Published online Apr 21, 2010. doi: 10.3748/wjg.v16.i15.1811
Revised: January 18, 2010
Accepted: January 25, 2010
Published online: April 21, 2010
Acute liver failure (ALF) is a devastating clinical syndrome characterised by progressive encephalopathy, coagulopathy, and circulatory dysfunction, which commonly leads to multiorgan failure and death. Central to the pathogenesis of ALF is activation of the immune system with mobilisation of cellular effectors and massive production of cytokines. As key components of the innate immune system, monocytes and macrophages are postulated to play a central role in the initiation, progression and resolution of ALF. ALF in humans follows a rapidly progressive clinical course that poses inherent difficulties in delineating the role of these pivotal immune cells. Therefore, a number of experimental models have been used to study the pathogenesis of ALF. Here we consider the evidence from experimental and human studies of ALF on the role of monocytes and macrophages in acute hepatic injury and the ensuing extrahepatic manifestations, including functional monocyte deactivation and multiple organ failure.
- Citation: Possamai LA, Antoniades CG, Anstee QM, Quaglia A, Vergani D, Thursz M, Wendon J. Role of monocytes and macrophages in experimental and human acute liver failure. World J Gastroenterol 2010; 16(15): 1811-1819
- URL: https://www.wjgnet.com/1007-9327/full/v16/i15/1811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i15.1811
Acute liver failure (ALF) is characterised by overwhelming hepatic injury with failure of hepatocyte function, resulting in a devastating clinical syndrome of coagulopathy, encephalopathy, and circulatory dysfunction[1,2]. Phenotypically, there are striking similarities between septic shock and ALF. Both conditions are characterised by activation of a systemic inflammatory response syndrome (SIRS), resulting in circulatory dysfunction with systemic vasodilatation and refractory multiorgan failure[3]. ALF, like septic shock, is associated with an overwhelming activation of the immune response, including the production of inflammatory cytokines and mobilisation of cellular components of the immune system. Monocytes and macrophages are key orchestrators of the innate immune system and are postulated to play a pivotal role in the initiation, propagation and resolution of ALF.
The paucity of effective clinical treatments for ALF, except for supportive care and liver transplantation, reflects our poor understanding of the pathogenesis of ALF. Clinical trials and studies on patients with ALF are limited by small numbers of subjects with heterogeneous causes of ALF and are confounded by inherent difficulties in studying patients with rapidly progressive pathology. There are a number of animal models of acute liver injury commonly used to study ALF: murine models of acetaminophen (APAP) toxicity, concanavalin-A (Con-A) T-cell mediated hepatitis, carbon tetrachloride (CCl4)-induced injury, and other models including galactosamine/lipopolysaccharide (Gal/LPS) (for a review[4]). Unfortunately, these are models of acute liver injury and cannot truly reflect the human syndrome of ALF.
In this article we consider the role of macrophages, monocytes, and the inflammatory cytokines they produce, in the progression of acute liver failure in human and experimental models. We consider monocyte/macrophage function in the hepatic and systemic compartments, including their role in initial injury, the process of recruitment of monocytes to the injured liver and the possible influence of hepatic inflammatory events on the functional monocyte deactivation encountered in ALF.
Following an acute hepatic insult, there is a rapid and marked increase in the number of inflammatory cells within the liver. Recent studies have shed light on the process by which circulating monocytes are recruited to the injured liver, where, due to their plasticity, macrophages are implicated in both tissue destructive and reparatory processes of the inflammatory response.
Karlmark et al[5] demonstrated, in a murine model of acute CCl4 hepatotoxicity, that a sub-population of infiltrating macrophages account for 10%-12% of total hepatic cells 24-48 h after the onset of liver injury. This massive recruitment of macrophages and their contribution to evolving acute liver failure have attracted much interest recently. Two distinct populations of macrophage have been described in both APAP and acute CCl4 models of hepatotoxicity[5,6]. The first is a CD11b+ F4/80- population that resembles the sub-group of circulating monocytes that are the origin of stable populations of tissue macrophages. This population shows only a modest increase in number following acute liver injury by CCl4. The second population is CD11b+ F4/80+ and this resembles the subset of circulating monocytes from which activated macrophages in inflammatory conditions are thought to be derived. It is this population that shows marked expansion in number following acute hepatic injury[5].
Holt et al[6] 2008, in an APAP model of hepatoxicity, showed that these “inflammatory macrophages” were derived from circulating monocytes rather than from proliferation of the resident Kupffer cell (KC) population. They noted preservation of the macrophage response to acute APAP toxicity when the KC population was depleted prior to APAP challenge. Conversely, when circulating monocytes were depleted by bone marrow irradiation prior to APAP, the number of intrahepatic macrophages seen after tissue injury was diminished.
Monocyte chemoattractant protein-1 (MCP-1) is a member of the C-C chemokine family, which acts on the chemokine (C-C motif) receptor 2 (CCR2) that is expressed on macrophages, monocytes, and to a lesser extent, T and NK cells[7]. Though initially described as a monocyte chemokine it also plays a role in the recruitment of NK cells and T cells in a wide range of inflammatory conditions[8]. An early increase in hepatic expression of MCP-1 and other chemokines is a consistent feature of murine models of ALF. An increase in MCP-1 mRNA or total hepatic levels of MCP-1 have been reported within 12 h of liver insult in APAP, Con-A, Gal/LPS, and CCl4 models of acute liver failure[5,9-12]. The origin of hepatic MCP-1 in ALF is thought to be both resident KCs and injured hepatocytes[13].
