Published online Apr 21, 2010. doi: 10.3748/wjg.v16.i15.1928
Revised: January 18, 2010
Accepted: January 25, 2010
Published online: April 21, 2010
Abdominal wall defects and incisional hernias represent a challenging problem. In particular, when a synthetic mesh is applied to contaminated wounds, its removal is required in 50%-90% of cases. Biosynthetic meshes are the newest tool available to surgeons and they could have a role in ventral hernia repair in a potentially contaminated field. We describe the use of a sheet of bovine pericardium graft in the reconstruction of abdominal wall defect in two patients. Bovine pericardium graft was placed in the retrorectus space and secured to the anterior abdominal wall using polypropylene sutures in a tension-free manner. We experienced no evidence of recurrence at 4 and 5 years follow-up.
- Citation: Cavallaro A, Lo Menzo E, Di Vita M, Zanghì A, Cavallaro V, Veroux PF, Cappellani A. Use of biological meshes for abdominal wall reconstruction in highly contaminated fields. World J Gastroenterol 2010; 16(15): 1928-1933
- URL: https://www.wjgnet.com/1007-9327/full/v16/i15/1928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i15.1928
Abdominal wall defects and incisional hernias represent a challenging problem. The risk of developing an incisional hernia after a midline laparotomy is up to 11%[1]. The size of the abdominal wall defect and the potential presence of contamination of the site can complicate this commonly performed surgical repair. Several techniques have been adopted over time. Primary repairs often lead to unacceptable high tension, and their recurrence rate has been reported as high as 12%-50%[1,2]. In patients whose fascial defect is significant, mesh repair is preferable in order to obtain a tension-free abdominal wall closure. In the latter case, the recurrence rates are reported between 3% and 24%[2-4]. According to the literature, the use of prosthetic mesh reduces the recurrence rate but is also associated with serious complications in 10%-15% of