Published online May 14, 2010. doi: 10.3748/wjg.v16.i18.2278
Revised: February 23, 2010
Accepted: March 1, 2010
Published online: May 14, 2010
AIM: To establish the real time fluorescence polymerase chain reaction (RT-PCR) with dual labeled probes for fast detection of SLC25A13 gene mutation 851del4.
METHODS: Four hundred infants (< 1 year of age) with unexplained intrahepatic cholestasis from 18 provinces or municipalities in China were enrolled in this study for detecting their SLC25A13 gene mutation 851del4. Suitable primers and fluorescence-labeled probes for detecting SLC25A13 gene mutation 841del4 were designed. Normal and mutant sequences were detected by PCR with two fluorescence-labeled probes. After a single RT-PCR, results were obtained by analyzing the take-off curves. Twenty-four positive and 14 negative samples were retested by direct sequencing.
RESULTS: Eight homozygous and 30 heterozygous mutations were detected in 46 mutant alleles with a 851del4 mutation rate of 5.8% (46/800). Twenty-six and 20 mutant alleles were observed respectively, in 474 and 242 alleles from the intermediate and southern areas of China. No mutant allele was detected in 84 alleles from northern China. Twenty-four positive samples including 4 homozygous and 20 heterozygous mutations, and 14 negative samples were retested by direct sequencing, which confirmed that the accuracy of RT-PCR was 100%.
CONCLUSION: RT-PCR can detect the mutation 851del4 in infants with intrahepatic cholestasis with an accuracy of 100%.
-
Citation: Fu HY, Zhang SR, Yu H, Wang XH, Zhu QR, Wang JS. Most common
SLC25A13 mutation in 400 Chinese infants with intrahepatic cholestasis. World J Gastroenterol 2010; 16(18): 2278-2282 - URL: https://www.wjgnet.com/1007-9327/full/v16/i18/2278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i18.2278
The SLC25A13 gene, localized in chromosome 7q21.3, encodes a 3.4kb transcript that is expressed most abundantly in liver[1,2]. SLC25A13 gene mutations can lead to citrin deficiency, which causes not only onset of type II citrullinemia (CTLN2) in adults[2], but also neonatal intrahepatic cholestasis (NICCD)[3,4]. Clinical manifestations of NICCD include intrahepatic cholestasis, fatty liver, failure to thrive, hyperaminoacidemia, liver dysfunction, hypoglycaemia, hypoproteinemia, and coagulation disorders. Symptoms of most NICCD patients disappear by 12 mo of age. Specific treatment, except for nutritional program including supplementation of fat soluble vitamins and formulas containing medium-chain triglycerides[3,5-7], is usually not required. However, NICCD is not always benign and a few NICCD patients may have a less favorable clinical course with relatively early liver dysfunction that necessitates management with liver transplantation under the age of 1 year[8]. Some infants with homozygous mutations may suffer from CTLN2 one or more decades later[9,10].
Since the full genomic sequence of the SLC25A13 gene was published in 1999, over 50 mutations have been identified[11]. It has been reported that more than 100 000 East Asians have two homozygous mutated SLC25A13 alleles, and the carrier rate in Chinese population is 1/63[11,12]. Mutation 851del4, is a 4-bp (GTAT) deletion from nt 851-854 in exon 9, leading to premature truncation of a protein. Population analysis has shown that mutation 851del4 accounts for 70% of all detected SLC25A13 gene mutations in general Chinese population[13]. However, its prevalence in infants with intrahepatic cholestasis is unclear.
Traditionally, mutation 851del4 is tested by direct agarose gel electrophoresis or GeneScan[2,12-14]. Since the differences in length between the mutant fragment and wild sequence are quiet small, direct agarose gel electrophoresis depends greatly on experience of the investigator, while GeneScan requiring expensive equipments is time consuming. In this study, real-time fluorescence polymerase chain reaction (RT-PCR) with dual labeled probes was used to detect the most common SLC25A13 gene mutation 851del4 in 400 Chinese infants with intrahepatic cholestasis.
