Published online Mar 28, 2010. doi: 10.3748/wjg.v16.i12.1506
Revised: January 7, 2010
Accepted: January 14, 2010
Published online: March 28, 2010
AIM: To investigate the efficacy of short-term peg-interferon (PEG-IFN) monotherapy for chronic hepatitis C patients who achieved an immediate virological response.
METHODS: Defining an “immediate virological response (IVR)” as the loss of serum hepatitis C virus (HCV) RNA 7 d after the first administration of PEG-IFN α, we conducted a 12-wk course of PEG-IFN α2a monotherapy without the addition of ribavirin for 38 patients who had low pretreatment HCV RNA load and exhibited IVR. The patients included 21 men and 17 women, whose ages ranged from 22 to 77 years (mean ± SD: 52.0 ± 17.8 years). There were 4 patients with HCV genotype 1b, 23 patients with genotype 2a and 4 patients with genotype 2b. HCV genotype was not determined for the remaining 7 patients. Patients were categorized into a sustained virological response (SVR) group, if serum HCV RNA remained negative for 24 wk after the end of treatment, or into a relapse group.
RESULTS: Based on the intention-to-treat analysis, 35 patients (92.1%) achieved SVR. One patient (2.6%) relapsed with serum HCV RNA 12 wk after the end of treatment. Two patients (5.3%) withdrew from the study during the 24-wk follow-up period. With regard to the HCV RNA genotype, the SVR rates were 100% (4/4) for genotype 1b, 95.7% (22/23) for genotype 2a and 100% (4/4) for genotype 2b. The SVR rate in 7 patients, whose HCV RNA genotypes were not determined, was 71.4% (5/7).
CONCLUSION: Short-term PEG-IFN α2a monotherapy is highly effective for chronic hepatitis C patients who have low pretreatment HCV RNA load and exhibit IVR.
- Citation: Yada M, Masumoto A, Yamashita N, Motomura K, Koyanagi T, Sakamoto S. Immediate virological response predicts the success of short-term peg-interferon monotherapy for chronic hepatitis C. World J Gastroenterol 2010; 16(12): 1506-1511
- URL: https://www.wjgnet.com/1007-9327/full/v16/i12/1506.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i12.1506
Hepatitis C virus (HCV) infection is a leading cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. About 170 million patients are chronically infected with HCV worldwide. Interferon (IFN) therapy is important for suppressing the progression of chronic liver disease derived from HCV infection. Currently, combination therapy with peg-interferon α (PEG-IFN α) and ribavirin (RBV) is the first-line therapy used to eliminate HCV in patients with chronic hepatitis C. The duration of treatment is determined based on the viral genotype, with treatment administered for 48 wk in patients with genotype 1 and 24 wk in patients with genotypes 2 or 3[1,2]. However, long-term administration of IFN and RBV increases the cost of treatment and the risk of severe adverse events.
The efficacy of IFN therapy depends on the HCV genotype, pretreatment viral load and early viral kinetics[3-7]. Thus, IFN therapy must be individualized and optimized according to the virological and clinical status of each patient. Several studies have shown that the duration of IFN therapy could be shortened in patients who achieve serum HCV RNA negativity during the early stages of treatment[7-9]. Therefore, it is essential that we reduce the treatment duration for patients who exhibit a quick virological response to IFN therapy. In the present study, we focused on the initial response to PEG-IFN α administration and studied the efficacy of short-term PEG-IFN α2a monotherapy for patients who had low pretreatment HCV RNA load.
We defined an “immediate virological response (IVR)” as the loss of serum HCV RNA 7 d after the first administration of PEG-IFN α. Patients with low pretreatment HCV RNA load (< 1.0 × 105 IU/mL) were monitored for HCV RNA in the serum 7 d after the first administration of PEG-IFN α2a. We scheduled a 12-wk treatment course of PEG-IFN α2a without the addition of RBV for patients who achieved IVR. The patients received subcutaneous injection of 180 μg of PEG-IFN α2a (Pegasys, Roche) once per week. According to the circumstance of each patient, the dose was reduced to 90 μg or the treatment course was terminated.