cases. Some of these complications, such as infection, fistula and skin erosion, often lead to mesh removal[4]. In particular, when a synthetic mesh is applied to contaminated wounds, its removal is required in 50%-90% of the cases[5]. Strength, flexibility, host tissue compatibility and ability to avoid infections should characterize an ideal mesh. Many synthetic and biological mesh tissues have been proposed over time but no single material, nor newer biosynthetic mesh, has fulfilled these requirements and gained universal acceptance. We present two cases in which biological meshes were used successfully in contaminated fields.
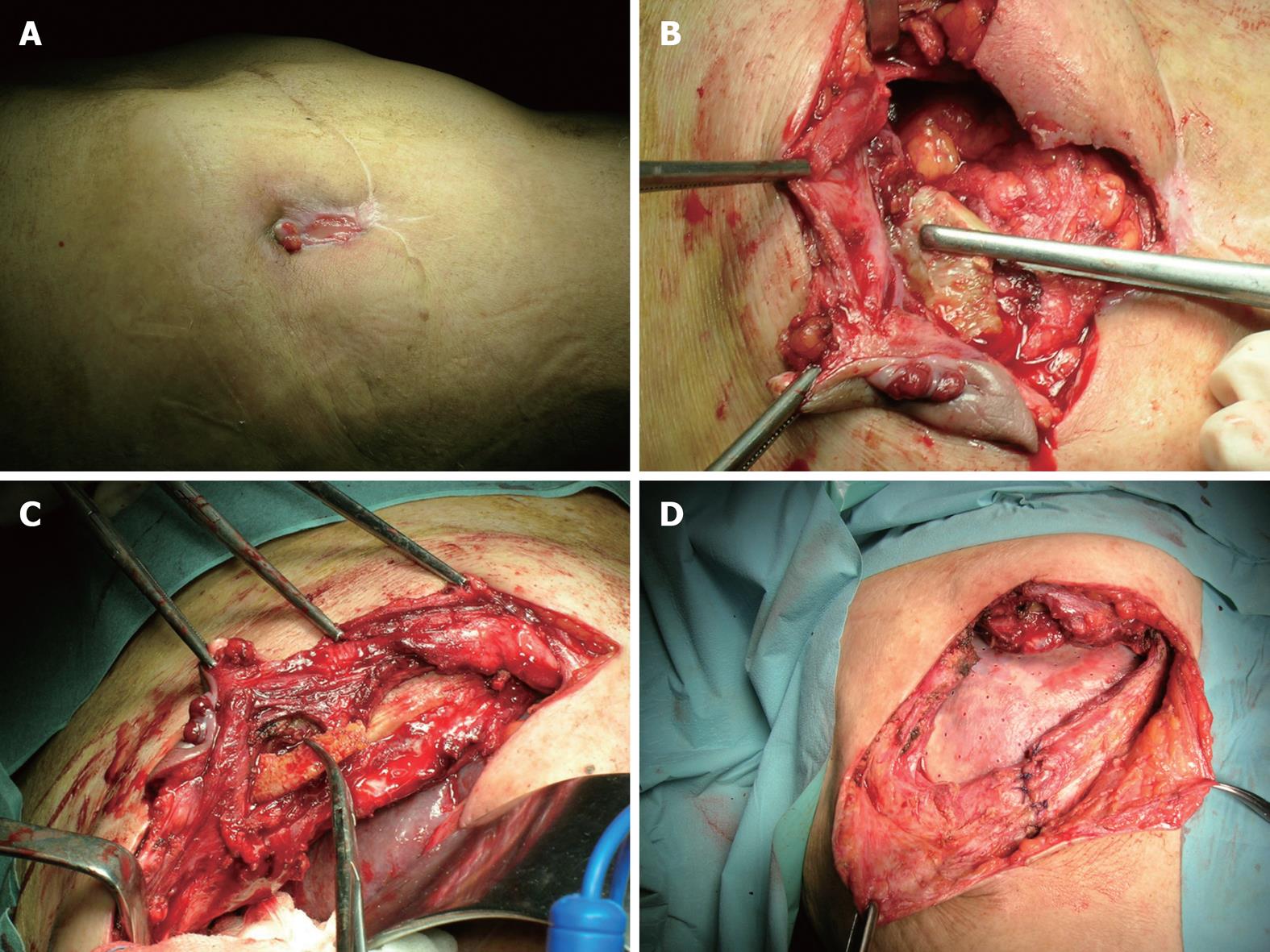
A 62-year-old white woman presented to the General Surgery and Senology Unit of our University with a 6-mo history of abdominal pain after right hemicolectomy for carcinoma through a midline laparotomy at another institution. Her medical history was also significant for obesity, hypertension and coronary artery disease. She stated that pain occurred soon after the surgical procedure, together with random fever. Six months later, she developed an abdominal wall abscess that spontaneously drained externally through a small incisional hernia in the right-upper abdomen, and formed a persistently draining sinus. Following this, she underwent incisional hernia repair with pre-peritoneal prolene mesh placement. After 4 mo, she developed a small fistula again (Figure 1A), therefore, she was admitted to our division for further care. On physical examination, we observed a well-healed midline incision with mild tenderness along the incision itself. Computed tomography (CT) of the abdomen showed a multiloculated collection around the mesh, which measured approximately 12 cm × 15 cm. A course of antibiotic therapy (imipenem) was initiated before bacterial cultures were obtained. The first culture was positive for Staphylococcus aureus and Pseudomonas aeruginosa. The second, after 1 wk of appropriate antibiotic administration, remained negative for any microorganism.