A gene test for mutation 851del4 in 14 infants with intrahepatic cholestasis was carried out by direct sequencing. Four cases were detected to be with mutant alleles, including one homozygote and three heterozygotes. These patients were used as samples to develop the method of RT-PCR. The other ten cases with wild sequence served as the control.
Then, RT-PCR was performed to detect the prevalence of 851del4 mutation in 386 infants with unexplained intrahepatic cholestasis. If a heterozygous mutation 851del4 was found in an infant, the second mutation was identified in all the 18 exons and their flanking sequences by direct sequencing.
The accuracy of RT-PCR was retested by direct sequencing in 24 infants with mutant sequences and 14 infants with normal sequence.
From June 2003 to May 2009, 400 infants with idiopathic intrahepatic cholestasis were enrolled in this study for testing their genes. The inclusion criteria were conjugated hyperbilirubinaemia with its serum total bilirubin (TBil) exceeding 5 mg/dL and conjugated bilirubin level > 20% of TBil or > 1 mg/dL if the total serum bilirubin < 5 mg/dL, and development of conjugated hyperbilirubinaemia under the age of 1 year. Those were excluded from the study if they had diseases affecting their extrahepatic biliary system (such as biliary atresia, choledochal cyst or tumor, inspissated bile, or haemangioma), low γ-glutamyl transpeptidase (GGT) level (no more than 50 U/L) that may indicate progressive familial intrahepatic cholestasis[15], and obvious extrahepatic abnormalities or positive serology other than cytomegalovirus (CMV) that may also indicate infection. CMV infection occurs frequently in infants with neonatal hepatitis in Mainland China[16,17], and the outcome is similar in those infected with or without CMV[18,19].
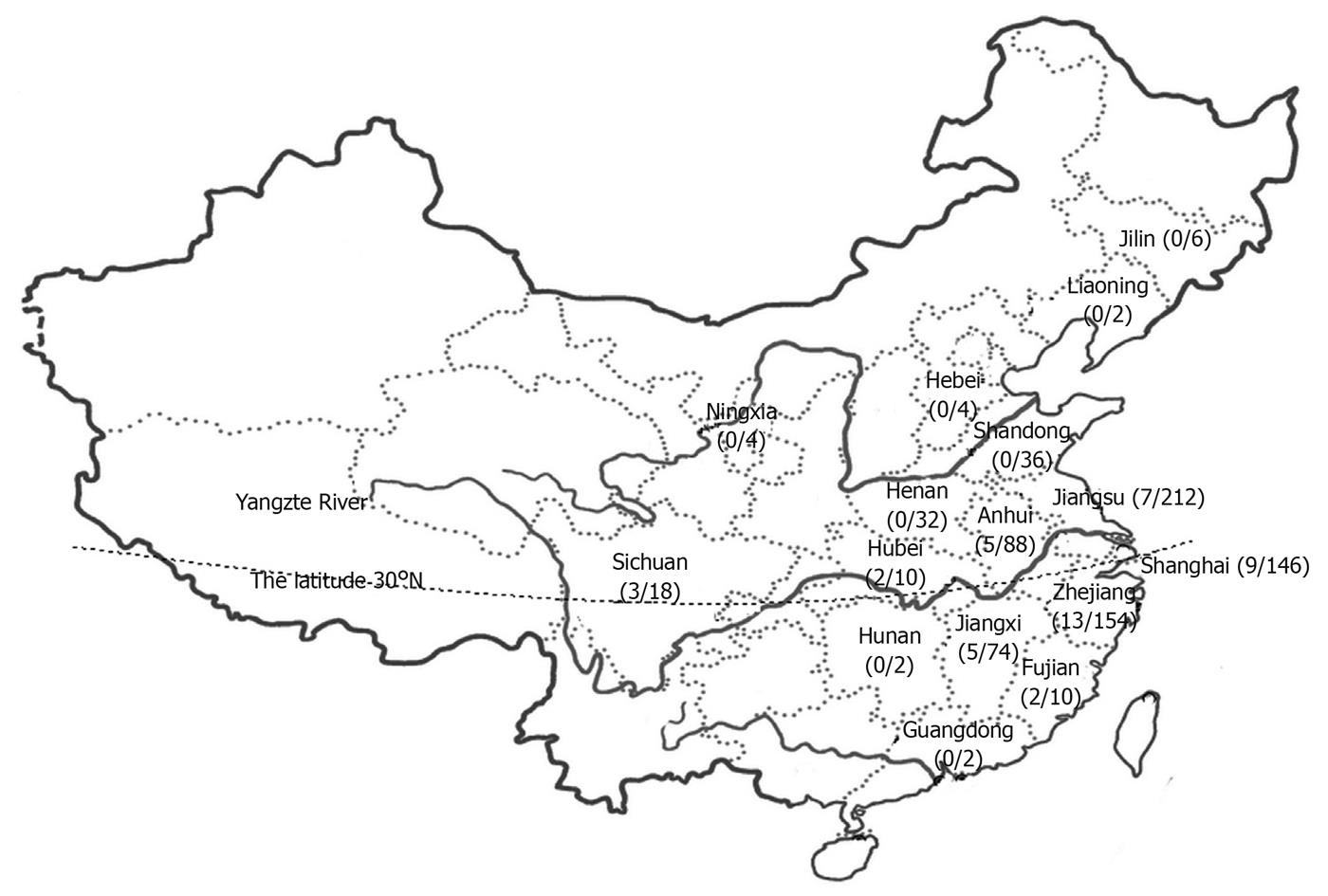
The infants included in this study came from 18 provinces or municipalities in China. Because the most likely boundary between northern and southern China was drawn at an altitude of 30°N, the Yangtze River was considered a historically significant border between northern and southern China as previously described[13,20]. According to such criteria, 237 infants came from a border area (Shanghai City, and Jiangsu, Anhui, Hubei, Sichuan Provinces), 121 from the southern area (Hunan, Jiangxi, Zhejiang, Fujian and Guangdong Provinces), and 42 from the northern area (Jilin, Liaoning, Hebei, Shandong, Ningxia and Henan Provinces) in our study (Figure 1).
