Published online Mar 28, 2010. doi: 10.3748/wjg.v16.i12.1482
Revised: December 19, 2009
Accepted: December 26, 2009
Published online: March 28, 2010
AIM: To investigate whether Recql5, a DNA helicase that plays an important role in the maintenance of genome integrity, is a tumor suppressor in the gastrointestinal tract in mice.
METHODS: We generated cohorts of both Recql5-proficient and Recql5-deficient Apcmin/+ mice and compared the tumor susceptibility in their gastrointestinal tracts.
RESULTS: Recql5 deficiency in Apcmin/+ mice resulted in a significant increase in the tumor incidence in both the colon (P = 0.0162) and the small intestine (P < 0.01). These findings have provided the first genetic evidence for a tumor suppression role of Recql5 in the gastrointestinal tract of mice. Importantly, since mouse Recql5 and human RECQL5 are highly conserved, these findings also suggest that RECQL5 may be a tumor suppressor for human colon cancer.
CONCLUSION: Recql5 has a tumor suppression role in the mouse gastrointestinal tract.
-
Citation: Hu Y, Lu X, Luo G. Effect of Recql5 deficiency on the intestinal tumor susceptibility of
Apcmin mice. World J Gastroenterol 2010; 16(12): 1482-1486 - URL: https://www.wjgnet.com/1007-9327/full/v16/i12/1482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i12.1482
Cancer is a complicated genetic disorder, which may result from a myriad of deleterious oncogenic events induced by both endogenous and environmental insults, which perturb the normal growth control and physiological functions of cells[1,2]. Most tumors are found to harbor genetic changes of either activation of proto-oncogenes, or inactivation of tumor suppressor genes (TSGs), or both[3]. In particular, inactivation of TSGs represents an important early event of carcinogenesis in colorectal cancer. Generally, TSGs can be categorized into two major types, so-called “gatekeeper” and “caretaker” genes[4,5]. Gatekeepers, such as the Retinoblastoma gene and the Adenomatous polyposis coli (APC) gene, have pivotal roles in cell proliferation by regulating cell cycle checkpoints, apoptosis and signaling transduction[6,7]. It has been hypothesized that the loss of caretakers provides the initial changes for the initiation of carcinogenesis, whereas mutations in gatekeepers provide the necessary “promotion” effect for the fully fledged development of cancer.
Chromosome instability (CIN) is one of the hallmarks of many cancer cells, and it has been suggested that CIN, both structural and numerical, contributes to the development of malignancies, and in particular, colorectal cancer[8,9]. CIN may occur through many different mechanisms, such as DNA breaks, centrosome amplification, chromatid cohesion instability and cell cycle checkpoint defects[9,10]. We have reported recently that deletion of Recql5, a member of the RecQ DNA helicase family, in mice resulted in a rearrangement type of CIN and an increased susceptibility to cancer in a number of organs and tissues, but not in the intestinal tract[11]. Nonetheless, given that CIN is known to play an important role in the development of colorectal cancer, we suspected that Recql5 might have a role in tumor suppression in the gastrointestinal (GI) tract but that such an effect could not be readily detected in our previous study using straight Recql5 knockout mice. Apcmin mice have been widely used as a sensitizing background for assessing the potential oncogenic effect in the GI tract of specific genetic alternations[12]. Apcmin mice carry a spontaneous point mutation in one of the two copies of the Apc, the mouse homologue of the human APC TSG. In humans, mutations in this APC TSG give rise to familial adenomatous polyposis syndrome[13]. In adult Apcmin mice, the loss of the remaining wild-type copy of the Apc gene in the colonic epithelium predisposes these cells to tumorigenesis, leading to development of adenomas both in the small intestine and colon. The number of tumors developed per mouse as well as the size of these tumors can be affected by the genetic background of the animals. Thus, they have been used extensively for assessing the potential oncogenic effects of specific genetic alterations[12].