Experiments using CCR2 -/- knockout mice suggest that functional CCR2 is necessary for egress of F4/80+ monocytes from the bone marrow. In the acute liver injury model induced by CCl4, CCR2 -/- mice demonstrated a decreased number of hepatic and serum CD11b+ F4/80+ cells following liver injury, but an increase in this cellular fraction in bone marrow when compared with wild-type mice[10]. When this population of monocytes was adoptively transferred into the circulation of CCR2 -/- mice they were able to traffic unhindered into the injured liver[5]. Similarly to the liver injury model, CCR2 has been shown to be essential for monocyte mobilisation from the bone marrow, but not for efflux into inflamed tissues, such as in murine CMV, L. monocytogenes, and urinary tract infection models[8,14,15].
This evidence suggests that hepatic-derived MCP-1 is able to stimulate the expansion of a bone marrow population of CD11b+ F4/80+ monocytes, and CCR2 is necessary for these cells to exit the bone marrow, but not essential for influx into the injured liver[5].
In human ALF, there is also evidence of early upregulation of hepatic chemokines. Leifeld et al[9] demonstrated serum MCP-1 levels that were, on average, five times higher in patients with ALF when compared to patients with self-limiting acute hepatitis. MCP-1 levels were also significantly elevated above healthy control populations and those with chronic liver disease. There was a trend suggesting that higher serum MCP-1 levels were associated with poor outcome (death or transplantation) from ALF. Upregulation of chemokines, including MCP-1, was demonstrated by reverse transcriptase-polymerase chain reaction (RT-PCR) and immunohistochemistry. The latter technique showed that MCP-1 expression was not limited to inflammatory cells only, but was also detected in hepatocytes, biliary epithelial cells, and sinusoidal endothelial cells (SEC) in human ALF.
These findings have been replicated in patients with acute severe alcoholic hepatitis with clinical liver failure. MCP-1 is seen to be upregulated throughout the liver, with expression observed in hepatocytes and sinusoidal endothelial cells[16]. Conversely, in control patients with stable alcoholic liver disease, MCP-1 expression is limited to non-parenchymal cells.
In paediatric patients with acute acetaminophen hepatotoxicity, serum MCP-1 levels showed a strong correlation with clinical parameters of severe liver injury, including elevated levels in patients in whom treatment with N-acetyl cysteine was delayed[17].
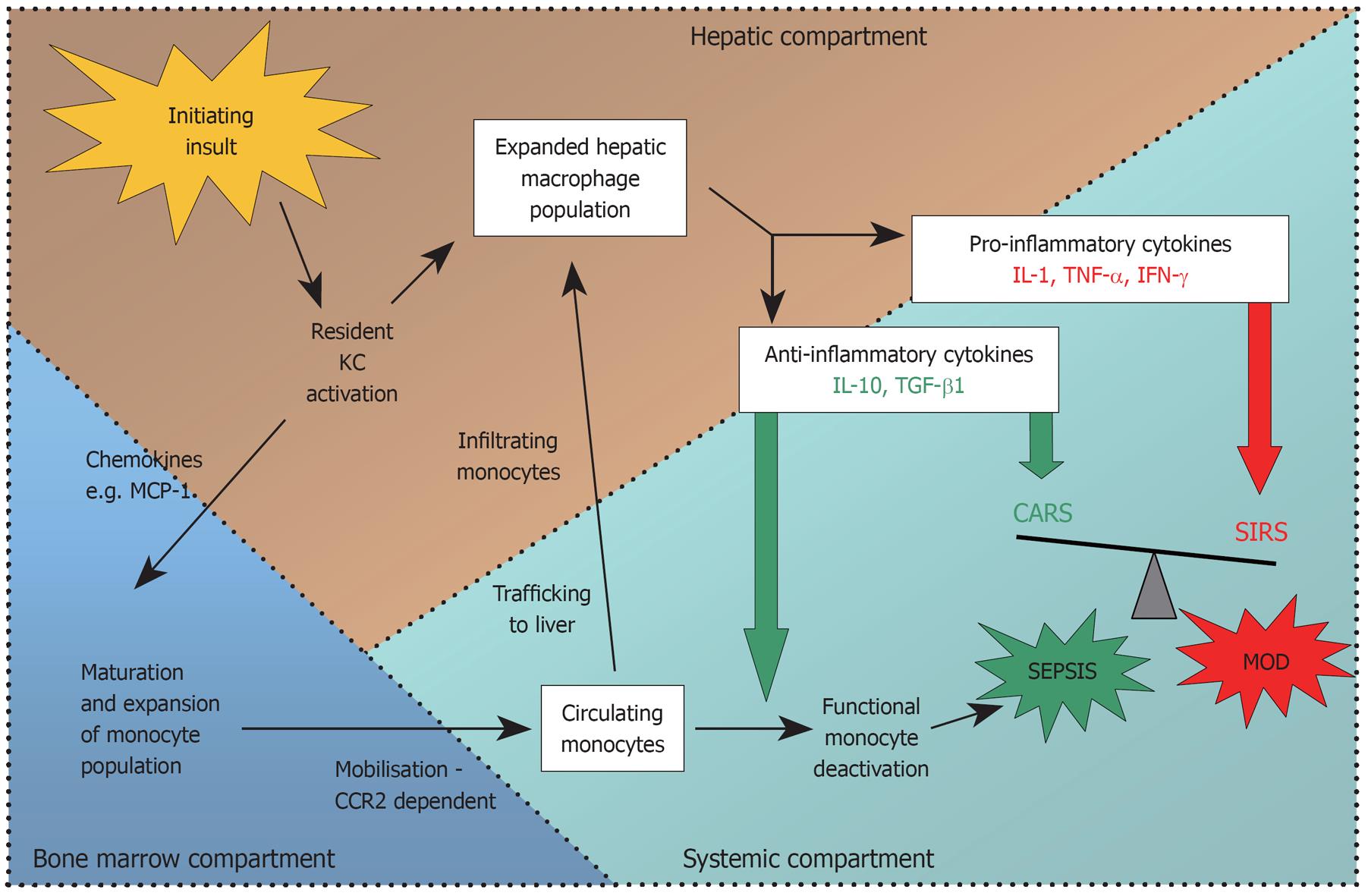
Thus evidence from both murine and human studies suggests that early release of chemokines from injured hepatocytes, SECs and KCs in ALF is responsible for the mobilisation and subsequent trafficking of a population of activated monocytes/macrophages to the liver from the bone marrow (summarised in Figure 1).