This study was approved by the Ethics Committee on Human Research of the Children’s Hospital of Fudan University. Informed consent was obtained from the parents or the guardians of each participant.
The sequence of upstream primer and downstream primer is 5'-TTGGTATATTTGTTGCTTGTGTTT-3' and 5'-AGAGGGAACTCTGCCCTTTAA-3', respectively. Their normal and mutated sequences were detected with the probe FAM-CCTACAGACGTATGACCTTAGCA-TAMRA and the probe HEX-CCTACAGACGACCTTAGCAGA-TAMRA, respectively. Twenty-five microlitre reaction mixture used in this study contained 2 μL of genomic DNA, 10 μL of 2.5* real Master Mix [Tiangen Biotech (Beijing) Co. Ltd.], 1.25 μL of 20*probe enhancer, 0.25 μL of each FAM- and HEX-labeled probe, 0.5 μL of each upstream primer and downstream primer (diluted to 10 μmol/L), and 10.25 μL of ddH2O. After overlaying 25 μL liquid, RT-PCR was performed under the following conditions: denaturation at 95°C for 2 min, followed by 40 thermal cycles, each at 95°C for 15 s, at 56°C for 30 s, and at 68°C for 40 s. The results were obtained by analyzing the curves.
To ensure the specificity and sensitivity of amplification, nested PCR was performed to amplify exons 8 and 9. Thirty cycles of PCR were performed in 30 μL reaction mixture at 94°C for 3 min followed by 25 cycles, each at 94°C for 30 s, at 50°C for 30 s, at 72°C for 1 min, and a final extension at 72°C for 4 min, using primers Ex8/9F(5'-GCCTGTAATCCCAGCACTT-3') and Ex8/9R(5'-GTTGAGAGATGTAGCGAAAGAA-3'). The outer PCR reaction mixture consisted of 2*Taq- plus 15 μL PCR Master MIX, 1 μL in each primer (10 μmol/L), 2 μL DNA template, and 11 μL ddH2O. After the initial outer PCR, inner PCR was conducted at 94°C for 3 min followed by 30 cycles, each 94°C for 30 s, at 50°C for 30 s, at 72°C for 1 min, and a final extension at 72°C for 4 min using 1 μL of primers IVS7F (5'-TCACTCATTCCAGTGCCTTG-3') and IVS9B (5'-GCAGTTGCCTTTGCGGCATTG-3') as previously described[2], 2 μL outer PCR product as template, 2*Taq- plus 15 μL PCR Master MIX, and 11 μL ddH2O.
