCR products was electrophoresed on 1.5% agarose gel. The results were recorded using a gel imaging system for semi-quantitative analysis by grey ratio of target gene to β-actin. Reverse transcription polymerase chain reaction (RT-PCR) reagents were purchased from Promega Company (San Luis Obispo, CA, USA) and primers were synthesized in Beijing Bioko Biotech Co., Ltd (Beijing, China).
An antigen-antibody complex was prepared. In brief, 10 μL of exosomes was obtained and mixed with an equal volume of rabbit anti-human HSP70 monoclonal antibody (1:50 dilution), mouse anti-human HLA-I monoclonal antibody (1:100 dilution) or mouse anti-human NY-ESO-1 monoclonal antibody (1:50 dilution), respectively. These samples were dropped onto a copper mesh surface and incubated for 1 h at room temperature. Twenty μL of SPA diluted at 1:15 was dropped onto the hydrophobic membrane to form liquid beads. The copper mesh was floated in SPA droplets with its membrane surface faced down at room temperature for 30 min. Then, 15 μL of uranyl acetate drops was put onto the copper mesh surface and stained at room temperature for 30 s. A SPA-coated copper mesh was taken as a control. Exosomes with black colloidal gold particles on the capsular membrane and cavity were marked as positive under transmission electron microscope.
Exosomes and their labeled colloidal gold particles were counted under each camera view of exosome samples. Each protein was represented by the number of colloidal gold particles in 100 exosomes. The data were expressed as mean ± SD for further statistical analysis.
The area under each photographic view was 1000 nm × 700 nm. The number of exosomes under each field of vision was counted. The average number of exosomes under each camera view was calculated in 10 randomly selected horizons. The number of exosomes in each mL supernatant of the cells was figured out by copper mesh diameter (2.5 mm) and area (1.25 mm × 1.25 mm × 3.14). The cells in each of experimental and control groups were counted three times and averaged for statistical analysis.
Forty microgramme of exosomes was taken and 10% SDS-PAGE electrophoresis was performed at a constant electric power. After electrophoresis, the gels were transferred to NC membrane, incubated with HSP70, HLA-I and NY-ESO-1 antibodies diluted at 1:500 overnight at 4°C. Then, horseradish peroxidase-tagged antibody was added and incubated at room temperature for 2 h. Photography was performed with a Kodak X-OMAT film (Eastman Kodak, Rochester, NY). The light absorption value A of target bands and β-actin was determined using the imaging analysis system. The results were indicated by the absorption ratio of target bands and β-actin, and averaged from three independent experiments.
Exosomes in two cell lines of experimental and control groups were taken to determine the protein concentration with a bicinchoninic acid (BCA) protein assay kit following its manufacturer’s instructions.
The data were expressed as mean ± SD. Statistical analysis was performed using the SPSS13.0 statistical software. P <0.05 was considered statistically significant.
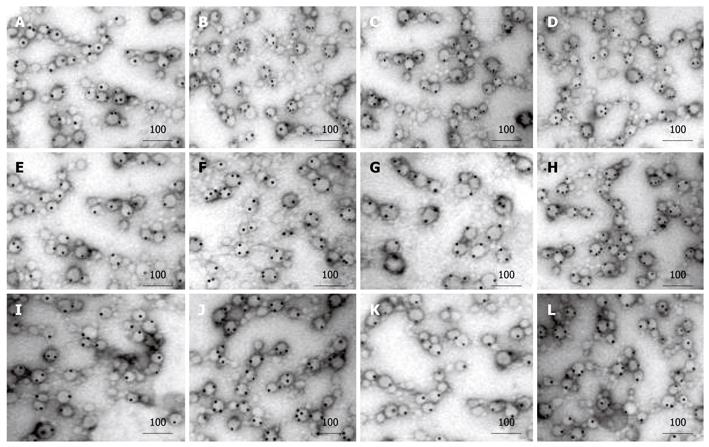
Immunoelectron microscopy showed that exosomes, secreted from HepG2 and Hep3B cells, had the membranous structure of microcapsules, 30-80 nm in diameter. They were round or oval and their cavities were full of components with a low electron density. After colloidal gold immunoelectron marking, dotted, granular colloidal gold markers could be observed in the capsular membranes and cavities. Each kind of immune molecules had no more than two gold-labeled particles in the control groups. However, after treatment with 5-Aza-CdR, 5, 4, and 3 gold-labeled HSP70, HLA-I and NY-ESO-1 molecules were observed in exosomes, respectively. The number of gold-labeled particles in each immune molecule was significantly greater in experiment groups than in control groups (P < 0.05, Figure 1, Table 1).
