Published online Apr 21, 2010. doi: 10.3748/wjg.v16.i15.1924
Revised: January 13, 2010
Accepted: January 20, 2010
Published online: April 21, 2010
The association of cystic fibrosis and Crohn’s disease (CD) is well known, but to date, there are very few cases in the literature of patients suffering from mucoviscidosis who have required treatment with infliximab. We report the case of a 23-year-old patient suffering from cystic fibrosis and severe CD treated successfully with infliximab without any infective complications or worsening of the pulmonary disease and with a long term (2 years) complete remission.
- Citation: Vincenzi F, Bizzarri B, Ghiselli A, de’ Angelis N, Fornaroli F, de’ Angelis GL. Cystic fibrosis and Crohn’s disease: Successful treatment and long term remission with infliximab. World J Gastroenterol 2010; 16(15): 1924-1927
- URL: https://www.wjgnet.com/1007-9327/full/v16/i15/1924.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i15.1924
The association of cystic fibrosis (CF) and Crohn’s disease (CD) is well known, but, to date, there are very few cases in the literature of patients suffering from mucoviscidosis who have required treatment with infliximab.
CF is the most common life-threatening autosomal recessive disease in Caucasian children; it has an incidence of 1 case in every 2500 children born alive[1]. CF involves an anomalous function of the exocrine glands, caused by a mutation of a gene (cystic fibrosis transmembrane conductance regulator, CFTR) located on chromosome 7, which codes for a protein involved in ion transport through the cell membrane[1]. More than 1000 mutations of CFTR have been identified, the most common of which is ΔF 508, found in 50% of CF patients. Pulmonary complications are the most common causes of mortality, but the presenting symptoms are very often linked to gastrointestinal and pancreatic biliary diseases. These are mainly caused by the unusual viscosity of the secretions in hollow organs and in the ducts of solid organs[1].
Meconium ileus, intussusception, appendicitis, rectal prolapse, gastro-esophageal reflux, CD and fibrosing colonopathy are the gastrointestinal diseases observed in patients suffering from CF[1,2].
CD is a chronic inflammatory bowel disease which may be localized throughout the gastrointestinal tract. The association between CD and CF is known; there are reports of a prevalence of CD in patients suffering from CF 17 times higher than in controls[3].
CD in patients suffering from CF is, therefore, not an exceptional event; the use of an immunosuppressant such as infliximab in patients suffering from CF may, however, be considered as uncommon, as seen in the lack of data available in the literature. Probably, underlying this lack of data is the fear that the immunosuppressive properties of such a biological treatment would be contraindicated by the characteristic infections in CF, especially of the lung.
We report the case of a 23-year-old patient suffering from CF and severe CD who was treated successfully with infliximab and who is in long term remission.
This is a report of the case of a 23-year-old female suffering from CF who, from the age of 16 years, started having recurrent abdominal pain associated with weight loss. She was regularly followed up by a centre for CF.
At the age of 14 years, she underwent explorative laparotomy with appendicectomy for suspected acute abdomen. The operation was complicated by the appearance of a cutaneous fistula at the site of the surgical wound. Because of persistence of symptoms associated with severe deterioration of nutritional condition, she was sent to our centre. A low digestive endoscopy carried out at our unit showed a condition of acute pancolitis compatible with chronic inflammatory bowel disease. The histological examination of the multiple biopsies taken confirmed the suspected diagnosis of CD.
An induction treatment cycle with prednisone, full dose for 4 wk, with mesalazine and metronidazole was initiated. Having obtained clinical and endoscopic histological remission, we reduced the prednisone dose, stopped metronidazole and continued maintenance treatment with mesalazine. The patient continued treatment with mesalazine alone with good clinical progress for about four years during which she did not come to us for check-ups but preferred to go to the hospital in her city of residence.
In 2003, she underwent emergency laparotomy at another centre following the appearance of acute abdomen. Surgery showed a pericolic abscess collection in the cecum and ascending colon; she was then subjected to ileocecal resection and ileotransverse colonic anastomosis. At two months following the surgery, the girl was treated with steroids, azathioprine and mesalazine for severe flare-up of disease. She continued with low dose steroid treatment (5-10 mg/d) for about two years; treatment with azathioprine was suspended owing to poor efficacy and was replaced with methotrexate (MTX).
During this period of time the patient presented stable respiratory function (spirometry, clinical outcome of exacerbation), but she developed chronic Pseudomonas aeruginosa airway infection. Therefore, ciprofloxacin or trimethoprim sulfamethoxazole or doxycycline was administered for 2 wk twice every 3 mo.