Having established that macrophages are recruited to the liver in acute injury, the challenge that remains is determining what role these cells play in liver damage or repair.
Two early studies in animal models of APAP-induced hepatotoxicity suggested that depletion of macrophage numbers with gadolinium chloride or dextran sulphate prior to APAP dosing offered protection from liver damage[18,19]. However, neither of these studies confirmed successful depletion of intrahepatic macrophages, and in one study, the number of intrahepatic F4/80+ cells post-APAP treatment was actually increased, suggesting their prior treatment with KC depleting agents had not prevented an expansion in the macrophage population following the toxic insult[19]. Subsequent studies have used the more effective technique of macrophage depletion with liposome/clodronate. An APAP model of acute liver failure has shown macrophage depletion increases sensitivity to toxic injury, as measured at 8 and 24 h post-APAP alanine transaminase (ALT) values[20]. Conversely, in an acute CCl4 model, macrophage depletion was reported to have no effect on ALT levels four and 24 h after toxin administration[5].
Prevention of liver injury-induced macrophage population expansion by inhibition of the MCP-1/CCR2 interaction has been demonstrated in a number of studies using CCR2 knockout mice. As described above, these animals show a marked reduction in intrahepatic macrophages following liver injury compared with wild-type (WT) mice, due to failure of monocyte recruitment from bone marrow. Two studies in CCR2 -/- mice treated with toxic doses of APAP have shown ALT levels in the first 24 h comparable to WT controls[6,13]. Histological evidence of liver damage was also comparable between CCR2 -/- and WT mice at 24 h in both studies. At later time points, the two studies showed divergent results, with Dambach et al[13] showing less inflammation in CCR2 -/- mice. However, Holt et al[6] described CCR2 -/- mice showing persisting necrosis and ongoing inflammation at 72 h, whereas the wild-type animals showed almost complete histological resolution. Hogaboam et al[12] used a similar model of acute APAP toxicity in CCR2 -/- mice and results seemed to concur with Holt’s study, showing a worsening of liver injury by ALT values and histology in the knockout animals at all time points when compared to WT controls.
Overall current evidence in murine models is conflicting (Table 1). It appears that depletion of resident KCs prior to, and at the onset of, liver injury reduces the extent of liver damage. Kupffer cells are partly responsible for the initial pro-inflammatory response to injury with cytokine production and the recruitment of multiple inflammatory effector cells including neutrophils, NK cells, NKT cells, and T cells that mediate tissue injury. However, there is some evidence to suggest that the population of hepatic macrophages derived from newly recruited infiltrating monocytes possess anti-inflammatory activity and may play a role in recovery from acute liver damage. Though it is beyond the scope of this review to discuss in detail, evidence also suggests that it is this same population of immigrant macrophages that is responsible for driving the fibrosis that follows acute liver injury[5,10].