| n | HepG2 | P | Hep3B | P | |||
| Control group | Experiment group | Control group | Experiment group | ||||
| HSP70 | 3 | 74.2 ± 3.1 | 99.8 ± 5.2 | < 0.01 | 70.2 ± 5.1 | 99.9 ± 3.8 | < 0.01 |
| HLA-I | 3 | 53.3 ± 2.5 | 79.6 ± 6.1 | < 0.01 | 40.6 ± 2.7 | 86.7 ± 3.6 | < 0.01 |
| NY-ESO-1 | 3 | 38.2 ± 4.2 | 58.2 ± 4.3 | < 0.01 | 32.1 ± 4.6 | 70.3 ± 2.9 | < 0.01 |
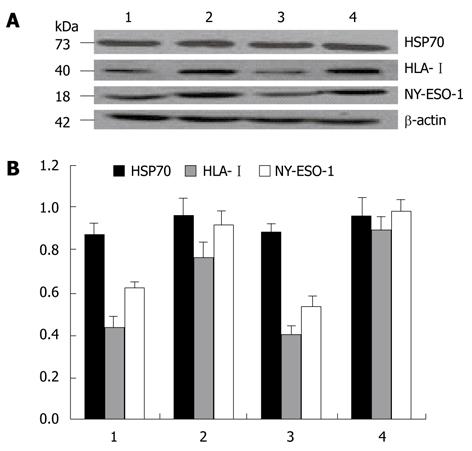
In the supernatant of exosomes from two cell lines, a clear specific protein band could be detected by Western blotting at the molecular weights of 73, 40 and 18 kDa. After treatment with 5-Aza-CdR, the HLA-I and NY-ESO-1 expression level was significantly higher in experimental groups than in control groups (P < 0.05), while the HSP70 expression did not increase significantly (Figure 2).
The number of exosomes secreted from both cell lines and the total protein in exosomes were significantly higher after treatment with 5-Aza-CdR than before treatment with 5-Aza-CdR (P < 0.05, Table 2).
| Subject | n | HepG2 con | HepG2 exp | P | Hep3B con | Hep3B exp | P |
| Exo No | 3 | 3.56 × 108± 2.6 × 102 | 4.53 × 109± 3.7 × 102 | 0.010 | 3.12 × 108± 6.2 × 102 | 4.13 × 109± 3.7 × 102 | 0.021 |
| Exo Pro | 3 | 0.92 ± 0.13 mg | 1.31 ± 0.16 mg | 0.012 | 0.89 ± 0.10 mg | 1.52 ± 0.16 mg | 0.011 |
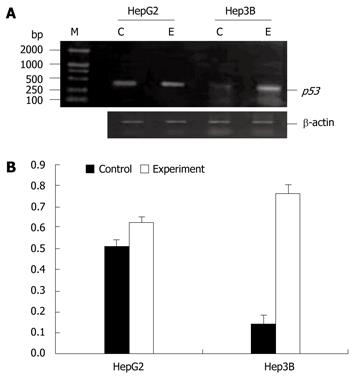
The p53 gene was moderately expressed in HepG2 cells but not in Hep3B cells before 5-Aza-CdR treatment. However, the p53 gene mRNA was significantly expressed in two cell lines after 5-Aza-CdR treatment (Figure 3).
