INTRODUCTION
Hepatitis B virus (HBV) is an enveloped hepatotropic DNA virus associated with significant morbidity and mortality. Globally, at least 2 billion people or one third of the world population have been infected with HBV, over 378 million people (or 6% of the world population) are chronic carriers, and approximately 620 000 people die each year from acute and chronic sequelae secondary to HBV infection[1]. As the pathogenic mechanisms of HBV remain to be elucidated, molecular biological studies for HBV are still required. Present options to treat chronic HBV infection are restricted to the use of interferon and nucleoside analogues; interferon was the first drug approved for the treatment of HBV although the response rates were not high and there were some side effects[2,3]. The recent development of nucleoside analogues to treat HBV infection has accelerated with the characterization of the molecular mechanism of HBV replication. Nucleoside analogues interfere with the viral reverse transcriptase and thus inhibit viral replication[4]. However, the development of drug resistance is a major limitation to long-term effectiveness of antiviral agents. Therefore, the current treatment is not ideal in clinical studies and it is necessary to develop new anti-HBV therapeutic options for chronic hepatitis B patients[5]. Understanding the mechanisms of drug resistance mutants is also important for designing new agents and devising strategies to manage and prevent the development of antiviral drug resistance[6-8].
As the host range of HBV is limited to humans and primates, most in vivo studies about the mechanism of HBV replication and HBV-associated liver diseases, and about the selection of anti-HBV drugs, have been performed by using HBV transgenic mice[9-12]. However, the acquisition of transgenic mice is very time-consuming and laborious, needing expensive microinjection equipment and complicated technologies. It is costly and inconvenient to establish HBV transgenic mice with every mutant[13]. Thus, the availability of a small model which can be easily established to study the in vivo efficacy of new antiviral drugs and mechanisms of HBV virulence would help to develop new options to control HBV replication. In our lab, a simple and convenient immunocompetent mouse model for HBV replication has been established by using a hydrodynamic-based procedure[14].
As our mouse model is a transient HBV transfection model with viral replication and expression lasting for 10 d and peaking at day 3 and day 4 after transfection, whether this HBV replication mouse model can meet the applicable requirement and be used in studies about HBV is unclear. The present study was performed to identify these issues by utilizing the HBV replication mouse model to evaluate the effect of polyIC and nucleoside analogues on HBV replication and to investigate the replication ability of drug-resistant HBV mutants and an HBx-minus mutant[15].
MATERIALS AND METHODS
Plasmids
Wild type pHBV4.1 is an HBV replication competent plasmid which contains 1.3 copies of the HBV genome (subtype ayw)[16,17]; the telbivudine resistance mutant named as pLdT is a plasmid at which the residue rt204 ATG (M, Methionine) is changed into ATT (I, Isoleucine); the adefovir resistance mutant named as pADV2 is a plasmid at which the residue rt181 GCT (A, Alanine) is changed into GTT (V, Valine) and the residue rt236 AAC (N, Asparagine) is changed into ACC (T, Threonine). Both mutants were derived from wild type pHBV4.1 by site-directed mutagenesis (QuikChange mutagenesis kit, Stratagene) according to the manufacturer’s instructions and were confirmed by sequencing. The plasmid payw1.2 is a replication competent construct that contains 1.2 copies of the wild type HBV genome (subtype ayw). The HBx-minus mutant payw*7, which contains an ochre termination signal (CAA to UAA) after codon 7 (at codon 8) in the HBx ORF and lacks the expression of HBx, was derived from payw1.2 by site-directed mutagenesis[18,19].
Animals
Balb/c male mice (6-8 wk old) at specific pathogen-free (SPF) level, weighing 18-20 g, were purchased from the Huaxi Laboratory Animal Center of Sichuan University, China. All mice drank tap water and were fed rat chow ad libitum and were kept within a 12 h light-dark cycle at constant temperature and humidity. All mice were cared for in compliance with institutional guidelines. For establishment of the mouse model, the mice were injected with 10 μg HBV plasmid DNA in 2 mL phosphate-buffered saline (PBS) via the tail vein within 5-8 s[20].