We administered PEG-IFN α2a without the addition of RBV to a total of 59 patients with low HCV RNA load from December 2004 to November 2007. They were monitored for serum HCV RNA 7 d after the first administration of PEG-IFN α2a. Among these, 38 patients who achieved IVR were enrolled in this study. The age, gender, HCV genotype, serum HCV RNA level, ALT level, hemoglobin (Hb), and the neutrophil and platelet (PLT) counts before treatment are shown in Table 1. The patients included 21 men and 17 women, whose ages ranged from 22 to 77 years (mean ± SD: 52.0 ± 17.8 years). There were 4 patients with genotype 1b, 23 patients with genotype 2a and 4 patients with genotype 2b. HCV genotype was not determined for the remaining 7 patients. Among the 38 patients enrolled in this study, 37 received IFN therapy for the first time for HCV infection. Pt. 16 is the same patient as Pt. 3, who received the same treatment course for HCV genotype 2a infection at 26 years of age. After the initial successful treatment, the patient was infected with genotype 2b HCV and re-treated at 28 years of age. Pt. 25 had been treated for hepatocellular carcinoma prior to this IFN treatment course.
| Pt. No. | Age (yr) | Gender | Genotype | Viral load (IU/mL) | ALT (IU/L) | Hb (g/dL) | Neutrophil (/mm3) | PLT (× 103/mm3) | Naive/ re-treatment |
| 1 | 51 | M | 2a | 2.4 × 104 | 83 | 10.5 | 2729 | 254 | Naive |
| 2 | 56 | M | 1b | 7.6 × 103 | 14 | 14.0 | 2565 | 268 | Naive |
| 3 | 26 | F | 2a | 2.7 × 104 | 157 | 15.6 | 5217 | 284 | Naive |
| 4 | 67 | M | 2a | < 5.0 × 103 | 63 | 15.2 | 3940 | 159 | Naive |
| 5 | 48 | F | 2a | 1.1 × 104 | 121 | 12.1 | 1851 | 146 | Naive |
| 6 | 48 | F | 2a | 7.0 × 104 | 48 | 12.7 | 3084 | 244 | Naive |
| 7 | 70 | M | 2a | 5.3 × 104 | 119 | 13.7 | 2002 | 157 | Naive |
| 8 | 64 | M | ND | < 5.0 × 103 | 65 | 14.5 | 2200 | 148 | Naive |
| 9 | 22 | M | ND | < 5.0 × 103 | 51 | 15.3 | 5003 | 308 | Naive |