5-Aza-CdR can recover some genes with CpG islands in their promoter regions, or significantly increase the expression of these genes by directly inhibiting the DNA methyltransferase activity[19]. It has been shown that DNA methylation can regulate cancer/testis antigen (CTA), MHC I, MHC II, and a variety of immune adhesion molecules[20,21]. 5-Aza-CdR can significantly increase their expression in tumor cells and improve anti-tumor immune response capacity [17].
Exosomes, originating as vesicles in some late endosomes, would release when these mature endosomes or multivesicular bodies (MVBs) are fused with cell membranes. Exosomes carry a variety of specific proteins, such as antigen-presenting-associated protein, T cell activation-associated protein, and some tumor antigens and antigen chaperones[7]. Exosomes themselves offer an antigen delivery system and can transfer tumor antigens to antigen-presenting cells, causing anti-tumor responses[22].
For these reasons, use of exosomes in immunotherapy and research requires high-quality exosomes. However, the mechanism underlying exosome secretion has not been well elucidated. Yu et al[11] found that activation of wild-type p53 increases exosome production by upregulating a transmembrane protein, TSAP6, which selectively transports proteins to exosomes and adjusts exosome formation. 5-Aza-CdR can increase p53 gene expression by damaging or methylating DNA[12,14]. In addition, it has been reported that 5-Aza-CdR increases the expression of CTA, HLA-I, and HLA-II through demethylation, thus improving anti-tumor specific immune response and decreasing the size of transplanted tumors in mice[15,16]. Therefore, this study investigated whether 5-Aza-CdR can increase the number of exosomes and the immune molecule level in exosomes secreted by hepatoma cells through p53 gene activation and DNA demethylation pathways.
Immunoelectron microscopy and Western blotting in this study showed that exosomes derived from HepG2 and Hep3B cells were rich in HSP70 protein both before and after 5-Aza-CdR treatment. However, after 5-Aza-CdR treatment, electron microscopy showed that the tagged HSP70 proteins in exosomes were increased, while Western blotting showed that they did not, possibly due to the fact that HSP70 gene expression is not regulated by DNA methylation. Moreover, HLA-I and NY-ESO-1 molecules were clearly regulated by DNA methylation, the expression of these molecules especially that of HLA-I in Hep3B cells, was relatively low in control groups. After 5-Aza-CdR treatment, these molecules were significantly increased in both cell lines. The number of exosomes secreted by treated and untreated hepatoma cells was calculated and changes of protein in exosome stock solutions treated and untreated with BCA were observed under electron microscope, showing that after 5-Aza-CdR treatment, the number of exosomes secreted by hepatoma cells and the protein in exosomes are significantly increased (P < 0.05). Furthermore, immune electron microscopy and Western blotting revealed that the number of exosomes was increased by almost an order of magnitude. Although there was a difference in p53 mRNA expression between the two hepatoma cell lines before treatment with 5-Aza-CdR, the p53 expression was significantly increased in both cell lines, especially in Hep3B cell line after 5-Aza-CdR treatment,. The number of exosomes and the expression of HSP70 protein were slightly higher in Hep3B cells than in HepG2 cells. However, the expression of HLA-I and NY-ESO-1 molecules was significantly higher in Hep3B cells than in HepG2 cells, indicating that exosomes secreted by hepatoma cells are regulated by the p53 gene, while HLA-I and NY-ESO-1 proteins in exosomes are mainly regulated by DNA methylation. As a “molecular chaperone”, HSP70 can actively deliver antigens through receptors on antigen-presenting cell membranes, thus increasing the efficiency of antigen presentation by 104-folds of pure pinocytosis or phagocytosis. Tumor-specific antigens are released from HSP within cells and presented to cytotoxic T cells, which are activated to execute immune responses to different tumors[23]. NY-ESO-1, belonging to the CTA family, is expressed in a variety of tumor tissues. Anti-NY-ESO-1 specific antibodies and sensitized lymphocytes can be found in serum of hepatoma patients[24,25]. Thus, NY-ESO-1 is currently thought to be a tumor-specific antigen with the strongest antigen immunogenicity[26]. However, NY-ESO-1 re-expression in tumor cells is not homogeneous and its low expression level is insufficient to stimulate cell-mediated immunity. To enhance tumor-specific immunity, sufficient tumor-specific antigens or immune-associated molecules are needed.