Study procedure
To evaluate the HBV replication mouse model in screening the efficacy of antiviral drugs in vivo, polyinosinic-polytidylin acid (polyIC) (Sigma USA) and nucleoside analogues adefovir (GlaxoSmithKline) and entecavir (Bristol-Myers Squibb) were administered by injection or oral gavage and their inhibiting efficacies on HBV replication (wild type HBV) were assessed. Twenty-four hours after the HBV replication mouse model was established, 200 μg polyIC in 200 μL PBS was given three times at 24 h intervals, and 200 μL PBS was used as a control. Twenty-four hours after hydrodynamic injection with pHBV4.1 plasmid, the nucleoside analogue-treated groups were administered adefovir by oral gavage at a dose of 1.5 mg/kg per day, and entecavir at a dose of 0.075 mg/kg per day in a medication course lasting 3 d, while the PBS group was used as a control. On the third day, the mice were sacrificed 4-6 h after the oral gavage. For each group there were at least 3 mice in parallel.
To identify the value of the HBV replication mouse model for assessing replication ability of HBV mutant strains, the drug-resistance mutants pLdT (rtM204I, ayw subtype) and pADV2 (rtA181V + rtN236T, ayw subtype) as well as HBx-minus mutant payw*7 were injected respectively. Three days after injection, all mice were sacrificed and samples of liver tissue and blood were collected.
Liver tissue was collected and frozen in liquid nitrogen. Blood samples were processed for collection of serum and stored at -20˚C until assayed. The corresponding HBV DNA replication intermediates in liver were assessed as follows.
Detection of hepatitis B surface antigen and hepatitis B e antigen by enzyme-linked immunosorbent assay: Hepatitis B e antigen (HBeAg) and hepatitis B surface antigen (HBsAg) qualitative analysis was performed with 50 μL mouse serum by using enzyme-linked immunosorbent assay (ELISA). HBeAg and HBsAg detection kits (Shanghai Shiye Kehua Company, China) were used respectively, according to the manufacturer’s instructions, and samples were considered positive at A450≥ 2.1.
Detection of HBV DNA replication intermediates by Southern blotting: The frozen liver tissue was mechanically pulverized in liquid nitrogen and HBV DNA replicative intermediates were isolated as described previously[21]. One hundred and twenty micrograms of liver tissue powder was lysed in 0.6 mL of 100 mmol/L Tris hydrochloride (pH 8.0) and 2 mL/L NP40. The supernatant was adjusted to 6.75 mmol/L magnesium acetate plus 200 μg DNaseI/mL, and incubated for 1 h at 37°C. The supernatant was readjusted to 10 mmol/L ethylenediaminetetraacetic acid (EDTA), 8 g/L sodium lauryl sulfate, 100 mmol/L NaCl and 1.6 mg pronase/mL, and was incubated for an additional 1 h at 37°C. The supernatant was extracted twice with phenol/chloroform, precipitated with 0.7 volume of isopropanol, and resuspended in 30 μL of 10 mmol/L Tris hydrochloride (pH 8.0) and 1 mmol/L EDTA. DNA Southern blotting hybridization analysis was performed with 30 μL viral replication intermediates, as previously described. Filters were probed with digoxigenin-labeled (Roche Applied Science) HBV ayw genomic DNA to detect HBV sequences. The level of HBV DNA replicative intermediates was calculated by the Quantity One software according to the manufacturer’s instructions.
RESULTS
The effect of polyIC on HBV replication
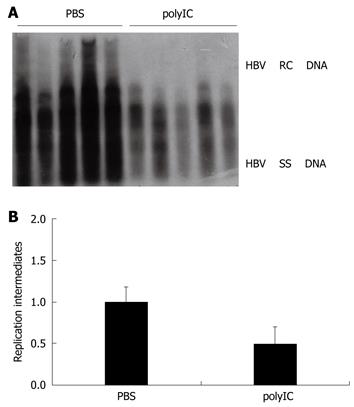
PolyIC is a strong inducer of internal interferon and its efficacy on HBV replication in vivo was studied. As shown in Figure 1A and B, the level of HBV DNA replication intermediates in liver tissue of the polyIC-treated group was inhibited by about one-fold, as compared with that of the PBS-treated group. This result suggested that polyIC injection decreased the level of viral replication in the liver.