| Study | Model | Method of macrophage depletion/inhibition | Affect on severity of acute liver injury | Conclusion |
| Laskin et al[18], 1995 | APAP (rat) | Gadolinium chloride/dextran sulphate | Decreased ALT at 24 h in treated groups; Decreased necrosis | Macrophage depletion was protective |
| Michael et al[19], 1999 | APAP (mouse) | Gadolinium chloride/dextran sulphate | Decreased ALT at 8 h in treated groups | Macrophage depletion was protective |
| Hogaboam et al[12], 2000 | APAP (mouse) | CCR2 -/- | ALT at 24 and 48 h, hepatic necrosis and TUNEL staining all increased in KO; Increase in IFN-γ and TNF-α | CCR2 KO - macrophage depletion worsened liver injury |
| Dambach et al[13], 2002 | APAP (mouse) | CCR2 -/- | ALT levels similar in WT and KO mice; Histologically KO mice showed less inflammation at 72 h | CCR2 KO - macrophage depletion, caused less inflammation at 72 h but no overall difference in outcome |
| Ju et al[20], 2002 | APAP (mouse) | Liposome/clodronate | Increased ALT at 8 and 24 h in treated group | Macrophage depletion increased liver damage |
| Holt et al[6], 2008 | APAP (mouse) | CCR2 -/- | ALT same at 10 and 24 h; Comparable histological necrosis at 24 h but delayed recovery at 48 and 72 h in CCR2 -/- | Reduction in infiltrating macrophage population causes delayed recovery |
| Karlmark et al[5], 2009 | CCl4 (mouse) | Liposome/clodronate | Unaltered ALT level at 4 and 24 h post-CCl4 | Reduction in infiltrating macrophages had no effect on severity of liver damage |
| Cytokine | Model | Source | Mechanism of action |
| TNF-α | Murine hepatic ischaemia[21,22] | Macrophage | Hepatocellular apoptosis |
| Murine α-amanitin or actinomycin D[23] | Cellular recruitment | ||
| Murine Gal/LPS[24] | |||
| Murine Con-A[25] | |||
| Rat Gal/LPS[26] | |||
| IFN-γ | Murine Gal/LPS[27] | Macrophages | Induce iNOS |
| Murine APAP[28] | NK and T cells | Upregulates Fas, sensitising hepatocytes to apoptosis | |
| Murine Con-A[29,30] | Upregulates adhesion molecules and chemokines resulting in leucocyte accumulation | ||
| MIF | Guinea pig halothane[31] | Preformed stores released from hepatocytes and KCs early in injury | Stimulate release of proinflammatory cytokines |
| Murine APAP[31] | Counter-act glucocorticoid anti-inflammatory effects | ||
| Murine LPS in BCG-primed mice[32] | Cellular recruitment | ||
| Rat ethanol[33] | |||
| IL-6 | Murine APAP[22,34,35] | Released from macrophages and T cells | Reduced TNF-α secretion |
| Murine alcohol and TNF-α[36] | Activation of STAT3 signalling pathway | ||
| Murine Con-A[37,38] | Induction of anti-apoptotic proteins e.g. Bcl-2, Bcl-XL, FLIP | ||
| Murine Acute CCl4[39] | |||
| Murine Fas-mediated apoptosis[40] | |||
| Murine ischemia/reperfusion[41] | |||
| IL-10 | Murine Gal/LPS[27,42,43] | Macrophages/monocytes and injured hepatocytes | Inhibition of TNF-α, IFN-γ secretion |
| Murine CCl4[44] | |||
| Rat CCl4[45] | |||
| Murine APAP[46] | |||
| Murine Con-A[47] |
Tumour necrosis factor-α: Tumour necrosis factor-α (TNF-α), a macrophage derived pro-inflammatory cytokine, has a central role in the pathogenesis of many inflammatory clinical syndromes. TNF-α can enhance the production of other pro-inflammatory cytokines and provoke cellular recruitment to sites of injury. It also has an important direct effector function by inducing hepatocellular apoptosis through its interaction with TNF-receptor 1. This cell surface receptor is connected to an intracellular death domain whose activation ultimately leads to the induction of effector caspases and the execution of cellular apoptosis.
In experimental models of acute liver failure, elevated serum levels of TNF-α and increased mRNA have been observed shortly after the initiating insult[21,25,48]. Studies in the murine Gal/LPS and Con-A models of hepatic failure have shown that treatment with neutralising anti-TNF antibodies provides protection against liver damage[24,25]. A recent study using targeted antisense oligonucleotides to inhibit expression of the TNF-α gene, limited the hepatic damage and subsequent mortality from Gal/LPS in a rat model of acute hepatic failure[26]. TNF-α-driven hepatocyte necrosis through the TNF-R1 pathway has been well described in a number of experimental models of acute liver injury[21,23,49].
Interferon-γ: Interferon-γ (IFN-γ) is a pro-inflammatory cytokine released by T-cells, NK cells, and macrophages. It has a variety of anti-viral, anti-bacterial and pro-inflammatory effects. In models of acute liver injury, increases in serum IFN-γ and intrahepatic IFN-γ mRNA are seen as part of the initial response to injury[28,29]. IFN-γ -/- knockout mice show resistance to toxic and T-cell mediated acute liver failure, with improved survival and reduced hepatic necrosis and apoptosis[30]. Neutralisation of IFN-γ with antibodies in WT mice confers a dose-dependent protective effect following APAP or Con-A administration[28,29].
IFN-γ deficient mice show a decrease in Fas induction after liver injury; hence it has been postulated that IFN-γ contributes to liver injury by the upregulation of Fas on hepatocytes, rendering them susceptible to apoptotic cell death[30].
Macrophage migration inhibitory factor: Macrophage migration inhibitory factor (MIF) is a multi-functional inflammatory cytokine that is constitutively expressed in many cell types, including centrilobular hepatocytes and Kupffer cells[50]. Pre-formed MIF is released early in response to stress (glucocorticoid) and infectious stimuli (LPS). It has an important role in stimulating the inflammatory response through cellular recruitment, stimulation of cytokine production, and counteracting the inhibitory effect of glucocorticoid on the production of pro-inflammatory cytokines. Though initially described as a T-cell cytokine, it has since been established that macrophages are an important source of MIF[51]. An increase in MIF mRNA is seen in KCs following acute injury[50].
MIF has a role in a number of experimental models of acute liver injury[31-33]. Significant increases in serum MIF are detectable in the early stages following acute liver injury by toxins or LPS[31,32]. MIF -/- knockout mice demonstrate resistance to APAP-induced hepatotoxicity, displaying lower ALT levels, less histological necrosis, and improved survival compared to WT counterparts[31]. In a BCG-LPS model of murine ALF pre-treatment with anti-MIF antibodies prevented the development of liver failure and death in response to LPS challenge[32].