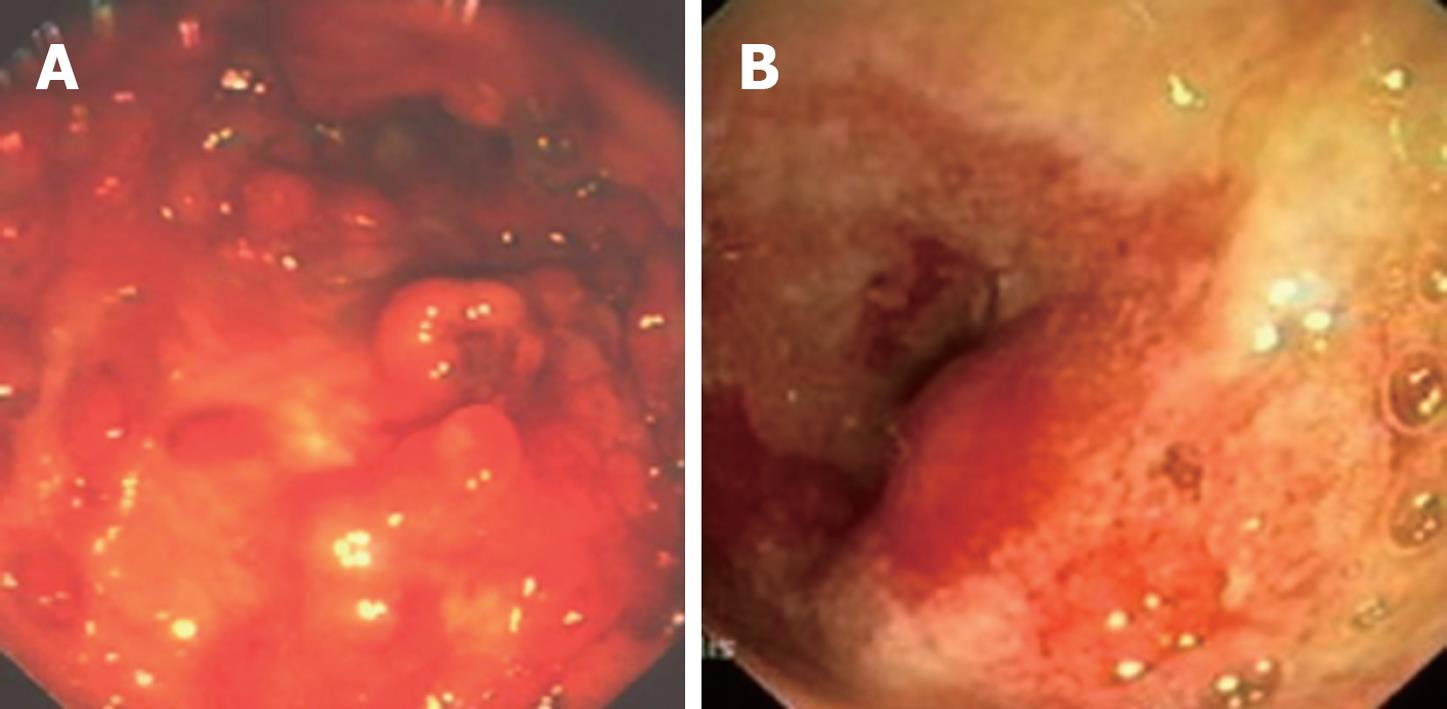
In August 2006, the patient returned to us spontaneously following recurrent episodes of abdominal pain and considerable weight loss (about 10 kg in the previous 2 mo). An ileocolonoscopy carried out under sedoanalgesia showed hyperacute disease of the sigmoid-rectum (Figure 1A and B). Multiple “tags” were present in the anal site with a big “knife-cut” lesion at the level of the posterior commissure. The histological examination confirmed a severe reactivation of her disease. The CD activity index (CDAI) was 390. Index values of 150 and below are associated with quiescent or non-active disease (i.e. “remission”); values over 150 are indicative of active disease, and over 450, extremely severe disease[4]. Total parenteral nutrition was therefore started with complete suspension of fractionated feeding by oral administration and medical treatment was resumed (cortisone, metronidazole as antibiotic and mesalazine as anti-inflammatory).
Due to the steroid therapy, fasting blood glucose pathologically increased (320 mg/dL) and therefore subcutaneous insulin therapy was started with an improvement of glucose intolerance.
Endoscopic histological examination performed after a short time (4 wk), showed only modest improvement of the lesions found earlier during the endoscopic examination, despite improvement of the general condition.