Figure 1 Evaluation of the effect of polyIC on hepatitis B virus (HBV) replication using the HBV replication mouse model.
A: The effect of polyIC on HBV DNA replication intermediates in mouse model. After hydrodynamic transfection, mice were treated with phosphate-buffered saline (PBS) (control) or polyIC; B: The relative intensity of HBV DNA replication intermediates of the mouse model for PBS group and polyIC group which inhibited the HBV replication by about one fold. The levels of HBV DNA replication intermediates in PBS-treated group were set to 1, and the relative levels of polyIC-treated group were calculated accordingly.
The effect of nucleoside analogues on HBV replication
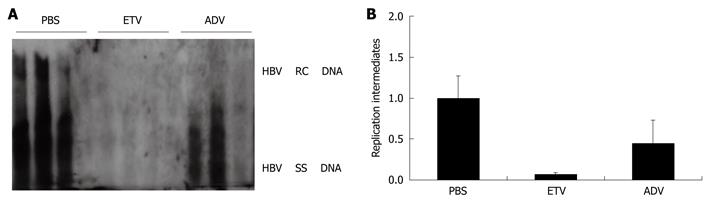
To investigate whether the HBV replication mouse model can be used in the selection of anti-HBV agents, we used this model to test the effect of entecavir and adefovir, two of the approved nucleotide analogues for chronic hepatitis B, on HBV replication. Figure 2A and B show the levels of HBV DNA replication intermediates in liver tissue of the mouse model. Compared with the PBS-treated group, the HBV DNA replication intermediate levels in the entecavir-treated group and the adefovir-treated group were inhibited by around 13.6-fold and 1.4-fold, respectively. In contrast to these results, it appeared that both nucleoside analogues did not influence the expression of HBV-associated antigens, as the HBeAg and HBsAg were both positive in the serum of the mouse model after nucleoside analogue treatment for 3 d.
Figure 2 Evaluation of the effect of nucleoside analogues entecavir (ETV) and adefovir (ADV) on HBV replication using the HBV replication mouse model.
After hydrodynamic transfection, mice were administered by oral gavage with two different nucleoside analogues: entecavir at a dose of 0.075 mg/kg per day and adefovir at a dose of 1.5 mg/kg per day for 3 d. A: HBV DNA replication intermediates in the mouse model treated with PBS (control), entecavir and adefovir, respectively; B: The relative levels of HBV DNA replication intermediates in the mouse model among different groups treated with PBS (control), entecavir and adefovir, respectively. The levels of HBV DNA replication intermediates in PBS treated group were set to 1, and the relative levels of treated groups were calculated accordingly.
Replication ability of drug-resistant HBV mutants
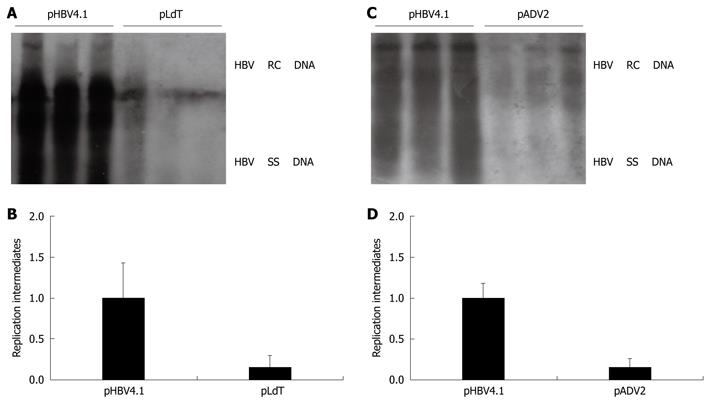
To investigate whether our HBV replication mouse model can be used in studies about replication ability of drug-resistant HBV mutants, the mouse model was used to study the replication ability of two drug-resistant HBV variants: an adefovir resistance mutant and a telbivudine resistance mutant. The HBV DNA replication ability of both drug-resistant HBV mutants decreased by a large amount compared with the wild type control mouse group. HBV DNA replication in the telbivudine resistance mutant pLdT (rtM204I) was decreased about 4.5-fold and in the adefovir resistance mutant pADV2 (rtA181V + N236T) was decreased about 5.6-fold, compared with the wild type mice (Figure 3A-D). This result suggested that these mutations in the drug-resistant HBV mutants could result in the decline of replication ability. However, the HBsAg and HbeAg were both positive in the serum of the mouse model as compared with the wild type control.