We scheduled the patient for exploration and mesh removal. We entered the abdominal cavity through her previous midline laparotomy. The cavity was then exposed and the fluid collection aspirated along with debris. The inflammatory process extended to the periostium of the tenth rib. Removal of the mesh from the anterior abdominal wall required extensive and tedious dissection. The abdomen was entered and all adhesions to abdominal organs (bowel and omentum) were removed. We noticed an anomaly of the rib profile, therefore, after further investigation, we discovered a bone fracture with surrounding tissue rearrangement. We excised the rib for histological examination and it was consistent with osteomyelitis (Figure 1B and C). After removal of the contracted mesh, we created skin flaps and re-positioned the posterior rectus sheath. We placed a sheet of bovine pericardium graft (Tutomesh®; Tutogen Medical, Germany) in the retrorectus space and secured the mesh in a tension-free manner to the abdominal wall, using polypropylene sutures. The anterior rectus sheath was then re-positioned with Vicryl, and the skin was closed with staples (Figure 1D). The patient had no significant postoperative complications, and she was discharged on postoperative day 6. We performed CT at 5 mo after surgery, which showed no seroma and excellent remodeling of the host tissue. There was no clinical evidence of recurrence at her 5-year follow-up visit.
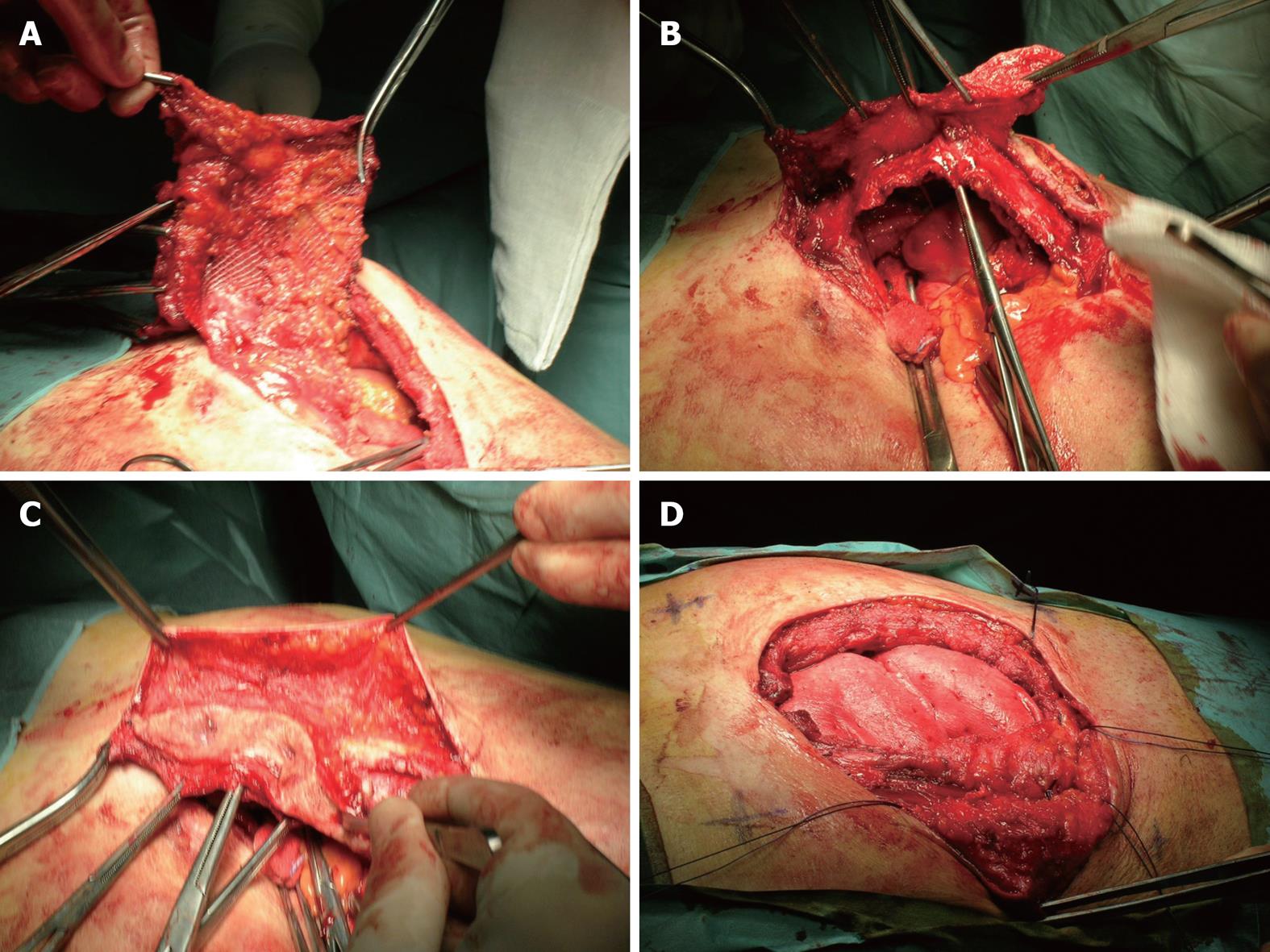
A 69-year-old man with a previous history of left hemicolectomy for diverticular disease underwent elective incisional hernia repair with intraperitoneal polytetrafluoroethylene (PTFE) mesh placement 8 mo later. Postoperatively, the patient developed a persistent infected seroma, which was unresponsive to antibiotic treatment. The patient underwent surgery again to drain the collection, debride the area, and substitute the contracted Goretex mesh with a polypropylene mesh in the retrorectus space. This attempt proved unsuccessful and led to abscess formation after only 1 mo. The patient was scheduled for elective