Because of the severity of the lesions, particularly of the anus, and after informing the patient and the pneumologists, we decided to start a biological treatment cycle with infliximab in addition to treatment with azathioprine (AZA) (2.5 mg/kg per day) in a single administration in the morning, associated with antibiotic treatment with 3rd generation cephalosporin and glycopeptide. The infusions of infliximab can be superimposed with this treatment regime, which is used in our centre, with an initial treatment at 0, 2, and 6 wk, followed by a maintenance phase with infusions every 8 wk.
At the discontinuation of steroids, insulin therapy was no longer necessary.
After the first three intravenous infusions of infliximab, endoscopic examination showed complete regression of anal lesions and distinct improvement of the lesions of the sigmoid rectum, with healing appearance of the mucosa and no continuous lesions up to the ileocolic anastomosis.
The appearance of the ileum, explored to about 40 cm, was within acceptable limits. Nine months after beginning the treatment and after 7 infusions of infliximab, the patient showed distinct improvement in her general condition and body weight increased by 13 kg.
Twelve months after commencement of the treatment, condition continues to be optimal and there has been no complication involving infection in the lung or septic episodes. The working of the lungs was kept stable during the course of treatment with infliximab (forced expiratory volume in 1 s (FEV1) 85% in April 2006 and FEV1 80% in July 2007), without any pulmonary exacerbations.
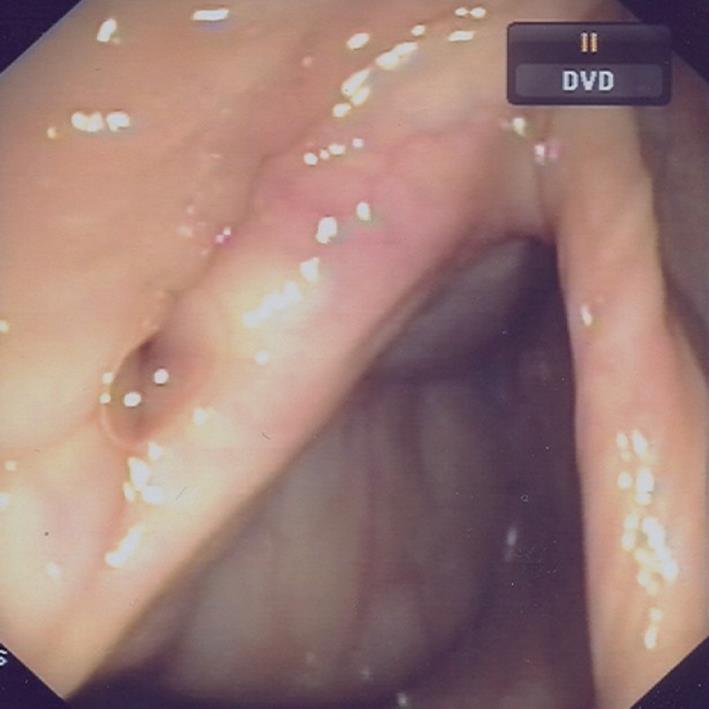
Suspension of the biological treatment was therefore programmed after the 10th infusion, while treatment with AZA and mesalazine as maintenance continued. Since the last infliximab infusion two years have passed and a complete remission of CD has been maintained both clinically and endoscopically (Figure 2).
This report deals with the case of a patient suffering from CF who, at the age of 16 years, was diagnosed as suffering from CD. The literature reports frequent cases of CD during the course of CF. The prevalence is about 17 times greater than that of controls[3]; this shows that there is a pathogenic relationship between the two diseases. The etiopathogenesis has not yet been identified but a number of mechanisms have been proposed, the most probable of which is an altered immune response to a chronic infection[4]. Furthermore, patients who are carriers of the ΔF 508 mutation seem to have an increased risk of developing gastrointestinal problems[5].