The PCR products were analyzed by direct sequencing, in which IVS7B and IVS9B were taken as sequence primers for forward and backward sequencing identification. Mutation 851del4 was identified using software (Bioedit, North Carolina State University, NC, USA) and checked by two of the investigators.
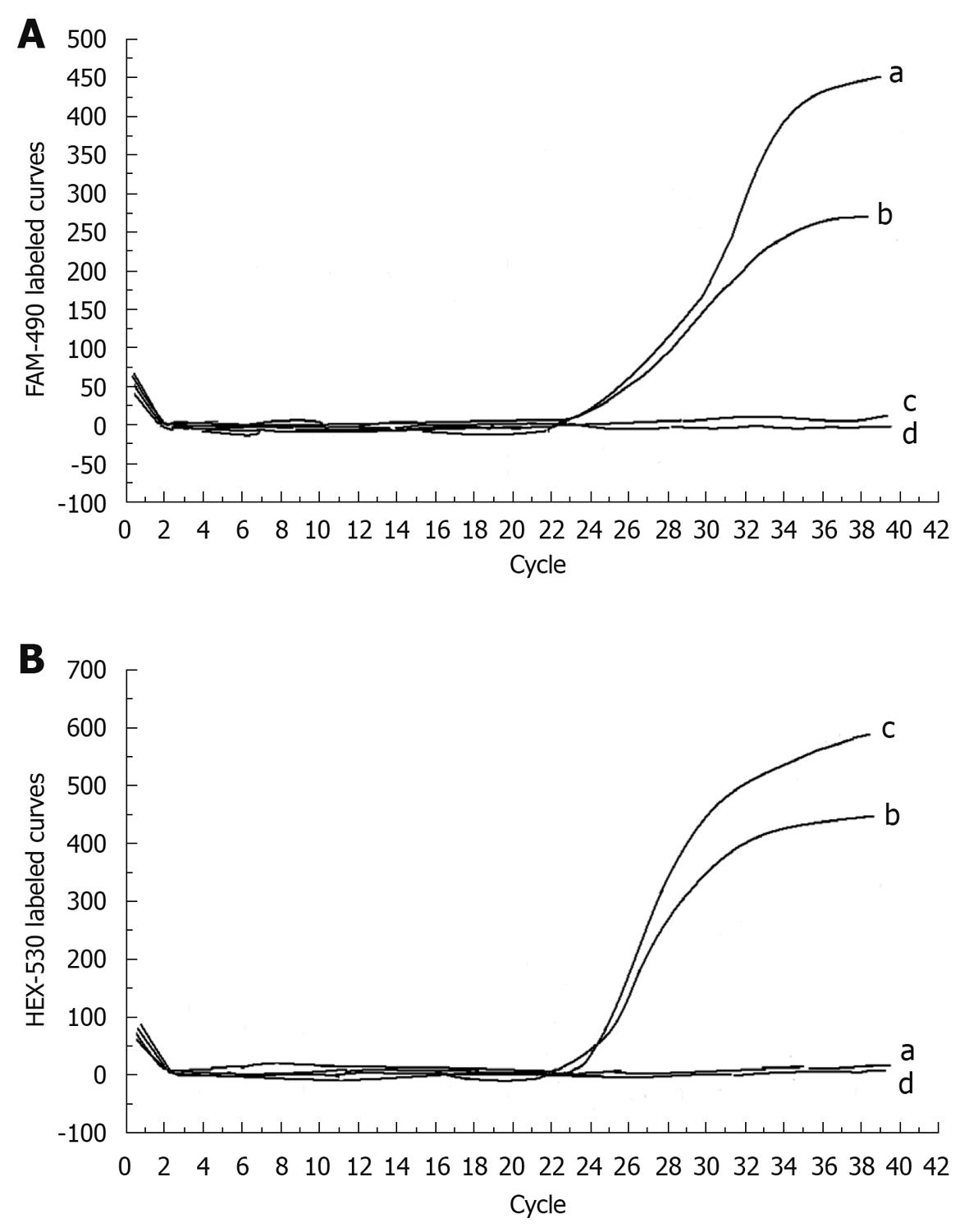
The curves were analyzed in accordance with the direct sequencing in 14 infants with known sequences. In infants with homozygous 851del4 mutation, positive fluorescence was observed only on the HEX-530 curve but not on FAM-490 curve. In those with heterozygous 851del4 mutation, positive fluorescence was found on both FAM-490 and HEX-530 curves, which was very close to each other, with the difference in Ct value always less than 2 (Figure 2). In those with the wild sequence, positive fluorescence was detected only on the FAM-490 curve. For any given sample, the experiment was considered a failure if negative fluorescence appeared on both HEX-530 and FAM-490 curves.
Positive mutation 851del4 was detected in 24 samples including 4 homozygous and 20 heterozygous mutations, and negative mutation 851del4 was retested in 14 infants by nested PCR and direct sequencing, which was consistent with that detected by RT-PCR.
Eight homozygous and 30 heterozygous mutations were detected in the 400 infants with intrahepatic cholestasis, with a 851del4 mutation rate of 5.8% (46 mutant alleles with 851del4 were detected in 800 tested alleles). Subsequent testing of 18 exons and their flanking sequences in 30 heterozygous mutations showed another 9 mutant alleles including 1638ins23 in 5, R184X in 1 and novel mutations (data will be reported separately, manuscript in preparation) in 3, respectively.
Twenty-six and 20 mutant alleles were detected, respectively in 474 and 242 alleles of the intermediate and southern areas,with a 851del4 mutation rate of 5.5% and 8.3%, respectively. No mutant allele was detected in alleles of northern China (Figure 1).