Interleukin-6: Interleukin-6 (IL-6) is a macrophage and T cell-derived multifunctional cytokine with both pro- and anti-inflammatory actions. In a number of acute liver injury models, IL-6 has been shown to play an important protective role. IL-6 -/- knockout mice display increased sensitivity to liver injury, impaired regeneration, and poor outcomes in APAP[22,34], alcohol and TNF-α-mediated liver cell apoptosis[36], acute CCl4 toxicity[39], Fas-mediated apoptosis[40], and ischaemia-reperfusion injury[41]. In a number of these experiments, the treatment of knockout and wild-type mice with recombinant IL-6 prior to the liver insult conferred protection against hepatic damage[37,39-41]. Experimental evidence suggests the hepatoprotective effects of IL-6 in acute liver damage are mediated through the activation of the STAT3 signalling pathway, with induction of anti-apoptotic proteins such as Bcl-2, Bcl-XL, and FLIP and inhibition of NKT cells via targeting of CD4+ T cells[38,39,52].
Interleukin-10: IL-10 is a pleiotropic anti-inflammatory cytokine that is capable of downregulating several aspects of the pro-inflammatory cascade, including activated macrophage function. IL-10 is secreted from various cell types, including monocytes and macrophages, in response to injury. The liver is a major source of IL-10 in systemic inflammatory conditions.
In experimental models of acute liver injury, IL-10 has consistently been shown to be protective against liver damage[27,42-47]. IL-10 serum levels and mRNA expression in liver tissue are seen to rise early after hepatic insult in APAP, Con-A, and Gal/LPS models[42,46,47]. The kinetics of IL-10 production parallel TNF-α secretion in a murine Gal/LPS model, suggesting both pro- and inflammatory cytokines are released together and act concurrently during the early acute inflammatory response[42].
In a Con-A model of ALF, neutralisation of IL-10 with monoclonal antibodies exacerbated liver damage, as measured by serum transaminases and liver histology. Serum levels of TNF-α, IFN-γ, and IL-12 were augmented by neutralisation of IL-10[47]. Similar findings were demonstrated in an APAP model utilising IL-10 knockout mice, where the absence of IL-10 was associated with increased ALT, worse histological necrosis, and reduced survival. Again this increased susceptibility seemed to be attributable to upregulation of pro-inflammatory cytokines and the effectors TNF-α, IL-1, and iNOS[46]. Treatment with recombinant IL-10 in Gal/LPS models caused a dose-dependent reduction in liver damage and hepatic and serum TNF-α expression[27,42,43]. In vitro work on isolated rat hepatocytes demonstrated that IL-10 was expressed early following LPS challenge. Treatment of KCs with exogenous IL-10 downregulated production of superoxide dismutase and TNF-α responses following LPS challenge; a phenomenon analogous to the functional monocyte deactivation described later[45].
Thus, in animal models of acute liver injury there is consistent evidence that IL-10 is expressed in the early phases of liver damage, simultaneous to pro-inflammatory effectors. It acts to abrogate liver injury by limiting the release of pro-inflammatory cytokines and effectors. In vivo it is likely to be the balance of these inter-related and mutually regulating factors that determine the extent and outcome of the hepatic insult.
As in experimental models of acute liver failure, ALF in humans is known to be associated with massive activation of pro- and anti-inflammatory mediators. A number of studies have looked at patient populations and correlated serum levels of inflammatory cytokines with clinical parameters of ALF severity. TNF-α has been shown in a number of clinical cohorts to be elevated in patients with ALF compared with controls with acute hepatitis or chronic liver disease[53-57]. In two of these studies, elevated TNF-α levels correlated with poor clinical outcomes[53,58]. IL-6 and IL-10 have been shown to possess hepatoprotective properties in experimental models of ALF models[53-56,59]. However, human studies of ALF have detected higher levels of IL-6 and -10 in non-surviving ALF patients compared to spontaneous survivors[53,56,59].
Interestingly, a number of recent studies have measured proxy markers of macrophage and monocyte activation and shown that increases in these markers correlate with poor outcome in patients with ALF. CD163 is a macrophage lineage-specific scavenger receptor that is expressed by activated macrophages (and to a lesser extent by circulating monocytes) and can be shed in the circulation as a soluble receptor[60]. Hiraoka et al[60] showed that, in a small population of patients with ALF, mean levels of serum CD163 were elevated when compared with either healthy controls or patients with acute hepatitis. There was a trend towards increasing CD163 in non-survivors, however this did not reach significance. Møller et al[61] used a larger cohort from the US ALF study group and replicated the finding of elevated CD163 in ALF patients compared with controls. They also showed a significant difference between CD163 levels on day three between survivors and non-survivors.
Infectious complications are a common feature of established acute liver failure and account for a significant proportion of overall mortality[1,62]. A number of recent studies have investigated the phenomenon of functional monocyte deactivation that occurs in patients with acute and acute-on-chronic liver failure, and that might be partially responsible for the observed susceptibility of these patients to overwhelming sepsis[53,63-65].