removal of the polypropylene mesh. After removal of the contracted mesh (Figure 2A-C), the posterior rectus sheath was re-positioned to the midline, and a sheet of bovine pericardium mesh (Tutomesh®) was implanted in the retrorectus space and secured to the anterior abdominal wall using polypropylene sutures in a tension-free manner. The anterior abdominal wall was then re-positioned with Vycril interrupted sutures and the skin was closed with staples (Figure 2D). The patient had no significant postoperative complications, and he was discharged on postoperative day 3. At his 4-year follow-up visit, there was no evidence of recurrence.
Incisional hernias are still a challenging problem for the surgeon. Their primary repair leads to high recurrence rates (up to 50%) because of the tension created and myocutaneous flap necrosis[1,2].
Many techniques have been proposed over time to reduce tension, such as relaxing incisions, and compartment release. Unfortunately, the results are far from being optimal[6,7]. Furthermore, large, full-thickness, abdominal wall defects secondary to wide resection of malignant tumors, traumatic injuries, or congenital abnormalities, cannot be closed primarily. The use of prosthetic meshes has then become necessary. Along with the traditional open techniques of mesh implantation, the more recent laparoscopic approach has gained popularity because of the decrease in wound infection, recurrence rates and recovery time.
Unfortunately the intrinsic property of tissue ingrowth of the prosthetic meshes currently used, such as polypropylene, polyester and PTFE, is also the cause of unwanted adhesions, chronic sinus (2%-6%), fistula formation (0%-2%) and wound infections (2%-17%)[8]. Other potential complications of synthetic meshes include mesh contraction, migration, folding of the edges with visceral contact, recurrence, inflammation, seroma, and chronic pain due to inflammatory response and/or nerve entrapment. As a result, complications are common following ventral hernia repair, and occur in 5%-30% of laparoscopic cases and 27%-34% after open cases[9].