To our knowledge, this is the first study to detect the most common mutation 851del4 of the SLC25A13 gene in Chinese infants with intrahepatic cholestasis in a relatively large sample size. In this study, we developed a simple, fast, and accurate RT-PCR for detecting the mutation 851del4, the most common mutation of the SLC25A13 gene, and detected 46 mutant alleles from 800 alleles with it. The prevalence of mutation 851del4 in Chinese infants with intrahepatic cholestasis was 1/17, which is much higher than that in general Chinese population (1/93)[13].
Compared with conventional nested PCR and agarose gel electrophoresis or GeneScan, RT-PCR could detect the mutation alleles and identify patients with homozygous or heterozygous 851del4 mutation, indicating that it can be used in diagnosis of intrahepatic cholestasis in infants.
In the present study, the mutation rate of 851del4 was about 5.8%, which was significantly higher than that in the general population, indicating that the SLC25A13 gene mutation plays an important role in the pathogenesis of intrahepatic cholestasis of Chinese infants and should be detected in such infants, especially in those with symptoms of NICCD.
Screening of mutation 851del4 by RT-PCR may contribute to the diagnosis of intrahepatic cholestasis in patients with homozygous mutation because testing 18 exons of the SLC25A13 gene can be omitted and diagnosis of citrin deficiency can be made. Early diagnosis and intervention of NICCD are extremely important[20] for the prevention of its serious consequences.
In this study, 30 heterozygous mutations of 851del4 were detected in 400 intrahepatic cholestasis infants with 9 compound heterozygous mutations detected by examining 18 exons of the SLC25A13 gene. It is noteworthy that if suspicious patients were found to be heterozygous or when no mutation was found, all the 18 exons and their flanking sequence of the SLC25A13 gene should be examined.