In the current study, we have examined the potential effect of Recql5 deficiency on tumorigenesis within the gastrointestinal tract in Apcmin/+ mice, taking advantage of the sensitized genetic background for analyzing tumorigenesis in the GI tract provided by this well established model[14].
Recql5+/+ and Recql5+/- mice were generated from crossings between Recql5+/- mice, which were maintained in a mixed genetic background (87.5% C57BL/6 and 12.5% 129sv) as described previously[15]. C57BL/6J (B6) and C57BL/6J-ApcMin/J (ApcMin/+) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were propagated in the Case Western Reserve University American Association of Laboratory Animals accredited barrier-free facility. Mice were fed a commercially available rodent breeder diet, 5010 (PMI LabDiet). All cages, food, bedding, and water were autoclaved before use. All procedures were approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Recql5+/- female mice were mated with B6-ApcMin/+ male mice. The resulting Recql5+/-ApcMin/+ progeny were intercrossed to obtain both Recql5+/+ApcMin/+ and Recql5-/-ApcMin/+ mice. Genotyping was carried out by standard PCR methods[16]. At 90 d of age, Recql5+/+ApcMin/+ and Recq5-/-ApcMin/+ mice were euthanized by CO2 asphyxiation for quantitative analysis of intestinal adenomas. The entire intestine tract from duodenum to anus was removed, washed in phosphate buffered saline (PBS), opened longitudinally and pinned luminal side up on a wax dissection plate. Intestinal adenomas (macroadenomas with maximal diameters ≥ 1 mm, microadenomas < 1 mm) along the entire intestine were counted by microscopic examination at 10 × magnification followed by fixation with 10% formalin in PBS (Fisher Scientific). Digital images of polyps and a