The literature contains case reports of diagnosis of CD in patients suffering from CF who complain of long periods of abdominal pain and most of all, loss of weight, in spite of appropriate nutritional and therapeutic support[6,7]. As in the case of our patient, protracted gastrointestinal symptoms and lack of response to basic treatment make it necessary to conduct instrumental examinations, in particular endoscopic examinations such as gastroscopy and colonoscopy, associated with perendoscopic biopsies, which allow a differential diagnosis between CD and fibrosing colonopathy[7,8]. This has led, in the last few years, to an increase in the diagnosis of CD during the course of CF[7], which was not suspected in the past. The aim of treatment during the course of CD is to induce and maintain remission of the disease. Corticosteroids have demonstrated a high efficiency in inducing clinical remission in patients with moderate to severe forms of disease[9,10]. In order to maintain the remission and to reduce the risk of dependence on corticosteroids, there is general agreement regarding the early introduction of immunosuppressants such as AZA, MTX, 6 mercaptopurine (MP) and also infliximab[11-13]. In the case of our patient, given the modest clinical and endoscopic response after one month of treatment with steroids, this therapeutic opportunity was also discussed with the pneumologists. Biological treatment with infliximab associated with AZA was therefore started, because of the presence of significant anal lesions, although the literature currently contains few case reports of patients suffering from CF treated with infliximab. However, numerous studies have shown the efficacy of infliximab in inducing or maintaining remission of disease in adults suffering from CD who have moderate-severe forms, with perianal localization or with fistulizing forms[9,10,14-17]. In patients with CF the presence of bronchiectasis and colonization with Pseudomonas, as in our patient, would normally represent a relative contraindication to the administration of infliximab, but numerous studies of bronchoalveolar lavage fluid from patients with CF have shown a high concentration of inflammatory mediators such as tumor necrosis factor α (TNFα)[18]. Indeed, drugs such as azithromycin ameliorate lung function in cystic fibrosis patients while also reducing the levels of TNFα[19].
In our experience with this patient, we observed a significant clinical improvement even after the first dose. This improvement was endorsed by the resolving of endoscopic and histological lesions, as is also reported in the literature[9,20,21]. The association of infliximab with AZA was justified by the fact that concurrent treatment with AZA, 6 MP or MTX may be helpful in maintaining the clinical response to infliximab, reducing the amount of circulating antibodies directed towards the latter; which are thought to be responsible for some cases of non response[22-25].
As shown in the literature[18,19], as well as in the case of our patient, the moment a treatment focused for CD was introduced, although not free of risks, a distinct improvement of the general condition was observed with weight increase of about 13 kg. After over 12 mo of treatment with infliximab and AZA, no pulmonary complication which would have compelled suspension of treatment was observed. In addition, the literature shows that no severe adverse events have occurred and that there was no reported increase in the prevalence of respiratory tract infections during infliximab administration in patients with chronic obstructive pulmonary disease[26].
The main questions arising from treatment with infliximab in this patient were, firstly, the possibility that the biological treatment could increase susceptibility to infections by opportunistic pathogens given the concurrent basic lung disease, since patients with cystic fibrosis already spontaneously suffer from such infections. Moreover, the infections most frequently reported during infliximab infusions are respiratory tract infections and urinary tract infections (35% infliximab-treated patients vs 25% placebo recipients)[27].
Secondly, given the excellent clinical, endoscopic, and histological progress of the patient, there was doubt regarding the best moment for suspension of the drug, especially taking into consideration the possibility of causing allergies or reduced efficacy in case of re-treatment because of the possible presence of antibodies against infliximab[9,22,23,25,28,29].
As regards the first challenge, the constant cover with antibiotic treatment was found to be effective in keeping the lung disease under control, as it did not worsen but rather remained stable as is shown by the spirometry tests taken during the course of immunosuppressive treatment.
As regards suspension of treatment, in the absence of precise bibliographic guidance, we believed that it was appropriate to suspend treatment in accordance with previously reported data after a period of not less than one year[30].