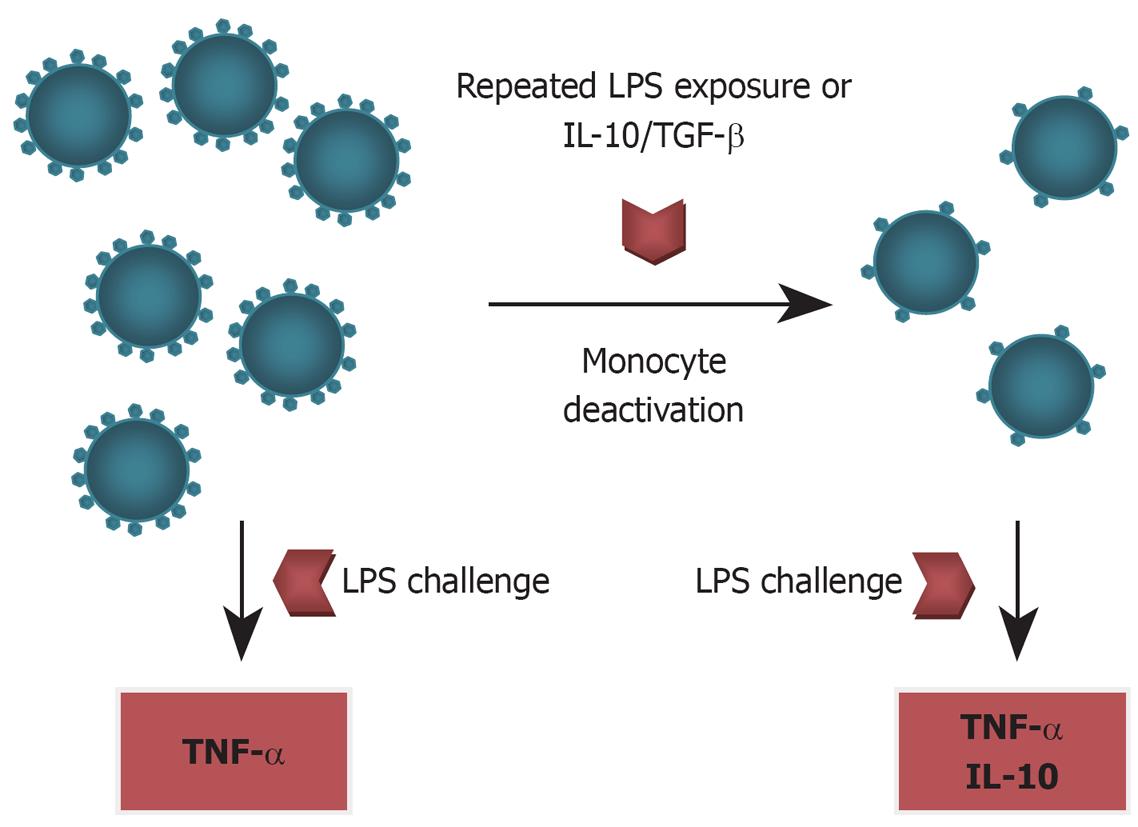
Monocyte deactivation was first described in the early 1990s in clinical populations of post-trauma and sepsis patients[58,66]. This phenomenon shares many features with the experimental model of “endotoxin tolerance”, which is characterised by a refractory monocyte phenotype with decreased expression of HLA-DR, impaired ability to present antigens, and a profoundly attenuated TNF-α response to LPS challenge (Figure 2). This monocyte phenotype can be induced experimentally by repeated LPS challenge (hence endotoxin tolerance) or exposure to IL-10 and TGF-β[66].
Antoniades et al[53] demonstrated monocyte deactivation in a group of patients with acetaminophen-induced acute liver failure (AALF). Patients with AALF who had adverse outcomes (death or transplantation) had significantly lower expression of HLA-DR than transplant-free survivors with AALF, control patients with chronic liver disease, and healthy controls. A reduction in the percentage and total number of HLA-DR positive monocytes was observed, as was a reduction in HLA-DR expression on individual monocytes. Longitudinal follow up of non-surviving and surviving patients showed the former group had persisting evidence of monocyte deactivation, with suppressed HLA-DR at days 3-6, whereas the later group showed recovery of monocyte function between day 1 and days 3-6.
Three studies have looked at this phenomenon in patients with acute-on-chronic liver failure and shown a similar reduction in monocyte HLA-DR in this group[63-65]. Furthermore, they all demonstrated that monocytes derived from acute-on-chronic liver failure patients show impaired ex vivo TNF-α secretion in response to LPS exposure. Berres et al[64] and Xing et al[65] also replicated the finding that low monocyte HLA-DR has a strong association with poor outcome in liver failure patients.
The confirmation of immune dysfunction with functional monocyte deactivation in ALF begs the question of what is driving this phenomenon and how might it be modulated to improve clinical outcomes. The presence of high levels of circulating anti-inflammatory cytokines, IL-10, TGF-β as part of the compensatory anti-inflammatory response (CARS) in ALF are obvious culprits, in that in vitro studies have clearly demonstrated their ability to induce monocyte deactivation (Figure 2). Thus it appears that the anti-inflammatory cytokines that provided early hepatic protection in acute liver failure, can, if unchecked, cause subsequent systemic immune paralysis that leads to a worse outcome.
Monocytes and macrophages are central players in the complex process of initiation, propagation, and resolution of acute liver injury. Current evidence from experimental and human models suggests that macrophages are activated early in the evolution of acute liver injury and respond with vigorous release of chemokines and pro-inflammatory cytokines that drive the acute inflammatory response to injury. Simultaneously, macrophages initiate a counter-acting anti-inflammatory cascade that moderates the degree of acute hepatic inflammation. As ALF progresses, circulating monocytes are recruited to the liver and are implicated in the resolution of inflammation and tissue repair processes. Meanwhile, the intricate balance between pro- and anti-inflammatory cytokines released from blood monocytes, hepatic macrophages, and other immune cells, is maintained through multiple interrelated regulatory mechanisms. However, in ALF, the immune response is skewed towards an anti-inflammatory-predominant environment, which could account for functional monocyte deactivation encountered and its attendant clinical sequelae of recurrent sepsis and multiple organ failure (Figure 3).