| 10 | 40 | F | 2a | 1.1 × 104 | 198 | 14.2 | 2424 | 190 | Naive |
| 11 | 68 | F | 2a | 9.3 × 103 | 31 | 11.8 | 3567 | 212 | Naive |
| 12 | 60 | M | 2a | < 5.0 × 103 | 18 | 14.9 | 1520 | 265 | Naive |
| 13 | 68 | F | 2a | 1.8 × 104 | 112 | 13.4 | 3338 | 190 | Naive |
| 14 | 76 | M | 2a | 1.8 × 104 | 39 | 13.6 | 2540 | 272 | Naive |
| 15 | 25 | F | 2a | 8.6 × 104 | 181 | 12.2 | 2649 | 260 | Naive |
| 16 | 28 | F | 2b | 1.9 × 104 | 19 | 14.4 | 3522 | 324 | Re-treatment |
| 17 | 23 | M | ND | < 5.0 × 103 | 456 | 15.0 | 3483 | 257 | Naive |
| 18 | 57 | F | 2b | 5.9 × 103 | 97 | 14.2 | 2080 | 306 | Naive |
| 19 | 48 | M | 2a | 6.4 × 104 | 41 | 15.1 | 1688 | 235 | Naive |
| 20 | 56 | M | 2b | 5.1 × 104 | 124 | 15.1 | 3810 | 82 | Naive |
| 21 | 34 | F | 2a | 2.8 × 104 | 72 | 13.4 | 4004 | 98 | Naive |
| 22 | 32 | F | 2a | 1.7 × 104 | 41 | 12.5 | 1853 | 259 | Naive |
| 23 | 74 | M | ND | < 5.0 × 103 | 37 | 10.6 | 1784 | 92 | Naive |
| 24 | 45 | M | 2a | 1.0 × 104 | 41 | 16.3 | 4606 | 147 | Naive |
| 25 | 76 | F | 2a | < 5.0 × 103 | 54 | 10.9 | 2948 | 192 | Naive |
| 26 | 53 | F | 1b | 5.0 × 103 | 13 | 11.9 | 3438 | 204 | Naive |
| 27 | 27 | F | ND | < 5.0 × 103 | 46 | 15.1 | 2205 | 169 | Naive |
| 28 | 71 | F | ND | 5.0 × 103 | 40 | 14.1 | 2022 | 98 | Naive |
| 29 | 70 | M | 2a | 5.1 × 104 | 81 | 14.5 | 2894 | 125 | Naive |
| 30 | 60 | M | 1b | 5.7 × 103 | 40 | 15.1 | 1502 | 297 | Naive |
| 31 | 57 | M | 2a | 2.8 × 104 | 61 | 15.5 | 3154 | 137 | Naive |
| 32 | 77 | M | 2a | < 5.0 × 103 | 68 | 10.3 | 1430 | 125 | Naive |
| 33 | 69 | F | 1b | 1.6 × 104 | 21 | 12.8 | 2772 | 186 | Naive |
| 34 | 37 | F | 2a | < 5.0 × 103 | 42 | 13.1 | 2343 | 314 | Naive |
| 35 | 23 | M | ND | < 5.0 × 103 | 46 | 14.6 | 2772 | 238 | Naive |
| 36 | 36 | M | 2a | 1.3 × 104 | 26 | 14.5 | 5126 | 242 | Naive |
| 37 | 57 | F | 2b | 4.7 × 104 | 50 | 14.9 | 1628 | 130 | Naive |