Even more challenging is the repair of complex contaminated abdominal wall defects, since no ideal operative technique or material for fascial reconstruction is currently available. The fear of fibrosis, erosions, infection and fistulas with the commonly used prosthetic meshes, has led engineers and surgeons to investigate alternative materials in order to achieve tension-free repair in a single-stage operation, even in highly contaminated fields. Biosynthetic grafts seem to offer a solution to this challenging problem. Their concept is to provide a collagen and other extracellular matrix scaffold, in which the host fibroblasts can create angiogenesis and deposit new collagen. The non-synthetic nature of these products allows them to be more resistant to infections. Several biological grafts are available on the market. Their classification is based on the species of origin (allogenic, xenogenic), type of collagen matrix utilized (dermis, pericardium, intestinal submucosa), decellularization process, presence or absence of cross-linking, storage requirements (need for refrigeration or not), and the need for rehydration (Table 1).
| Species of origin | Allogenic | Xenogenic | |
| Type of collagen matrix | Dermis | Intestinal submucosa | Bovine pericardium |
| Decellularization process | Yes | Yes | Yes (TUTOMESH®) |
| Cross-linking | Yes | No | No |
| Sterilization method | Salt solution, patented non-denaturing agents | H2O2, NAOH γ irradiation | |
| Storage (need for refrigeration) | Yes | No | No |
| Rehydration requirement | Yes | No | No |
Bovine pericardium (TUTOMESH®) is a cadaveric bovine pericardium surgical mesh that is processed by solvent dehydration followed by gamma irradiation. It has no risk for transmission of viruses or prions. The graft consists of collagenous connective tissue with three-dimensional intertwined fibers, it has multidirectional mechanical strength, and can be implanted regardless of the direction of the graft. Collagenous connective tissue with multidirectional fibers retains the mechanical strength and elasticity of the native tissue, while providing the basic structure to support replacement by new endogenous tissue (known as remodeling). It is indicated for use in general, gynecological, cardiac and plastic surgery, such as repair of pericardial structures, soft tissue deficiencies, rectal and vaginal prolapse, and hernias (including diaphragmatic, femoral, incisional, inguinal, lumbar, paracolostomy, and umbilical hernias), gastric banding, muscle flap reinforcement, and reconstruction of the pelvic floor.
A bioprosthesis derived from human acellular dermal matrix (Alloderm®; Lifecell Corporation, Brachburg, NJ, USA) has been used as a tissue substitute for skin grafting over the past 20 years, but its use for abdominal wall reconstruction is relatively recent. At present, there are not enough data or long-term reports to establish universal consensus for its use over other bioprostheses, but it is the material with the most published literature. Some authors have described concerns about the laxity of the material and its ability to stretch over time, with the development of eventration or pseudo-recurrences[9].
Another available product is derived from porcine dermal collagen (Permacol™; Tissue Science Laboratories plc, Covington, GA, USA). It is a flexible acellular cross-linked porcine dermal collagen that is approved by the US Food and Drug Administration for use as a tissue substitute. It has been used in the United Kingdom since 1998. In addition to its three-dimensional collagen architecture that is similar to human dermis, it supports fibroblast infiltration and neovascularization. As a result of its manufacturing peculiarity of cross-linking, its remodeling process is delayed in the host tissue, which provides additional strength, but also possible incomplete remodeling. The porcine dermal collagen graft is easy to handle and has demonstrated comparable tensile strength to polypropylene. Integration into host tissue and neovascularization allow for antibiotic diffusion and in turn, greater resistance to infections, as in other similar biological grafts[10].
Four types of incisional hernia repair with mesh have been commonly described.
In the inlay technique, the fascial defect cannot be approximated, so the prosthetic material is secured to the edges of the fascia to achieve abdominal wall closure. The onlay technique places the mesh above a primary fascial closure. The more recently described sublay technique (Rives Stoppa repair) applies the concepts of a tension-free technique in which the prosthetic mesh is secured in the retrorectus space after closure of the posterior rectus sheath/peritoneum. The aim is to prevent contact between the mesh and abdominal viscera. The coverage of the mesh provided by the muscle decreases the complications related to the mesh proximity to the subcutaneous tissue (seroma, wound infection)[11,12]. Finally, with the advent of meshes designed with an anti-adhesive barrier on one side, the mesh position has been moved one layer deeper, into the peritoneal cavity; the so-called intraperitoneal technique. In the latter, the mesh is secured to the abdominal wall with stitches, staples or tacks, and this is commonly performed laparoscopically.