metric ruler were captured using SPOT software 3.2.5 for Macintosh and an RT color SPOT camera mounted on a Leica MZFLIII workstation (Diagnostic Instruments). The percentages were calculated by dividing numbers of mice with more than 100 macroadenomas against total numbers of mice.
Statistical analyses were performed with the two-sample student’s t-test using the Prism software package (GraphPad Software).
To investigate whether Recql5 deficiency may affect the adenoma multiplicity by either accelerating the loss of wild type Apc or by promoting the progression of tumors, we introduced the Recql5 knockout allele into the ApcMin/+ background and analyzed the phenotype of intestinal adenomas at 90 d of age when mice are not severely affected by other complications, such as anemia.
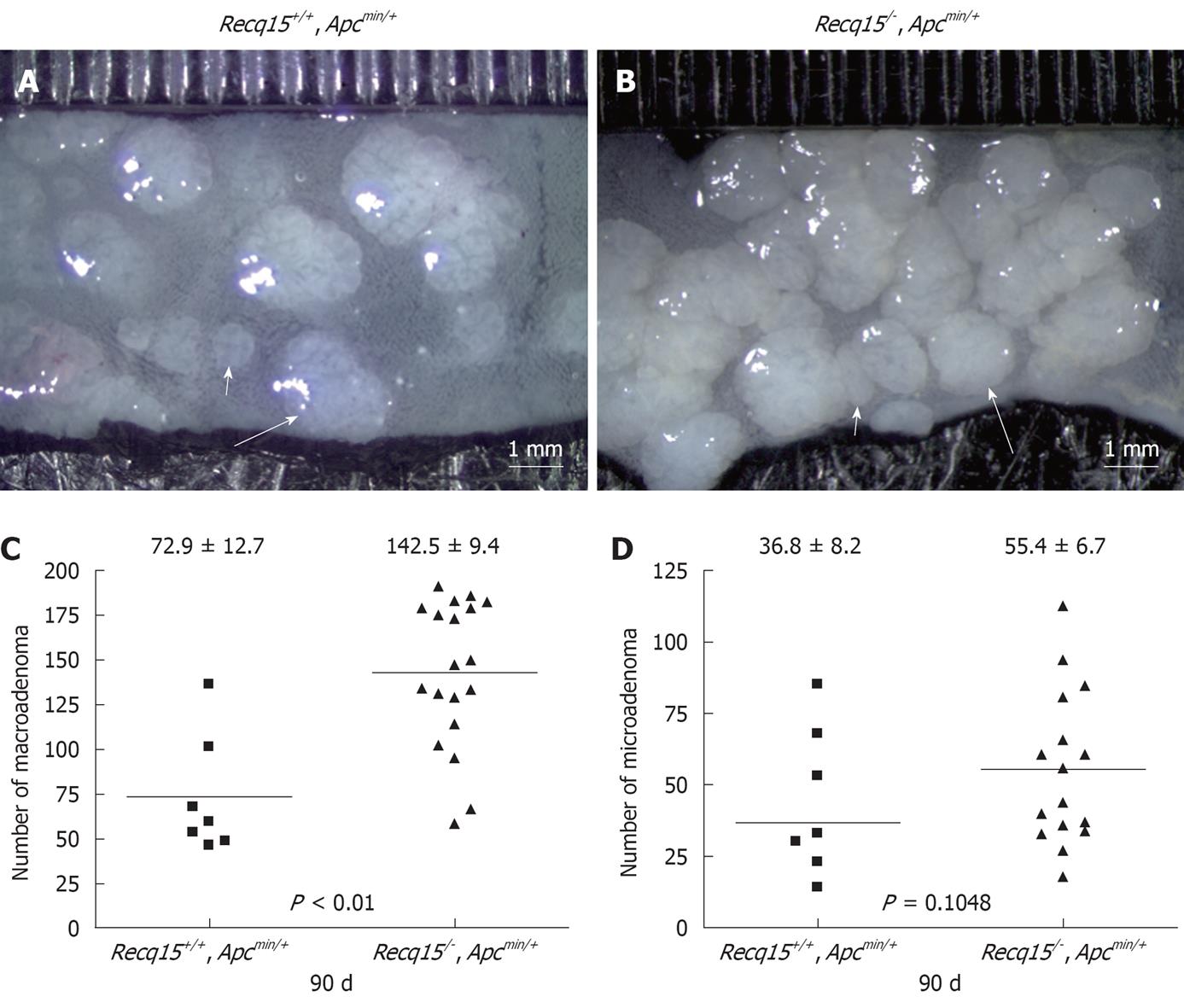
We found that under our specific experimental conditions and a C57BL/6J × 129Sv mixed genetic background, Recql5+/+ApcMin/+ mice developed 72.9 ± 12.7 (mean ± SE) macroadenomas (diameter ≥ 1 mm) along the entire intestinal tract at 90 d (Figure 1A and C). Remarkably, Recql5-/-ApcMin/+ littermates developed 142.5 ± 9.4 macroadenomas at the same age, which is significantly higher than that observed in their Recql5+/+ApcMin/+ littermates (P = 0.0032) (Figure 1A and C, Table 1). In particular, 15 out of 19 Recql5-/-ApcMin/+ mice (78.9%) had more than 100 macroadenomas (Figure 1B and C) compared with only 1 out of 7 (14.2%) of such individuals in the Recql5+/+ApcMin/+ cohort. Interestingly, however, although the average number of microadenomas (diameter < 1 mm) was higher in the Recql5-/-ApcMin/+ cohort than in mice of the corresponding Recql5+/+ApcMin/+ cohort, the difference did not reach a significant level (P = 0.1048, Figure 1D, Table 1).