Further studies should aim to dissect the temporal relationship of macrophage recruitment and activation, in both circulating and hepatic inflammatory compartments, during the evolution of acute liver failure. Insight into the mechanisms that influence macrophage function during the process of acute hepatic injury will enable the development of therapeutic strategies that can be administered in a timely fashion to modulate the activity of this pivotal immune cell during acute liver failure.
Peer reviewer: Robert Christiaan Verdonk, MD, PhD, Department of Gastroenterology and Hepatology, University Medical Centre Groningen, Hanzeplein 1, Groningen, 9700 RB, The Netherlands
S- Editor Tian L L- Editor Stewart GJ E- Editor Lin YP
| 1. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. |
| 2. | Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217-226. |
| 3. | Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845-861. |
| 4. | Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086-3098. |
| 5. | Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261-274. |
| 6. | Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410-1421. |
| 7. | Frade JM, Mellado M, del Real G, Gutierrez-Ramos JC, Lind P, Martinez-A C. Characterization of the CCR2 chemokine receptor: functional CCR2 receptor expression in B cells. J Immunol. 1997;159:5576-5584. |
| 8. | Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311-317. |
| 9. | Leifeld L, Dumoulin FL, Purr I, Janberg K, Trautwein C, Wolff M, Manns MP, Sauerbruch T, Spengler U. Early up-regulation of chemokine expression in fulminant hepatic failure. J Pathol. 2003;199:335-344. |
| 10. | Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Rénia L, Pol S, Mallet V, Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174:1766-1775. |
| 11. | Ajuebor MN, Hogaboam CM, Le T, Swain MG. C-C chemokine ligand 2/monocyte chemoattractant protein-1 directly inhibits NKT cell IL-4 production and is hepatoprotective in T cell-mediated hepatitis in the mouse. J Immunol. 2003;170:5252-5259. |
| 12. | Hogaboam CM, Bone-Larson CL, Steinhauser ML, Matsukawa A, Gosling J, Boring L, Charo IF, Simpson KJ, Lukacs NW, Kunkel SL. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245-1252. |
| 13. | Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093-1103. |
| 14. | Crane MJ, Hokeness-Antonelli KL, Salazar-Mather TP. Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: role for type I interferons in localized induction of CCR2 ligands. J Immunol. 2009;183:2810-2817. |
| 15. | Engel DR, Maurer J, Tittel AP, Weisheit C, Cavlar T, Schumak B, Limmer A, van Rooijen N, Trautwein C, Tacke F. CCR2 mediates homeostatic and inflammatory release of Gr1(high) monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. J Immunol. 2008;181:5579-5586. |
| 16. | Afford SC, Fisher NC, Neil DA, Fear J, Brun P, Hubscher SG, Adams DH. Distinct patterns of chemokine expression are associated with leukocyte recruitment in alcoholic hepatitis and alcoholic cirrhosis. J Pathol. 1998;186:82-89. |
| 17. | James LP, Simpson PM, Farrar HC, Kearns GL, Wasserman GS, Blumer JL, Reed MD, Sullivan JE, Hinson JA. Cytokines and toxicity in acetaminophen overdose. J Clin Pharmacol. 2005;45:1165-1171. |
| 18. | Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045-1050. |
| 19. | Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186-195. |
| 20. | Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504-1513. |
| 21. | Rüdiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202-210. |
| 22. | Masubuchi Y, Bourdi M, Reilly TP, Graf ML, George JW, Pohl LR. Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem Biophys Res Commun. 2003;304:207-212. |
| 23. | Leist M, Gantner F, Naumann H, Bluethmann H, Vogt K, Brigelius-Flohé R, Nicotera P, Volk HD, Wendel A. Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. Gastroenterology. 1997;112:923-934. |
| 24. | Hishinuma I, Nagakawa J, Hirota K, Miyamoto K, Tsukidate K, Yamanaka T, Katayama K, Yamatsu I. Involvement of tumor necrosis factor-alpha in development of hepatic injury in galactosamine-sensitized mice. Hepatology. 1990;12:1187-1191. |
| 25. | Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21:190-198. |
| 26. | Dong L, Zuo L, Xia S, Gao S, Zhang C, Chen J, Zhang J. Reduction of liver tumor necrosis factor-alpha expression by targeting delivery of antisense oligonucleotides into Kupffer cells protects rats from fulminant hepatitis. J Gene Med. 2009;11:229-239. |
| 27. | Nagaki M, Tanaka M, Sugiyama A, Ohnishi H, Moriwaki H. Interleukin-10 inhibits hepatic injury and tumor necrosis factor-alpha and interferon-gamma mRNA expression induced by staphylococcal enterotoxin B or lipopolysaccharide in galactosamine-sensitized mice. J Hepatol. 1999;31:815-824. |
| 28. | Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227-1236. |
| 29. | Mizuhara H, Uno M, Seki N, Yamashita M, Yamaoka M, Ogawa T, Kaneda K, Fujii T, Senoh H, Fujiwara H. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology. 1996;23:1608-1615. |
| 30. | Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(-/-) mice, but not in TNF-alpha(-/-) mice: role for IFN-gamma in activating apoptosis of hepatocytes. J Immunol. 1997;159:1418-1428. |