Figure 3 Assessment of HBV replication ability of drug-resistant HBV mutants using the HBV replication mouse model.
The levels of HBV DNA replication intermediates in wild type group were set to 1, and the relative levels of mutant groups were calculated accordingly. A, B: The HBV DNA replication intermediates and relative intensity between wild type injected with pHBV4.1 and telbivudine resistance mutant injected with pLdT (rtM204I); C, D: The HBV DNA replication intermediates and relative intensity between wild type injected with pHBV4.1 and adefovir resistance mutant injected with pADV2 (rtA181V + N236T).
Replication ability of HBx-minus mutant
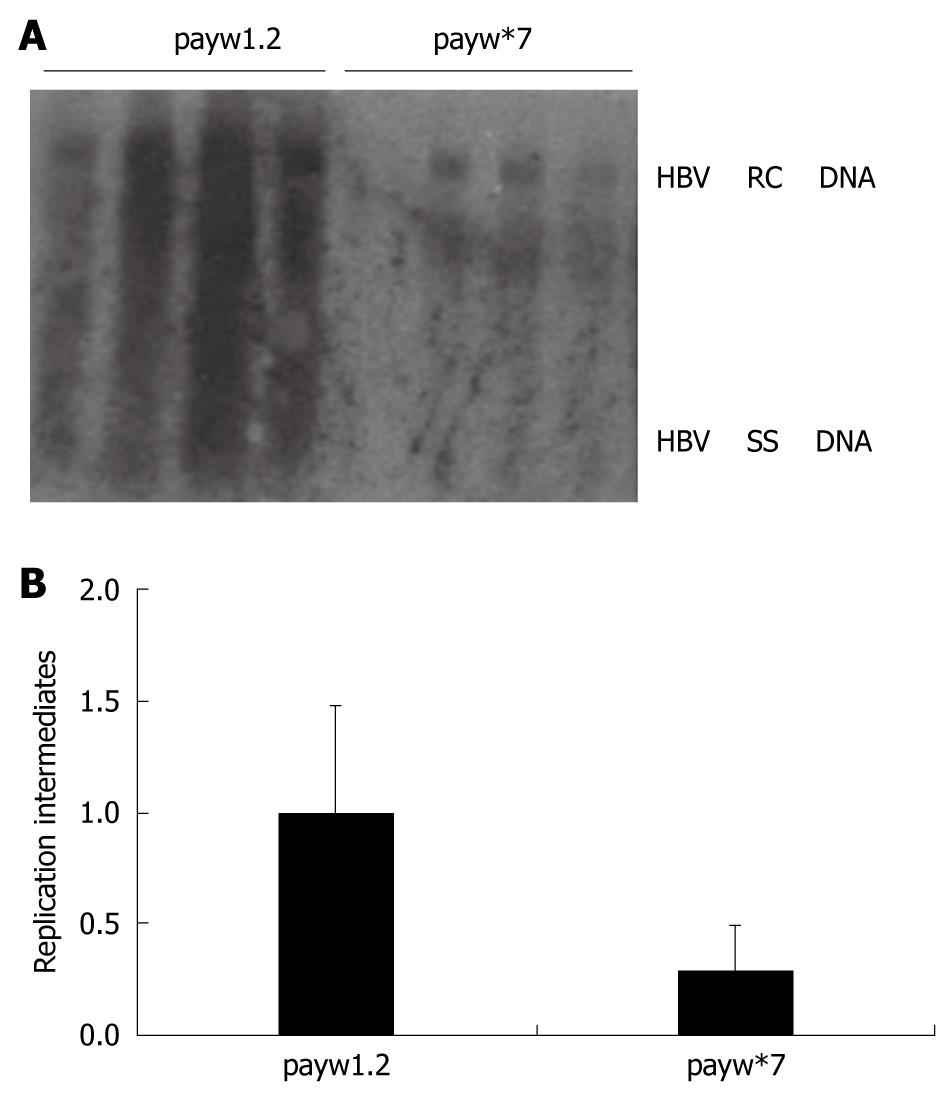
In order to evaluate whether the HBV replication mouse model can be used in the study of the mechanism by which HBV replicates, we utilized this model to identify the role of the HBx-minus mutant in HBV replication. Plasmids of wild type and HBx-minus mutant were transferred into Balb/c mice, respectively. The levels of HBV DNA replication intermediates from these two constructs were compared (Figure 4A). In both wild type and HBx-minus mutant groups HBV DNA replicated in this mouse model, which suggested that both plasmids had ability to replicate in vivo. However, the levels of HBV DNA replication intermediates of the HBx-minus mutant were decreased by nearly 2.9-fold compared with the payw1.2 control in this mouse model (Figure 4B).