The SLC25A13 gene mutation 851del4 has geographical variations in China and can be frequently found in southern China (1/70) but rarely in northern China (1/940)[13]. In this study, mutant alleles were detected in most infants from the intermediate and southern areas but not in those from northern China, showing that the distribution of the SLC25A13 gene mutation 851del4 is different in south and north areas of China. Our finding is consistent with the reported data[13]. Thus, different methods should be used in dignosis of intrahepatic cholestasis in infants according to their geographical origin.
RT-PCR was established and the SLC25A13 gene mutation 851del4 was detected in 400 infants using it in this study. However, it was used in detecting only one specific SLC25A13 gene mutation, its prevalence might be underestimated. Further study is needed to highlight the importance of the SLC25A13 gene mutations in infants with intrahepatic cholestasis and the application of RT-PCR in clinical practice.
In conclusion, RT-PCR can detect the most common SLC25A13 gene mutation 851del4 in patients with intrahepatic cholestasis, thus providing a useful and effective tool for the diagnosis of NICCD.
SLC25A13 gene mutations lead to citrin deficiency, which causes onset of type II citrullinemia (CTLN2) in adults and neonatal intrahepatic cholestasis (NICCD). Up to date, over 50 mutations of SLC25A13 have been identified, in which 851del4 is the most common mutation type. Traditionally, mutation 851del4 is screened by direct agarose gel electrophoresis or GeneScan. Since these methods are highly dependent on personal experience or need special equipments, a simple and accurate method for screening the mutation 851del4 is needed.
Population analysis has shown that mutation 851del4 accounts for 70% of all mutations of the SLC25A13 gene in general Chinese population. Mutation 851del4 is a 4-bp (GTAT) deletion from nt 851-854 in exon 9, leading to premature truncation of a protein. Early diagnosis of citrin deficiency is extremely important.
Real-time fluorescent polymerase chain reaction (RT-PCR) with dual-labeled probes was established, which could detect the mutation 851del4. The homozygosity, heterozygosity and wild sequence can be differentiated clearly. Compared with conventional nested PCR and agarose gel electrophoresis or GeneScan, RT-PCR does not require expertise in experimental operation.
The prevalence of mutation 851del4 in Chinese infants with intrahepatic cholestasis is 1/17, which is much higher than that in general Chinese population (1/93). RT-PCR may be used in diagnosis of intrahepatic cholestasis in infants.
The authors obtained infants with intrahepatic cholestasis from various parts of China. Using RT-PCR with dual-labeled probes, they identified patients with homozygous or heterozygous 851del4 mutations and found that the mutation is more prevalent in southern than in northern part of China, indicating that RT-PCR can also be used in diagnosis of other genetic disorders in pediatric patients.
Peer reviewer: Meenakshisundaram Ananthanarayanan, Associate Professor, Department of Pediatrics, Annenberg Bldg, Rm.14-24A, Box 1664, The Mount Sinai Medical Center, One Gustave L. Levy Place, New York, NY 10029, United States
S- Editor Wang JL L- Editor Wang XL E- Editor Lin YP
| 1. | Sinasac DS, Crackower MA, Lee JR, Kobayashi K, Saheki T, Scherer SW, Tsui LC. Genomic structure of the adult-onset type II citrullinemia gene, SLC25A13, and cloning and expression of its mouse homologue. Genomics. 1999;62:289-292. |
| 2. | Kobayashi K, Sinasac DS, Iijima M, Boright AP, Begum L, Lee JR, Yasuda T, Ikeda S, Hirano R, Terazono H. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet. 1999;22:159-163. |