| 31. | Bourdi M, Reilly TP, Elkahloun AG, George JW, Pohl LR. Macrophage migration inhibitory factor in drug-induced liver injury: a role in susceptibility and stress responsiveness. Biochem Biophys Res Commun. 2002;294:225-230. |
| 32. | Kobayashi S, Nishihira J, Watanabe S, Todo S. Prevention of lethal acute hepatic failure by antimacrophage migration inhibitory factor antibody in mice treated with bacille Calmette-Guerin and lipopolysaccharide. Hepatology. 1999;29:1752-1759. |
| 33. | Nanji AA, Lau GK, Tipoe GL, Yuen ST, Chen YX, Thomas P, Lan HY. Macrophage migration inhibitory factor expression in male and female ethanol-fed rats. J Interferon Cytokine Res. 2001;21:1055-1062. |
| 34. | James LP, Lamps LW, McCullough S, Hinson JA. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 2003;309:857-863. |
| 35. | Bourdi M, Eiras DP, Holt MP, Webster MR, Reilly TP, Welch KD, Pohl LR. Role of IL-6 in an IL-10 and IL-4 double knockout mouse model uniquely susceptible to acetaminophen-induced liver injury. Chem Res Toxicol. 2007;20:208-216. |
| 36. | Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21:32-43. |
| 37. | Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med. 1994;179:1529-1537. |
| 38. | Sun R, Tian Z, Kulkarni S, Gao B. IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4+ T cell- and STAT3-dependent manners. J Immunol. 2004;172:5648-5655. |
| 39. | Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem. 2001;276:26605-26613. |
| 40. | Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149-159. |
| 41. | Camargo CA Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513-1520. |
| 42. | Louis H, Le Moine O, Peny MO, Gulbis B, Nisol F, Goldman M, Devière J. Hepatoprotective role of interleukin 10 in galactosamine/lipopolysaccharide mouse liver injury. Gastroenterology. 1997;112:935-942. |
| 43. | Santucci L, Fiorucci S, Chiorean M, Brunori PM, Di Matteo FM, Sidoni A, Migliorati G, Morelli A. Interleukin 10 reduces lethality and hepatic injury induced by lipopolysaccharide in galactosamine-sensitized mice. Gastroenterology. 1996;111:736-744. |
| 44. | Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607-1615. |
| 45. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. |
| 46. | Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289-298. |
| 47. | Louis H, Le Moine O, Peny MO, Quertinmont E, Fokan D, Goldman M, Devière J. Production and role of interleukin-10 in concanavalin A-induced hepatitis in mice. Hepatology. 1997;25:1382-1389. |
| 48. | Dambach DM, Durham SK, Laskin JD, Laskin DL. Distinct roles of NF-kappaB p50 in the regulation of acetaminophen-induced inflammatory mediator production and hepatotoxicity. Toxicol Appl Pharmacol. 2006;211:157-165. |
| 49. | Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220-1234. |
| 50. | Bacher M, Meinhardt A, Lan HY, Mu W, Metz CN, Chesney JA, Calandra T, Gemsa D, Donnelly T, Atkins RC. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997;150:235-246. |
| 51. | Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895-1902. |
| 52. | Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest. 2003;112:978-980. |
| 53. | Antoniades CG, Berry PA, Davies ET, Hussain M, Bernal W, Vergani D, Wendon J. Reduced monocyte HLA-DR expression: a novel biomarker of disease severity and outcome in acetaminophen-induced acute liver failure. Hepatology. 2006;44:34-43. |
| 54. | Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. 1994;98:71-77. |
| 55. | Wigmore SJ, Walsh TS, Lee A, Ross JA. Pro-inflammatory cytokine release and mediation of the acute phase protein response in fulminant hepatic failure. Intensive Care Med. 1998;24:224-229. |
| 56. | Nagaki M, Iwai H, Naiki T, Ohnishi H, Muto Y, Moriwaki H. High levels of serum interleukin-10 and tumor necrosis factor-alpha are associated with fatality in fulminant hepatitis. J Infect Dis. 2000;182:1103-1108. |
| 57. | Streetz K, Leifeld L, Grundmann D, Ramakers J, Eckert K, Spengler U, Brenner D, Manns M, Trautwein C. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology. 2000;119:446-460. |
| 58. | Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC Jr. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204-207. |
| 59. | Izumi S, Hughes RD, Langley PG, Pernambuco JR, Williams R. Extent of the acute phase response in fulminant hepatic failure. Gut. 1994;35:982-986. |
| 60. | Hiraoka A, Horiike N, Akbar SM, Michitaka K, Matsuyama T, Onji M. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol. 2005;40:52-56. |
| 61. | Møller HJ, Grønbaek H, Schiødt FV, Holland-Fischer P, Schilsky M, Munoz S, Hassanein T, Lee WM. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671-676. |
| 62. | Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Gimson A, Casewell M, Fagan E, Williams R. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11:49-53. |
| 63. | Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol. 2005;42:195-201. |
| 64. | Berres ML, Schnyder B, Yagmur E, Inglis B, Stanzel S, Tischendorf JJ, Koch A, Winograd R, Trautwein C, Wasmuth HE. Longitudinal monocyte human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. 2009;29:536-543. |
| 65. | Xing T, Li L, Cao H, Huang J. Altered immune function of monocytes in different stages of patients with acute on chronic liver failure. Clin Exp Immunol. 2007;147:184-188. |