| Genotype | Size | Upper small intestine (duodenum and jejunum) | Lower small intestine (ileum) | Large intestine (colon) |
| Recql5+/+Apcmin/+ | Macroadenoma (≥ 1 mm) | 23.70 ± 4.04 | 52.20 ± 10.90 | 1.20 ± 0.65 |
| Microadenoma (< 1 mm) | 10.80 ± 2.57 | 25.20 ± 7.77 | 0.80 ± 0.65 | |
| Recql5-/-Apcmin/+ | Macroadenoma (≥ 1 mm) | 28.20 ± 3.38 (P = 0.270) | 108.00 ± 11.50 (P = 0.006) | 2.80 ± 0.47 (P = 0.041) |
| Microadenoma (< 1 mm) | 13.10 ± 2.04 (P = 0.360) | 41.40 ± 4.82 (P = 0.070) | 0.90 ± 0.33 (P = 0.450) |
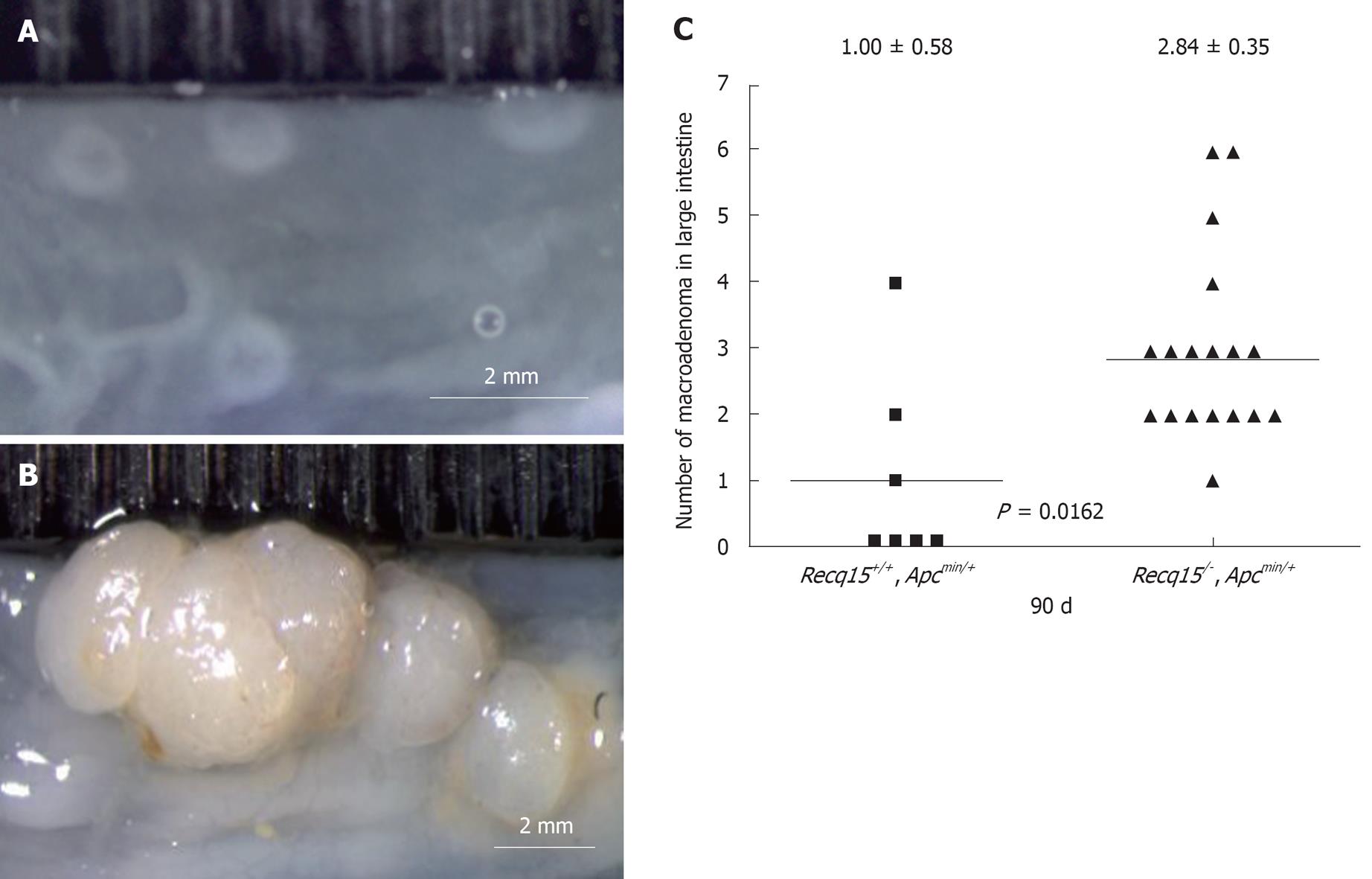
Previous studies have shown that although ApcMin/+ mice are highly prone to intestinal adenomas, colonic tumors were relatively infrequent (< 50% penetrance) in these mice[16]. We found, however, that Recql5 deficiency had a significant impact on the incidence of colonic tumors in these mice. It resulted in an increase of the colonic tumor incidence to 94.1% (16 out of 17 mice) (Figure 2). Moreover, microscopic examination revealed that some Recql5+/+ApcMin/+ mice developed only small polyp-like nodules in their colons (Figure 2A, Table 1), whereas most Recql5-/-ApcMin/+ mice had multiple macroadenomas in each colon (Figure 2B, Table 1). Together, this data clearly indicate that Recql5 deficiency could play an important role in the development of intestinal adenomas in ApcMin/+ mice.