| 38 | 71 | M | 2a | 3.5 × 104 | 102 | 14.4 | 3852 | 109 | Naive |
| mean ± SD | 52.0 ± 17.8 | 76.8 ± 77.6 | 13.7 ± 1.5 | 2838.20 ± 972.50 | 212.9 ± 79.1 |
Pretreatment serum HCV RNA levels were determined by RT-PCR using an Amplicor HCV monitor v2.0 series kit (Roche Diagnostics Co.), which had a detection limit of 5.0 × 103 IU/mL. When the serum HCV RNA level was below the detection limit, qualitative analysis of the HCV RNA was performed by RT-PCR using a COBAS Amplicor HCV test kit v2.0 (Roche Diagnostics Co.), which had a detection limit of 50 IU/mL. Qualitative analysis of HCV RNA was performed 7 d after the first administration for the evaluation of IVR, and at 24 wk after the end of treatment for the evaluation of the SVR. HCV genotype was determined using an HCV Genotyping SMITEST kit (Roche Diagnostics Co.).
Patients were categorized into the sustained virological response (SVR) group, if serum HCV RNA remained negative for 24 wk after the end of PEG-IFN α2a therapy. Patients were categorized into the relapse group if serum HCV RNA reappeared after the end of the treatment course.
All patients provided informed consent prior to their participation in this study. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
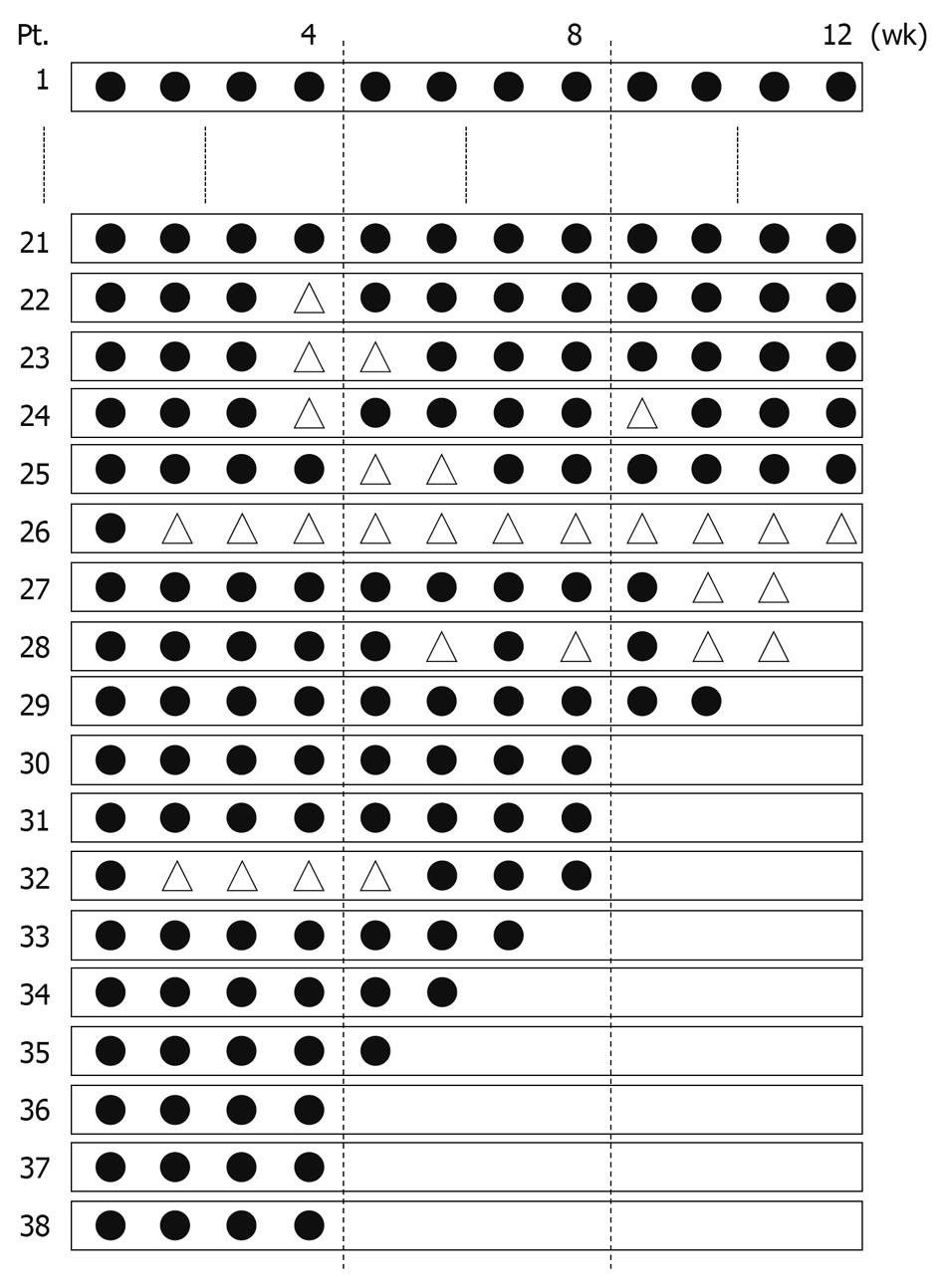
Figure 1 presents the dose and duration of PEG-IFN α2a administration for each patient. Of 38 patients enrolled in this study, 26 (Pts. 1-26, 68.4%) completed a 12-wk treatment course. It was necessary to reduce the dose of PEG-IFN α2a in 5 (Pts. 22-26) of these 26 patients. Twelve patients (Pts. 27-38, 31.6%) discontinued treatment prior to completion of the 12-wk course. In 6 (Pts. 30, 31 and 35-38, 15.8%) of these 12 patients, either self-withdrawal or financial problem was the cause for discontinuation. In 3 cases (Pts. 27, 28 and 32), the dose was reduced during the treatment, but the treatment was discontinued before completion of the 12-wk course.