| 3. | Tazawa Y, Kobayashi K, Ohura T, Abukawa D, Nishinomiya F, Hosoda Y, Yamashita M, Nagata I, Kono Y, Yasuda T. Infantile cholestatic jaundice associated with adult-onset type II citrullinemia. J Pediatr. 2001;138:735-740. |
| 4. | Ohura T, Kobayashi K, Tazawa Y, Nishi I, Abukawa D, Sakamoto O, Iinuma K, Saheki T. Neonatal presentation of adult-onset type II citrullinemia. Hum Genet. 2001;108:87-90. |
| 5. | Ohura T, Kobayashi K, Abukawa D, Tazawa Y, Aikawa J, Sakamoto O, Saheki T, Iinuma K. A novel inborn error of metabolism detected by elevated methionine and/or galactose in newborn screening: neonatal intrahepatic cholestasis caused by citrin deficiency. Eur J Pediatr. 2003;162:317-322. |
| 6. | Tazawa Y, Kobayashi K, Abukawa D, Nagata I, Maisawa S, Sumazaki R, Iizuka T, Hosoda Y, Okamoto M, Murakami J. Clinical heterogeneity of neonatal intrahepatic cholestasis caused by citrin deficiency: case reports from 16 patients. Mol Genet Metab. 2004;83:213-219. |
| 7. | Ohura T, Kobayashi K, Tazawa Y, Abukawa D, Sakamoto O, Tsuchiya S, Saheki T. Clinical pictures of 75 patients with neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD). J Inherit Metab Dis. 2007;30:139-144. |
| 8. | Tamamori A, Okano Y, Ozaki H, Fujimoto A, Kajiwara M, Fukuda K, Kobayashi K, Saheki T, Tagami Y, Yamano T. Neonatal intrahepatic cholestasis caused by citrin deficiency: severe hepatic dysfunction in an infant requiring liver transplantation. Eur J Pediatr. 2002;161:609-613. |
| 9. | Tomomasa T, Kobayashi K, Kaneko H, Shimura H, Fukusato T, Tabata M, Inoue Y, Ohwada S, Kasahara M, Morishita Y. Possible clinical and histologic manifestations of adult-onset type II citrullinemia in early infancy. J Pediatr. 2001;138:741-743. |
| 10. | Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD). J Hum Genet. 2002;47:333-341. |
| 11. | Kobayashi K, Ushikai M, Song YZ, Gao HZ, Sheng JS, Tabata A, Okumura F, lkeda S, Saheki T. Overview of Citrin Deficiency: SLC25A13 Mutations and the Frequency. Shiyong Linchuang Erke Zazhi. 2008;23:1553-1557. |
| 12. | Tabata A, Sheng JS, Ushikai M, Song YZ, Gao HZ, Lu YB, Okumura F, Iijima M, Mutoh K, Kishida S. Identification of 13 novel mutations including a retrotransposal insertion in SLC25A13 gene and frequency of 30 mutations found in patients with citrin deficiency. J Hum Genet. 2008;53:534-545. |
| 13. | Lu YB, Kobayashi K, Ushikai M, Tabata A, Iijima M, Li MX, Lei L, Kawabe K, Taura S, Yang Y. Frequency and distribution in East Asia of 12 mutations identified in the SLC25A13 gene of Japanese patients with citrin deficiency. J Hum Genet. 2005;50:338-346. |
| 14. | Yamaguchi N, Kobayashi K, Yasuda T, Nishi I, Iijima M, Nakagawa M, Osame M, Kondo I, Saheki T. Screening of SLC25A13 mutations in early and late onset patients with citrin deficiency and in the Japanese population: Identification of two novel mutations and establishment of multiple DNA diagnosis methods for nine mutations. Hum Mutat. 2002;19:122-130. |
| 15. | Wang JS, Wang ZL, Wang XH, Zhu QR, Zheng S. The Prognostic Value of Serum Gamma Glutamyltransferase Activity in Chinese Infants with Previously Diagnosed Idiopathic Neonatal Hepatitis. HK J Paediatr. 2008;13:39-45. |
| 16. | Guo HM, Wang XH, Zhu QR. Monitoring cytomegalovirus infection in infant with pp65 antigenemia assay. Linchuang Erke Zazhi. 2005;23:441-442. |
| 17. | Wang F, Feng WL. Study on the cause of 186 cases with infant hepatitis syndrome. Zhongguo Fuyou Baojian. 2005;10:1243-1244. |
| 18. | Chang MH, Hsu HC, Lee CY, Wang TR, Kao CL. Neonatal hepatitis: a follow-up study. J Pediatr Gastroenterol Nutr. 1987;6:203-207. |