Previous studies have shown that ApcMin/+ mice on a congenic C57BL/6 background develop 30 to 50 macroadenomas at 90 to 120 d of age, respectively[16]. We found that under the mixed genetic background resulting from intercrossing between B6/129.Recql5-/- and B6.ApcMin/+ mice, Recql5+/+ApcMin/+ mice developed 72.9 ± 12.7 macroadenomas (diameter > 1 mm) along the entire intestinal tract at 90 d. This elevated adenoma development in Recql5+/+ApcMin/+ mice suggests the existence of possible modifier(s) in this particular genetic background or a difference in environmental factors, such as diet. Importantly, comparing with cohorts of mice that were selected based on sibling pairs allows us to clearly show that the loss of Recql5 in Apcmin mice has a great impact on intestinal adenoma susceptibility, providing compelling evidence for an important role of Recql5 in tumor suppression in the gastrointestinal tract, particularly in the colon. This finding is consistent with our previous report that showed Recql5 has a tumor suppression role in a number of other organs/tissues[11]. Therefore, our study once again illustrates the recognized limitation of using straight knockout mouse models for assessing the tumorigenic potential of a specific genetic mutation in the GI tract and the usefulness of the Apcmin mice system in overcoming this limitation.
Mouse Recql5 and human RECQL5 are highly conserved. Thus, the findings reported here have also raised a question regarding the possibility that RECQL5 may be a tumor suppressor for human colon cancer. It should be noted, however, that information derived from studies based on mouse models may not be extrapolated for humans. Rather, results must be validated by future studies involving human samples.
The exact mechanism or mechanisms by which Recql5 affected the tumor susceptibility in these Apcmin mice remains to be determined. It should be noted, however, that both perturbation in the homeostasis of crypt stem cells as well as genome instability could affect the tumor susceptibility in this mouse model. We have reported previously that Recql5 has an important role in genome stability, more specifically in suppressing the gross rearrangement type of CIN, suggesting that the effect in CIN could be a contributing factor to the increase in tumor susceptibility as the result of Recql5 deficiency. Intriguingly, human RECQL5 has been implicated in RNAPII transcription ([17,18] and unpublished data from our laboratory), raising the possibility that Recql5 deficiency could contribute to carcinogenesis in the Apcmin mice by affecting RNAPII transcription and hence the homeostasis of the Apc-deficient intestinal and/or colonic stem cells through transcription. Importantly, mouse Recql5 and human RECQL5 are highly conserved. Thus it is possible that human RECQL5 is also an important suppressor for colon cancer. In this regard, it also worth noting that we have recently shown that both Recql5-deficient mouse cells[19] and RECQL5-deficient human colorectal cancer cells are hypersensitive to camptothecin which is the prototype of irinotecan, a drug approved by the FDA for treating colon cancer patients.
Colorectal cancer is a major type of human cancer. Knowledge regarding the molecular basis for the etiology of this disease can help in identifying novel biomarkers for its early diagnosis or in improving the efficacy of intervention regimens.