Figure 4 Assessment of HBV replication ability of HBx-minus mutant using the HBV replication mouse model.
A: The levels of HBV DNA replication intermediates between wild group injected with payw1.2 and HBx-minus mutant group injected with payw*7; B: The relative intensity of HBV DNA replication intermediates between wild group injected with payw1.2 and HBx-minus mutant group injected with payw*7. The levels of HBV DNA replication intermediates were reported to the levels of the mice injected with payw1.2, which were set at 1.
DISCUSSION
The development of animal models for HBV infection is crucial for understanding viral replication, disease pathogenesis, and selecting candidate drugs for the treatment of HBV infection[22]. However, the narrow range of hosts for the virus has limited the availability of animal models. Chimpanzees are susceptible to HBV infection, but experiments with chimpanzees are limited by cost, availability and ethical considerations. Hepadnaviruses such as ground squirrel hepatitis virus, woodchuck hepatitis virus and duck HBV have offered opportunities to investigate some mechanisms of viral pathogenesis, but these hepadnaviruses have some differences to HBV in terms of genome structure, transcription and replication regulation. Therefore, they cannot serve as a substitute for research into HBV[23]. Recently, much attention has been paid to HBV transgenic mice, especially HBV replication-competent transgenic mice with 1.3 copies of HBV genome. These transgenic mice have provided information on pathogenesis and virus replication and are widely used in studies of HBV. However, a plasmid needs to be transferred into a male pronucleus, then injected embryos are implanted into pseudopregnant females and live births tested for transgene, before transgenic mice can be cultivated; this process requires a great deal of time, cost and labor[24,25]. Furthermore, in HBV transgenic mice all viral RNA is synthesized from a chromosomally integrated copy of the virus, whereas no cccDNA, the natural template of viral transcription in vivo, is produced[26,27]. By using a hydrodynamic-based procedure, our laboratory has established a rapid and convenient mouse model with a high level of HBV replication. In this study, this mouse model was used to evaluate the inhibitory effect of some anti-HBV agents on HBV replication, the replication ability of HBV-resistant mutants and the effect of HBx on HBV replication; the results shown above suggesting that this model can be used for studies of HBV biology.
The principal treatments for chronic hepatitis B include the use of interferon and nucleoside analogues. PolyIC is a strong inducer of internal α/β interferon which could inhibit the HBV replication. In our study, it was shown that after treating the animal models with polyIC, the level of HBV replication decreased; there were at least 3 mice in parallel for each group to ensure the reliability and accuracy of results. The effect of polyIC injections on HBV replication in these mice was less than that in HBV transgenic mice reported previously[28] but supportive of the suggestion that an inhibitory effect of α/β interferon can be achieved in this mouse model. Similarly, we found that entecavir and adefovir, two of the approved nucleoside analogue treatments for chronic hepatitis B in the clinic, could also inhibit HBV replication in this mouse model. Furthermore, there are different inhibition levels according to the two different drugs as entecavir showed greater effects than adefovir in our study; this corresponds with the clinical report that entecavir has the greatest inhibitory effect on HBV[29]. These results demonstrate that this animal model can be used to study existing and potential therapeutic approaches directed against HBV.