In conclusion, hepatoma cells secrete more exosomes after 5-Aza-CdR treatment. Moreover, the expression of tumor-specific antigen, NY-ESO-1, and tumor-specific immune stimulating molecule, HLA-I, increase significantly. While 5-Aza-CdR up-regulates p53 gene expression, it may play a more important role in DNA demethylation. Exosomes secreted from hepatoma cells after 5-Aza-CdR treatment are more able to stimulate anti-tumor-specific immune response, which may be useful for the preparation of a new cancer therapeutic vaccine for hepatoma.
Human hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. However, no effective curative therapy for it is currently available. It is therefore necessary to develop a novel strategy against HCC. Tumor-derived exosomes, containing tumor-associated antigens and chaperones, are membrane nanoparticles, which make them stable, easy to maintain and susceptible to uptake by immune cells. However, cancer/testis antigens (CTAs) with a strong immunity and immune-associated molecules with adjuvant anti-cancer effects are heterogeneous and insufficient in cancer cells to emit specific immune reactions. Therefore, getting enough quantity and quality of exosomes for a sufficient immune effect remains a great challenge for tumor immunotherapy. 5-Aza-CdR, an inhibitor of DNA methyltransferase, can restore or increase CTAs and HLA-I/II expression in cancer cells by demethylation and induce p53 gene expression by damaging DNA, while p53 can increase exosome production. The authors hope to get more exosomes with a large number of CTAs and immune-associated molecules by modifying hepatoma cells with 5-Aza-CdR.
Exosomes secreted by tumor or dendritic cells are a recent hot spot in tumor immunology research. However, insufficient tumor-associated antigens and tumor-derived immune stimulated molecules are still a problem in such a research. Various methods, such as IL-2 gene transfection and curcumin- modified exosomes, have been used to improve exosome immune stimulation. In this study, hepatoma was treated with 5-Aza-CdR, and more powerful exosomes were produced.
5-Aza-CdR, a DNA methyltransferase inhibitor and a demethylation promoter of CpG in many genes including the p53 gene, can significantly restore or increase their expression. It has been shown that 5-Aza-CdR can significantly increase the expression of molecules necessary for anti-tumor cellular immunity such as HLA-I and HLA-II, and the therapeutic effect of anti-tumor immunity in vitro and in animal experiments. However, no report is available on the effect of 5-Aza-CdR on the number of exosomes in cancer cells. This study showed that 5-Aza-CdR could increase exosomes in hepatoma cells and immune-related molecules of exosomes. Although its mechanism remains unclear, it may be related to demethylation and the p53-induction effect of 5-Aza-CdR. These findings suggest that 5-Aza-CdR can modify exosomes, thus offering an interesting possibility for developing a cancer vaccine.
This study has confirmed that 5-Aza-CdR-modified exosomes from hepatoma cells contain more CTAs, chaperones and immune-stimulating molecules, which may be used in preparation of a new cancer therapeutic vaccine in vitro for hepatoma.
Exosomes, secreted by many kinds of cells including blood and tumor cells, have a membrane structure, 30-100 nm in size, consisting of proteins and RNA, and function as a vehicle for messages from cell to cell.
The authors studied the effect of 5-Aza-CdR on the number of exosomes and immune-associated proteins in them produced by hepatoma cell lines, HepG2 and Hep3B, showing that exosomes function as a vehicle for messages from cell to cell, which may be used in preparation of a new cancer therapeutic vaccine for hepatoma.