This is the first report about the role of Recql5, a DNA helicase that plays an important role in the maintenance of genome integrity, in suppressing tumorigenesis in the gastrointestinal (GI) tract in mice.
In this article, the authors reported that Recql5 has a role in suppressing tumorigenesis in the GI tract in mice. Since mouse Recql5 and its human homologue (RECQL5) are highly conserved, these new findings have implicated RECQL5 as a suppressor for colorectal cancer in humans. Thus, RECQL5 may be used as a biomarker for this disease. Moreover, they have recently shown that mutations in Recql5 resulted in a significantly enhanced sensitivity to anticancer drug camptothecin, the prototype of irinotecan that is currently used to treat colorectal cancer patients. Thus, the expression of RECQL5 could be used as a criterion for selecting patients for irinotecan-based chemotherapies.
No direct application may be derived based on the findings from this study. However, these findings should justify further investigation of the potential role of RECQL5 mutations in human GI cancers and the potential use of RECQL5 as a colorectal cancer biomarker or drug target.
This is a well conceived, concisely written article, which certainly deserves publication.
Peer reviewers: Dr. Inti Zlobec, PhD, Institute for Pathology, University Hospital Basel, Schoenbeinstrasse 40, Basel, CH-4031, Switzerland; Nathalie Wong, PhD, BSc (Hons), Professor, Department of Anatomical and Cellular Pathology, The Chinese University of Hong Kong, Shatin NT, Hong Kong, China; Ralph Graeser, PhD, Group Leader, Molecular & Cellular Biology, ProQinase GmbH, Breisacher Str. 117, Freiburg, 79106, Germany
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
| 1. | Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138-141. |
| 3. | Nowell PC. Tumor progression: a brief historical perspective. Semin Cancer Biol. 2002;12:261-266. |
| 4. | Kinzler KW, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997;386:761, 763. |
| 5. | Levitt NC, Hickson ID. Caretaker tumour suppressor genes that defend genome integrity. Trends Mol Med. 2002;8:179-186. |
| 6. | Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910-917. |
| 8. | Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773-785. |
| 9. | Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643-649. |
| 10. | van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2:196-206. |
| 11. | Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, Zheng L, Stark JM, Barnes EL, Chi P, Janscak P. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073-3084. |
| 12. | McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract. 2008;204:479-490. |
| 13. | Näthke IS. The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annu Rev Cell Dev Biol. 2004;20:337-366. |
| 14. | Dove WF, Cormier RT, Gould KA, Halberg RB, Merritt AJ, Newton MA, Shoemaker AR. The intestinal epithelium and its neoplasms: genetic, cellular and tissue interactions. Philos Trans R Soc Lond B Biol Sci. 1998;353:915-923. |
| 15. | Hu Y, Lu X, Barnes E, Yan M, Lou H, Luo G. Recql5 and Blm RecQ DNA helicases have nonredundant roles in suppressing crossovers. Mol Cell Biol. 2005;25:3431-3442. |
| 16. | Mann MB, Hodges CA, Barnes E, Vogel H, Hassold TJ, Luo G. Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund-Thomson syndrome. Hum Mol Genet. 2005;14:813-825. |
| 17. | Izumikawa K, Yanagida M, Hayano T, Tachikawa H, Komatsu W, Shimamoto A, Futami K, Furuichi Y, Shinkawa T, Yamauchi Y. Association of human DNA helicase RecQ5beta with RNA polymerase II and its possible role in transcription. Biochem J. 2008;413:505-516. |
| 18. | Aygün O, Xu X, Liu Y, Takahashi H, Kong SE, Conaway RC, Conaway JW, Svejstrup JQ. Direct inhibition of RNA polymerase II transcription by RECQL5. J Biol Chem. 2009;284:23197-23203. |
| 19. | Hu Y, Lu X, Zhou G, Barnes EL, Luo G. Recql5 plays an important role in DNA replication and cell survival after camptothecin treatment. Mol Biol Cell. 2009;20:114-123. |