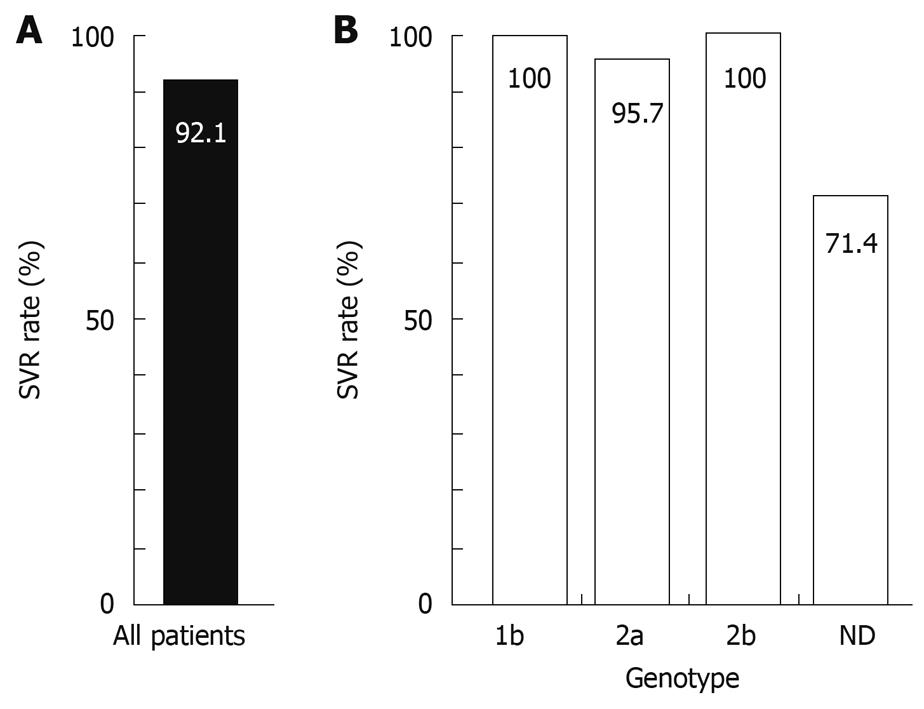
An intention-to-treat analysis was performed. Two patients (Pts. 27 and 35, 5.3%) withdrew from this study during the 24-wk follow-up period because they did not attend the hospital appointments. Thirty-five patients (92.1%) were negative for serum HCV RNA 24 wk after the end of treatment and were categorized into the SVR group (Figure 2). One patient (Pt. 6, 2.6%) was positive for serum HCV RNA 24 wk after the end of treatment and was categorized into the relapse group.
With regard to the HCV RNA genotype, the SVR rates were 100% (4/4) for genotype 1b, 95.7% (22/23) for genotype 2a and 100% (4/4) for genotype 2b (Figure 2). The SVR rate in 7 patients, whose HCV RNA genotypes were not determined, was 71.4% (5/7), as we could not evaluate the treatment effect for 2 patients because of self-withdrawal during the follow-up period.
The most frequent adverse events were flu-like symptoms, such as fever and pain. Fatigue appeared as the second most frequent. Other adverse events are summarized in Table 2. Some patients who complained of insomnia were treated with hypnotics. Thrombocytopenia (< 50 000/mm3) occurred in 1 case (Pt. 23) and the dose was reduced for the 4th and 5th administration during the treatment course. Neutropenia (< 750/mm3) appeared in 3 cases, and in 1 case (Pt. 32) it was necessary to reduce the dose of PEG-IFN α2a from the 2nd to 5th administration. Anemia (Hb < 8.5 g/dL) did not occur in any of the patients. In 6 cases, it was necessary to reduce the dose of PEG-IFN α2a because of fatigue (Pts. 22, 25 and 28), emotional lability (Pts. 26 and 27) or vertigo (Pt. 24). None of the patients discontinued the treatment course because of bone marrow suppression. In 5 cases it was necessary to discontinue the treatment course for the following reasons: 2 with fatigue (Pts. 28 and 34), 1 with stomatitis (Pt. 27), 1 with skin symptoms and diarrhea (Pt. 29) and the other with viral influenza infection (Pt. 32). These adverse events were not particularly severe. One case (Pt. 33) discontinued the treatment course because of bacterial pneumonia, and recovered after antibiotic administration.
| Adverse event | n (%) |
| Flu-like symptoms | |
| Fever | 20 (52.6) |
| Pain | 12 (31.5) |
| Fatigue | 13 (34.2) |
| Insomnia | 7 (18.4) |
| Neutropenia (< 750/mm3) | 3 (7.9) |
| Skin symptoms | 3 (7.9) |
| Emotional lability | 3 (7.9) |
| Gastrointestinal symptoms | 2 (3.3) |
| Thrombocytopenia (< 50 000/mm3) | 1 (2.6) |
| Bacterial pneumonia | 1 (2.6) |
| Influenza viral infection | 1 (2.6) |
| Vertigo | 1 (2.6) |
| Stomatitis | 1 (2.6) |
In the present study, we defined an “immediate virological response (IVR)” as the loss of serum HCV RNA 7 d after the first administration of PEG-IFN α. We then conducted a 12-wk treatment course of PEG-IFN α2a in a population of patients who had low pretreatment HCV RNA load and who achieved IVR. This short-term PEG-IFN α2a monotherapy without the addition of RBV exhibited an extremely high SVR rate.