Peer reviewer: Jing-Yuan Fang, MD, PhD, Professor, Shanghai Institute of Digestive Disease, Shanghai Jiaotong University School of Medicine, Renji Hospital, Shanghai 200001, China
S- Editor Wang YR L- Editor Wang XL E- Editor Ma WH
| 1. | Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15:3210-3216. |
| 2. | Mendizabal M, Reddy KR. Current management of hepatocellular carcinoma. Med Clin North Am. 2009;93:885-900, viii. |
| 3. | Wörns MA, Weinmann A, Schuchmann M, Galle PR. Systemic therapies in hepatocellular carcinoma. Dig Dis. 2009;27:175-188. |
| 4. | Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009;27:80-92. |
| 5. | Verslype C, Van Cutsem E, Dicato M, Arber N, Berlin JD, Cunningham D, De Gramont A, Diaz-Rubio E, Ducreux M, Gruenberger T. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann Oncol. 2009;20 Suppl 7:vii1-vii6. |
| 6. | Hao S, Moyana T, Xiang J. Review: cancer immunotherapy by exosome-based vaccines. Cancer Biother Radiopharm. 2007;22:692-703. |
| 7. | Mignot G, Roux S, Thery C, Ségura E, Zitvogel L. Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med. 2006;10:376-388. |
| 8. | Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005;11:7554-7563. |
| 9. | Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, Slattery K, Qureshi M, Wei Y, Deng Y. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumor immunity than exosomes released from heat-shocked tumor cells expressing cytoplasmic HSP70. J Cell Mol Med. 2009;Epub ahead of print. |
| 10. | Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE, Kimberly RP, Barnes S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta. 2007;1773:1116-1123. |
| 11. | Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795-4801. |
| 12. | Hurt EM, Thomas SB, Peng B, Farrar WL. Reversal of p53 epigenetic silencing in multiple myeloma permits apoptosis by a p53 activator. Cancer Biol Ther. 2006;5:1154-1160. |
| 13. | Liu LH, Xiao WH, Liu WW. Effect of 5-Aza-2’-deoxycytidine on the P16 tumor suppressor gene in hepatocellular carcinoma cell line HepG2. World J Gastroenterol. 2001;7:131-135. |
| 14. | Karpf AR, Moore BC, Ririe TO, Jones DA. Activation of the p53 DNA damage response pathway after inhibition of DNA methyltransferase by 5-aza-2’-deoxycytidine. Mol Pharmacol. 2001;59:751-757. |
| 15. | Karpf AR. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics. 2006;1:116-120. |
| 16. | Setiadi AF, David MD, Seipp RP, Hartikainen JA, Gopaul R, Jefferies WA. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol Cell Biol. 2007;27:7886-7894. |
| 17. | Natsume A, Wakabayashi T, Tsujimura K, Shimato S, Ito M, Kuzushima K, Kondo Y, Sekido Y, Kawatsura H, Narita Y. The DNA demethylating agent 5-aza-2’-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int J Cancer. 2008;122:2542-2553. |
| 18. | Bu N, Li QL, Feng Q, Sun BZ. Immune protection effect of exosomes against attack of L1210 tumor cells. Leuk Lymphoma. 2006;47:913-918. |
| 19. | Fandy TE. Development of DNA methyltransferase inhibitors for the treatment of neoplastic diseases. Curr Med Chem. 2009;16:2075-2085. |
| 20. | Manning J, Indrova M, Lubyova B, Pribylova H, Bieblova J, Hejnar J, Simova J, Jandlova T, Bubenik J, Reinis M. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology. 2008;123:218-227. |
| 21. | Setiadi AF, David MD, Seipp RP, Hartikainen JA, Gopaul R, Jefferies WA. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol Cell Biol. 2007;27:7886-7894. |
| 22. | Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297-303. |
| 23. | Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238-5247. |
| 24. | Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, Qin LL, Fei R, Mei MH, Leng XS. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clin Cancer Res. 2004;10:6946-6955. |
| 25. | Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332-4341. |
| 26. | Nakamura S, Nouso K, Noguchi Y, Higashi T, Ono T, Jungbluth A, Chen YT, Old LJ, Nakayama E, Shiratori Y. Expression and immunogenicity of NY-ESO-1 in hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:1281-1285. |