Long-term treatment of HBV with nucleoside analogues is associated with the emergence of resistant virus strains. The rate of development of lamivudine resistance increases progressively during treatment with rates of 20% to 25% reported annually and approaching more than 80% after 4 to 5 years of therapy[30,31]. The resistance rate to adefovir is 2% at 2 years and 29% at 5 years. It was previously demonstrated that the HBV mutation resistant to adefovir is the rtA181V + N236T[32-34], which was associated with a 5 to 10 fold decrease in sensitivity to adefovir and a moderately reduced replication capacity observed in in vitro assays[35-37]. The viral mutant resistant to telbivudine is the rtM204I mutation. It has been reported that the presence of resistant variants is one of the factors that causes severe liver damage and even fulminant liver failure, while the mechanisms remain unclear[38-40]. Therefore, the biological properties and mechanisms by which HBV-resistant variants cause deteriorated liver function need to be explored, and the effective agents against these variants need to be selected[41]. If HBV transgenic mice are used, it is very impractical to construct transgenic mice with every mutant. In contrast, it was demonstrated that the properties of HBV-resistant variants could be tested in a convenient HBV replication mouse model. Our results suggest that the mutations in drug-resistant HBV mutants could affect the replication process and result in the decline of replication ability but that they do not affect the transcription process and the expression of HBsAg and HbeAg. This could be because mutations may cause allosteric changes in the catalytic region of polymerase and affect the enzyme function but the transcription of viral messenger RNA which is translated into viral proteins is not affected. Thus, this mouse model of HBV replication will greatly facilitate studies about HBV drug-resistant mutants.
To understand the mechanism of HBV transcription, replication and pathogenesis, it has been necessary to mutate the HBV genome and then survey the transcription, replication and expression levels of viral mutants. In this way, the function of different HBV genome sequences can be understood. However, this research would be very time-consuming, costly and laborious if HBV transgenic mice are used. In the present study, the results were consistent with previous observations in HBV transgenic mice and cell culture as mentioned above, suggesting that this mouse model could be used in functional studies about HBV and that this model of HBV replication will also provide a useful tool for mechanism studies of HBV.
From these data we can draw the conclusion that this rapid and convenient model of HBV replication affords new opportunities and can be applied widely to many aspects of the study of HBV such as selection of antiviral candidates against HBV, the properties of drug-resistant variants, and the studies of HBV replication mechanisms. Furthermore, the model is reliable and simple for the study of HBV genomic function, and especially for investigating the mechanisms involved in HBV replication through the mutating HBV genome and for selection of new agents against HBV mutants.
COMMENTS
Background
More than 378 million people are chronic carriers of hepatitis B virus (HBV), and approximately 620 000 people die each year related to HBV infection. The lack of convenient and reliable animal models has hindered progress in HBV research. Recently, the authors have established a simple HBV replication mouse model using hydrodynamic tail vein injection of HBV replication competent plasmid (pHBV4.1) into Balb/c mice.
Research frontiers
An HBV mouse model established by using a hydrodynamic in vivo transfection method is useful and convenient. Chronic HBV mouse models have been established by using immunodeficient mice. With the progress of tissue engineering and transgenic technology, some liver cell humanized transgenic mice for studying human HBV have also been constructed.
Innovations and breakthroughs
In this study using an HBV replication model, the authors found that the in vivo ability of HBV DNA replication of drug-resistant HBV mutants decreased greatly, while the HBsAg and HbeAg were both positive in the serum compared with the wild type. It was further discovered that the lack of HBx also decreased the ability of HBV DNA replication in vivo. The authors demonstrated that the properties of HBV mutant variants could be tested in this convenient HBV replication mouse model without constructing transgenic mice.
Applications
The HBV replication mouse model established in this study is a rapid and convenient tool to detect the efficacy of antiviral agents and to study the replication ability of HBV mutants in vivo.
Peer review
The work done by Gao et al has a great value and the studied issue is applicable. It includes HBV gene expression and replication by HBV plasmids, which were delivered to the mouse liver by hydrodynamic injection. Development of methods that allow an efficient expression of exogenous genes in animals would provide tools for gene function studies, treatment of diseases and for obtaining gene products.