The current standard therapy to eliminate HCV in patients with chronic hepatitis C is combination therapy with PEG-IFN α and RBV. While the SVR rate for 24 wk of PEG-IFN α/RBV therapy in patients with genotypes 2 and 3 is 78%-93%, the SVR rate for 48 wk of treatment for genotype 1 is 40%-51%[1,2,10-13]. New methods of determining the adequate dose and duration of PEG-IFN α/RBV administration have been devised to increase the probability of SVR in the treatment for patients with genotype 1 and high pretreatment viral load[9,14]. High dose and long-term IFN treatments, however, are expensive and contribute to additional risk for many adverse events.
Major contributory factors for SVR are viral genotype (except genotype 1), low pretreatment viral load (< 1.0 × 105 IU/mL) and early loss of serum HCV RNA[3-7]. Early virological response (EVR) and rapid virological response (RVR), which are indicated by loss of serum HCV RNA at weeks 12 and 4, respectively, are closely related to the SVR rate[6,7,15,16]. Furthermore, Mangia et al[8] have reported that 12 wk of administration of PEG-IFN α/RBV to patients with HCV genotype 2 or 3 and who achieve RVR, results in a high probability of SVR. Tabaru et al[17] reported that the SVR rate was 100% after treatment with IFN α2b for 6 wk for patients infected with HCV genotype 2a and low viral load. Establishing the minimum, and yet sufficient, IFN therapy period is important in terms of financial efficiency and for reduction of the risk of adverse events. Therefore, it is essential to establish a guideline to make the treatment period shorter than the standard length for patients who have a high probability of achieving SVR. For patients with low pretreatment HCV RNA load, the current standard IFN therapy which is allowed by Japanese National Medical Insurance, is 24-48 wk of PEG-IFN α2a monotherapy without the addition of RBV. RBV causes hemolytic anemia, and severe anemic symptoms sometimes appear in PEG-IFN α/RBV combination treatment[18]. PEG-IFN α monotherapy can avoid these adverse events induced by RBV.
Thus, in the current study, we studied the efficacy of short-term PEG-IFN α2a monotherapy for patients who had a low pretreatment HCV RNA load and exhibited IVR. Remarkably, 35 (97.2%) out of 36 cases that we were able to follow up to 24 wk after the last administration, were categorized into the SVR group. With regard to the HCV RNA genotype, SVR rates were 100% (4/4) for genotype 1b, 95.7% (22/23) for genotype 2a and 100% (4/4) for genotype 2b (Figure 2). These data might suggest that the efficacy of short-term PEG-IFN α2a monotherapy for patients exhibiting IVR is independent of HCV genotype. Further analyses with a large population of patients should be conducted for each genotype of HCV, because the number of patients enrolled in this study, especially for genotype 1b, was small.
The patient (Pt. 6) who relapsed during this treatment course subsequently received 24 wk of PEG-IFN α2a therapy and succeeded in achieving SVR. We consider that if short-term treatment failed to induce SVR, those non-SVR patients could be re-treated with long-term PEG-IFN α monotherapy (24-48 wk) or PEG-IFN α/RBV combination therapy. Thus, it is suggested that we should select short-term monotherapy at the first approach for patients with low pretreatment HCV RNA load and IVR.
Nine out of 35 cases who achieved SVR received less than 9 wk (4-8 wk) of drug administration. Therefore, there is the possibility that the treatment period could be shortened to less than 12 wk for a certain group of patients. Further analyses in randomized controlled trials with a large population of patients should be conducted to examine the efficacy of shorter treatment courses, such as 4 wk or 8 wk.
Frequent adverse events that generally appear during IFN therapy were seen in this study group of patients. However, no severe events were observed. None of the patients required discontinuation of therapy because of bone marrow suppression. While 1 case exhibited bacterial pneumonia as a severe complication, the patient recovered with antibiotic treatment. Thus, we consider that short-term monotherapy is safe.
PEG-IFN α monotherapy clearly has the advantage of avoiding adverse events induced by RBV. However, we have to consider the possibility that adding RBV to this short term PEG-IFN α therapy could offer shorter treatment duration or higher SVR rates. This option should be investigated in larger studies.
In conclusion, short-term PEG-IFN α2a monotherapy is highly effective for chronic hepatitis C patients who have low pretreatment HCV RNA load and exhibit IVR. IVR is a simple and useful indicator of early viral kinetics to predict the high probability of SVR.