The data available on biological mesh implants for ventral hernia repair are very recent, and mostly in the form of case series. Lindermann et al[13] have published their experience using bovine pericardium graft in 37 incisional and two parastomal hernias. They have described four recurrences in the incisional group treated with the inlay technique (fascial defect cannot be approximated). They have suggested that the high elasticity of the mesh during the remodeling phase could be the likely cause of recurrence. Post et al[14], in their series of 13 successful incisional hernias with sublay bovine pericardium, have described one case in which the mesh had to be re-attached after it had pulled off the suture line during a coughing spell. No infection, pain, foreign body feeling or recurrence was seen. Parker et al[10] have reported their experience using Permacol in nine patients affected by complicated abdominal defects with either a contaminated wound or a history of a hernia mesh infection. In all cases, the mesh was placed in the retromuscular underlay position, and in spite of the presence of active infection or gross contamination, no infectious complications occurred. Baghai et al[15] have evaluated 20 biological mesh implants in 17 patients who required recurrent ventral hernia repair following synthetic mesh explantation due to infection. Different biological grafts were utilized in the study (including porcine intestinal submucosa, human and porcine dermis). Also, all the implants were carried out with an open technique in the inlay, onlay or retrorectus position. All the patients treated with the inlay or onlay technique (n = 17) experienced wound infection and/or dehiscence within 2 wk of surgery. The three remaining biological implants, placed in the retrorectus space with closure of the anterior fascia, were successful with no infection, dehiscence or reccurrences at 6 mo follow-up. Therefore, Baghai et al[15] have suggested that biological mesh implants are not effective when used with an inlay or onlay technique. According these preliminary results, the mesh position, regardless of the type of biological mesh composition and texture, may play a key role in the incidence of hernia recurrence. Patients whose defect is corrected by a bridging technique (inlay technique) seem to have an increased tendency to develop eventration and a higher recurrence rate, compared with those whose defect is reinforced after fascia closure (sublay technique)[9]. According to some authors, the use of biological meshes in a bridging manner (inlay technique) provides only a temporary means for restoration of abdominal wall integrity in highly contaminated fields, which leads to future implantation of a more durable prosthesis to reestablish the integrity of the abdominal wall. Conversely, when the biological mesh is used to reinforce a primary fascia closure (sublay or underlay technique), the rate of recurrence seems comparable with other prosthesis[16,17]. In the setting of a contaminated wound, delayed closure has been proposed to prevent a closed environment infection and limit recurrence, but its routine use has not gained wide support[18].
The scarcity of literature comparing the different types of biological grafts precludes an evidenced-based decision about which one to use. Therefore, we have to decide according to the characteristics of the grafts to choose the appropriate product.
Permacol is available in larger sizes than cadaveric graft (up to 18 cm × 28 cm), it is less expensive than human cadaveric dermal graft, and does not require rehydration before being placed[10].
Bovine pericardium meshes have been used safely even for the repair of inguinal or paraesophageal hernias, in which the constant movement of structures is a risk factor for recurrence[19]. In our opinion, one of the main advantages of bovine pericardium graft resides in the relative lack of elastin compared to dermal products (both allogenic and xenogenic). This, in turn, results in a higher ratio of mature collagen/elastin at the end of the remodeling, possibly minimizing eventration and pseudo-recurrence[9]. Also, these products are easy to handle, do not require refrigeration, have a long shelf life, and can be used immediately after opening without long rehydration processes. Finally, they tend to be less expensive than their human tissue counterparts. However, further studies with larger numbers of patients and longer follow-up are required to guide better the right choice of material[9,20].