Peer reviewers: Dr. Marco Scarpa, PhD, Department of Surgical & Gastroenterological Sciences (Gastroenterology section), University of Padova, via Giustiniani 2, Padova 35128, Italy; Stefan Riss, MD, Department of General Surgery, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria
S- Editor Wang JL L- Editor Logan S E- Editor Lin YP
| 1. | Chaudry G, Navarro OM, Levine DS, Oudjhane K. Abdominal manifestations of cystic fibrosis in children. Pediatr Radiol. 2006;36:233-240. |
| 2. | Fields TM, Michel SJ, Butler CL, Kriss VM, Albers SL. Abdominal manifestations of cystic fibrosis in older children and adults. AJR Am J Roentgenol. 2006;187:1199-1203. |
| 3. | Dobbin CJ, Moriarty C, Bye PT. Granulomatous diseases in a patient with cystic fibrosis. J Cyst Fibros. 2003;2:35-37. |
| 4. | Lerner A, Gal N, Mares AJ, Maor E, Iancu TC. Pitfall in diagnosis of Crohn's disease in a cystic fibrosis patient. J Pediatr Gastroenterol Nutr. 1991;12:369-371. |
| 5. | Lloyd-Still JD. Crohn's disease and cystic fibrosis. Dig Dis Sci. 1994;39:880-885. |
| 6. | Baxter PS, Dickson JA, Variend S, Taylor CJ. Intestinal disease in cystic fibrosis. Arch Dis Child. 1988;63:1496-1497. |
| 7. | Modolell I, Alvarez A, Guarner L, De Gracia J, Malagelada JR. Gastrointestinal, liver, and pancreatic involvement in adult patients with cystic fibrosis. Pancreas. 2001;22:395-399. |
| 8. | Lowe ME, Ameen N, Freedman S, Mulberg AE, Werlin SL. Research agenda for pediatric gastroenterology, hepatology and nutrition: cystic fibrosis and pancreatic diseases. Report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition for the Children's Digestive Health and Nutrition Foundation. J Pediatr Gastroenterol Nutr. 2002;35 Suppl 3:S258-S262. |
| 9. | Hyams JS, Markowitz JF. Can we alter the natural history of Crohn disease in children? J Pediatr Gastroenterol Nutr. 2005;40:262-272. |
| 10. | Travis SP, Stange EF, Lémann M, Oresland T, Chowers Y, Forbes A, D'Haens G, Kitis G, Cortot A, Prantera C. European evidence based consensus on the diagnosis and management of Crohn's disease: current management. Gut. 2006;55 Suppl 1:i16-i35. |
| 11. | Markowitz J, Grancher K, Kohn N, Daum F. Immunomodulatory therapy for pediatric inflammatory bowel disease: changing patterns of use, 1990-2000. Am J Gastroenterol. 2002;97:928-932. |
| 12. | Jacobstein DA, Mamula P, Markowitz JE, Leonard M, Baldassano RN. Predictors of immunomodulator use as early therapy in pediatric Crohn's disease. J Clin Gastroenterol. 2006;40:145-148. |
| 13. | Jaspers GJ, Verkade HJ, Escher JC, de Ridder L, Taminiau JA, Rings EH. Azathioprine maintains first remission in newly diagnosed pediatric Crohn's disease. Inflamm Bowel Dis. 2006;12:831-836. |
| 14. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. |
| 15. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. |
| 16. | Hyams JS, Markowitz J, Wyllie R. Use of infliximab in the treatment of Crohn's disease in children and adolescents. J Pediatr. 2000;137:192-196. |
| 17. | Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, Winter HS. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn's disease. Am J Gastroenterol. 2003;98:833-838. |
| 18. | Casserly B, Donat W. Stabilization of lung function and clinical symptoms in a patient with cystic fibrosis (CF) after institution of infliximab: a monoclonal antibody that binds tumor necrosis factor alpha. Lung. 2009;187:149-152. |
| 19. | Cigana C, Assael BM, Melotti P. Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrob Agents Chemother. 2007;51:975-981. |
| 20. | Borrelli O, Bascietto C, Viola F, Bueno de Mesquita M, Barbato M, Mancini V, Bosco S, Cucchiara S. Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn's disease. Dig Liver Dis. 2004;36:342-347. |
| 21. | D'haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999;116:1029-1034. |
| 22. | Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601-608. |
| 23. | Miele E, Markowitz JE, Mamula P, Baldassano RN. Human antichimeric antibody in children and young adults with inflammatory bowel disease receiving infliximab. J Pediatr Gastroenterol Nutr. 2004;38:502-508. |
| 24. | Lémann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, Laharie D, Moreau J, Cadiot G, Picon L. Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054-1061. |
| 25. | Ricart E, Panaccione R, Loftus EV, Tremaine WJ, Sandborn WJ. Infliximab for Crohn's disease in clinical practice at the Mayo Clinic: the first 100 patients. Am J Gastroenterol. 2001;96:722-729. |
| 26. | van der Vaart H, Koëter GH, Postma DS, Kauffman HF, ten Hacken NH. First study of infliximab treatment in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:465-469. |
| 27. | Blonski W, Lichtenstein GR. Safety of biologic therapy. Inflamm Bowel Dis. 2007;13:769-796. |
| 28. | Rodrigo L, Pérez-Pariente JM, Fuentes D, Cadahia V, García-Carbonero A, Niño P, de Francisco R, Tojo R, Moreno M, González-Ballina E. Retreatment and maintenance therapy with infliximab in fistulizing Crohn's disease. Rev Esp Enferm Dig. 2004;96:548-554; 554-558. |
| 29. | Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, Olson A, Bao W, Rutgeerts P. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol. 2004;2:542-553. |
| 30. | Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;CD006893. |