The efficacy of interferon (IFN) therapy for chronic hepatitis C depends on the hepatitis C virus (HCV) genotype, pretreatment viral load and early viral kinetics. Therefore, IFN therapy must be individualized and optimized according to the virological and clinical status of each patient.
Focusing on the initial response to PEG-IFN α administration, the authors proposed a new concept, the “immediate virological response (IVR)”. Then the efficacy of short-term PEG-IFN α2a monotherapy was investigated for patients who had low pretreatment HCV RNA load and exhibited IVR.
A 12-wk treatment course of PEG-IFN α2a without the addition of RBV was highly effective for patients who achieved IVR. SVR rate for all patients in this study was 92.1% (35/38).
The present study has shown that short-term PEG-IFN α2a monotherapy is an excellent treatment regimen for chronic hepatitis C patients who have low pretreatment HCV RNA load and exhibit IVR. It is also suggested that IVR is a simple and useful indicator of early viral kinetics to predict the high probability of SVR.
An “IVR” was defined as the loss of serum HCV RNA 7 d after the first administration of PEG-IFN α.
Yada et al reported that a 12-wk treatment course of PEG-IFN α2a alone in a population of patients who had low pretreatment HCV RNA load and achieved immediate virological response results in extremely high SVR rates. The data are encouraging and important from a cost-effectiveness point of view.
Peer reviewers: Yukihiro Shimizu, MD, PhD, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, Kyoto 615-8256, Japan; Dr. Radha Krishna Yellapu, MD, DM, Department of Hepatology, Mount Sinai Hospital, 121 E 97 Street, NY 10029, United States; Can Gonen, MD, Department of Gastroenterology, Kutahya State Hospital, 43100 Kutahya, Turkey
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36:S1-S2. |
| 2. | Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350-1359. |
| 3. | Yoshioka K, Kakumu S, Wakita T, Ishikawa T, Itoh Y, Takayanagi M, Higashi Y, Shibata M, Morishima T. Detection of hepatitis C virus by polymerase chain reaction and response to interferon-alpha therapy: relationship to genotypes of hepatitis C virus. Hepatology. 1992;16:293-299. |
| 4. | Matsumoto A, Tanaka E, Suzuki T, Ogata H, Kiyosawa K. Viral and host factors that contribute to efficacy of interferon-alpha 2a therapy in patients with chronic hepatitis C. Dig Dis Sci. 1994;39:1273-1280. |
| 5. | Nomura H, Kimura Y, Tada H, Hisano C, Morita C, Okamoto O, Shiraishi G, Kashiwagi S. Predictive factors of a response to interferon therapy in chronic hepatitis C. J Clin Gastroenterol. 1996;23:185-190. |
| 6. | Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645-652. |
| 7. | Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, Ferenci P, Ackrill AM, Willems B. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954-960. |
| 8. | Mangia A, Santoro R, Minerva N, Ricci GL, Carretta V, Persico M, Vinelli F, Scotto G, Bacca D, Annese M. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609-2617. |
| 9. | Satoh T, Masumoto A. Accordion Index: A new tool for the prediction of the efficacy of peg-interferon-alpha-2b and ribavirin combination therapy for chronic hepatitis C. Hepatol Res. 2008;38:315-318. |
| 10. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. |
| 11. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, DhumeauxD . Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. |
| 12. | Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. |
| 13. | Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, Sarrazin C, Harvey J, Brass C, Albrecht J. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993-999. |
| 14. | Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, Buggisch P, Goeser T, Rasenack J, Pape GR. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086-1097. |
| 15. | Yu JW, Wang GQ, Sun LJ, LixG , Li SC. Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J Gastroenterol Hepatol. 2007;22:832-836. |
| 16. | Poordad F, Reddy KR, Martin P. Rapid virologic response: a new milestone in the management of chronic hepatitis C. Clin Infect Dis. 2008;46:78-84. |
| 17. | Tabaru A, Narita R, Hiura M, Abe S, Otsuki M. Efficacy of short-term interferon therapy for patients infected with hepatitis C virus genotype 2a. Am J Gastroenterol. 2005;100:862-867. |
| 18. | Martin P, Jensen DM. Ribavirin in the treatment of chronic hepatitis C. J Gastroenterol Hepatol. 2008;23:844-855. |