In conclusion, complex reconstruction of the abdominal wall can be challenging. Biosynthetic meshes are the newest tool available in the surgeon’s armamentarium and they could have a role in ventral hernia repair in clean and contaminated fields. Their use is based on the concept of providing a collagen and extracellular matrix scaffold in which the host fibroblasts can create angiogenesis and deposit new collagen. In reviewing our results, the bovine pericardium as a xenogenic implant is a useful alternative to allogenic materials in incisional hernia repair. Further experience and longer follow-up is necessary to determine the results of bioprosthesis when used for abdominal wall reconstruction.
Peer reviewers: Dr. Yuk Him Tam, Department of Surgery, Prince of Wales Hospital, Shatin, NT, Hong Kong, China; Keiji Hirata, MD, Surgery 1, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578-583; discussion 583-585. |
| 2. | Millikan KW. Incisional hernia repair. Surg Clin North Am. 2003;83:1223-1234. |
| 3. | Matthews BD, Kercher KW. Bioprosthetic materials in hernia repair. Probl Gen Surg. 2002;19:7-13. |
| 4. | Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg. 2004;52:188-194. |
| 5. | Szczerba SR, Dumanian GA. Definitive surgical treatment of infected or exposed ventral hernia mesh. Ann Surg. 2003;237:437-441. |
| 6. | Mathes SJ, Steinwald PM, Foster RD, Hoffman WY, Anthony JP. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg. 2000;232:586-596. |
| 7. | Vrijland WW, Jeekel J, Steyerberg EW, Den Hoed PT, Bonjer HJ. Intraperitoneal polypropylene mesh repair of incisional hernia is not associated with enterocutaneous fistula. Br J Surg. 2000;87:348-352. |
| 8. | Chrysos E, Athanasakis E, Saridaki Z, Kafetzakis A, Dimitriadou D, Koutsoumpas V, Chalkiadakis G, Xynos E, Zoras O. Surgical repair of incisional ventral hernias: tension-free technique using prosthetic materials (expanded polytetrafluoroethylene Gore-Tex Dual Mesh). Am Surg. 2000;66:679-682. |
| 9. | Jin J, Rosen MJ, Blatnik J, McGee MF, Williams CP, Marks J, Ponsky J. Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg. 2007;205:654-660. |
| 10. | Parker DM, Armstrong PJ, Frizzi JD, North JH Jr. Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg. 2006;63:255-258. |
| 11. | Bauer JJ, Harris MT, Gorfine SR, Kreel I. Rives-Stoppa procedure for repair of large incisional hernias: experience with 57 patients. Hernia. 2002;6:120-123. |
| 12. | Stoppa RE. The treatment of complicated groin and incisional hernias. World J Surg. 1989;13:545-554. |
| 13. | Lindermann M, Urbach V, Paolucci V. Incisional hernia repair with a bioimplant derived from bovine pericardium. 81 Annual Meeting of the Association of Bavarian Surgeons. 2004 July 21-23; Munich, Germany. |
| 14. | Post C, Koziol D, Doering A, Kahle M. Bovine pericardium for the repair of large abdominal wall hernias. 81 Annual Meeting of the Association of Bavarian Surgeons. 2004 July 21-23; Munich, Germany. |
| 15. | Saettele TM, Bachman SL, Costello CR, Grant SA, Cleveland DS, Loy TS, Kolder DG, Ramshaw BJ. Use of porcine dermal collagen as a prosthetic mesh in a contaminated field for ventral hernia repair: a case report. Hernia. 2007;11:279-285. |
| 16. | Gupta A, Zahriya K, Mullens PL, Salmassi S, Keshishian A. Ventral herniorrhaphy: experience with two different biosynthetic mesh materials, Surgisis and Alloderm. Hernia. 2006;10:419-425. |
| 17. | Kim H, Bruen K, Vargo D. Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg. 2006;192:705-709. |
| 18. | Bellows CF, Albo D, Berger DH, Awad SS. Abdominal wall repair using human acellular dermis. Am J Surg. 2007;194:192-198. |