Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1221
Revised: December 29, 2009
Accepted: January 5, 2010
Published online: March 14, 2010
AIM: To assess the treatment and tumor-related variables associated with outcome after treatment of spontaneously ruptured hepatocellular carcinoma (HCC).
METHODS: Patients with ruptured HCC were identified. The complications, mortality and survival were assessed. The relationship between tumor size and the severity of hemoperitoneum and between tumor size and grade were examined.
RESULTS: From January 1993 to January 2008, 556 patients with HCC with or without cirrhosis were evaluated; of which, 16 (2.87%) presented with spontaneous rupture. All but 1 patient had cirrhosis. Twelve patients underwent surgical resection while 4 underwent trans-cutaneous arterial catheter embolization (TAE) (trans-cutaneous arterial embolization). Early mortality (< 30 d) was 25% (4 of 16) and was inversely related to Child-Pugh score; 3 of the 4 early deaths occurred in patients treated with TAE with 1 of 12 occurring in the resected group. There was no correlation between tumor size and grade or between size and severity of hemoperitoneum.
CONCLUSION: Tumor size did not correlate with severity of the hemoperitoneum. There was an inverse relationship between G1-G3 (grade of cellular differentiation) HCC and dimensions.
- Citation: Bassi N, Caratozzolo E, Bonariol L, Ruffolo C, Bridda A, Padoan L, Antoniutti M, Massani M. Management of ruptured hepatocellular carcinoma: Implications for therapy. World J Gastroenterol 2010; 16(10): 1221-1225
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1221.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1221
Hepatocellular carcinoma (HCC) is a hypervascular tumor that almost always occurs in the setting of liver cirrhosis. It is the fifth most common cancer in the world and is gradually increasing[1]. Spontaneous rupture is a complication seen in 3%-15% of cases, with an apparent lower incidence reported in Western compared with Asian centers[2]. The exact mechanism leading to rupture is not clearly defined although it appears to be more frequent when the tumor protrudes from the glissonian capsule[3]. Spontaneous rupture is the third leading cause of HCC-related death, after tumor progression and liver failure, and is more common than rupture of oesophageal varices. Previously considered a problem of only large tumors, this is not always the case, and smaller tumors, particularly those with an aggressive behavior, are at risk. The symptoms are related to the position of the tumor. In fact, rupture of deep tumors may be asymptomatic or produce pain while a peripheral lesion will give rise to hemoperitoneum, often with peritonitis and hemodynamic instability.
Appropriate management of this problem remains the subject of some debate. In patients with advanced liver disease or multifocal HCC, trans-cutaneous arterial catheter embolization (TAE) is clearly the most appropriate approach. However, in patients with preserved liver function and resectable tumors, resection may be an option. Although burdened by high mortality in the acute phase and not always feasible, surgical resection offers the indisputable advantage of definitively treating the cancer, in addition to obtaining hemostasis, and may offer a better prognosis[3]. This study analyzes the management of ruptured HCC in a single, tertiary referral center with experience of this disorder, specifically assessing peri-operative outcome with operative and non-operative therapy.
Patients with HCC treated at the authors’ institution were identified from a prospectively maintained hepato-biliary database and registry of Hepato-Biliary surgery. Patients who presented with ruptured tumors were taken from the database for in-depth analysis. Symptoms and clinical status on presentation were recorded and analyzed. Initial treatment was directed at patient resuscitation and hemodynamic stabilization according to the advanced trauma life support protocol. Blood products were administered as necessary. Cross-sectional imaging was used to determine the disease extent, and standard laboratory studies were used to assess hepatic function.
Definitive therapy was selected based on liver functional reserve according to Child-Pugh score, tumor extent and overall performance status. In patients with advanced cirrhosis (Child-Pugh C) or multifocal HCC, TAE was used as definitive therapy. In cases of surgical treatment, a right ‘hockey-stick’ incision was used as the favoured access; median laparotomy was used in one case. Procedures were classified based on Couinaud’s classification[4]. Non-anatomic resection (or wedge resection) denotes a non-segmental resection of the liver surrounding the cancer. The hepatic parenchyma was divided using the crush-clamping method and each vessel encountered was ligated or clipped. Hepatic inflow occlusion was carried out using the Pringle manoeuvre. Argon beam coagulator was used on the cut surface of the liver.
All patients, regardless of treatment, were monitored in the intensive care unit over the immediate post-treatment period. Macroscopic and microscopic histopathology tests were carried out on the resected specimens. The grade of cell differentiation was classified according to the Edmondson and Steiner system[5]. Maximal diameter of the specimen was taken as the tumor size in patients submitted to resection, while radiological images were used to measure HCC in patients treated with TAE. Postoperative complications were documented and classified as minor or major. Peri-operative mortality was defined as death during the same stay in hospital or within 30 d of the date of operation.
Data were processed using Excel. The G1 and G3 groups were compared with the Student’s t test for continuous data; the level of significance was assumed as P < 0.05.
From January 1993 to January 2008, 556 patients with HCC and cirrhosis were seen in our institution. One-hundred and four patients were treated with surgical resection, and 145 underwent percutaneous ethanol injection or radiofrequency ablation. Sixty were treated with TAE with or without chemotherapy. Sixteen patients presented with ruptured HCC, representing 2.87% of the entire population.
Twelve patients underwent surgical resection and 4 were treated with TAE (Table 1). There were 10 males and 6 females with a mean age of 67 years (range: 42-84 years). On admission, hemoperitoneum and abdominal pain were the first presentation in 14/16 cases. Hypovolemic shock was present in 10 patients. Abdominal ultrasonography (US) and/or computed tomography (CT) showed hemoperitoneum in all 14 patients and delineated the liver disease in all but 1 case. Underlying cirrhosis was present in 15 patients (alcoholic in 8, hepatitis C infection in 6 and both in 1). Anemia with a haemoglobin level less than 10 g/dL was present in 7 patients. Four patients had alanine transaminase and aspartate transaminase levels more than twice the upper limits of normal, platelet count less than 100 000 mm3, pro-thrombin time less than 70% and albumin level less than 3.5 g/dL. These four patients were excluded from surgery and treated with TAE. One patient was referred to our unit after urgent laparotomy and packing performed in another hospital.
| Patient | Age (yr) | Sex | Cirrhosis | HCC size | Grading | Treatment |
| 1 | 54 | F | C | 80 | 1 | Resection |
| 2 | 42 | M | - | 80 | 1 | Resection |
| 3 | 59 | F | Alc | 40 | 1 | Resection |
| 4 | 76 | M | Alc | 30 | 3 | Resection |
| 5 | 65 | M | Alc | 40 | 1 | Resection |
| 6 | 84 | M | Alc | 20 | 3 | Resection |
| 7 | 74 | M | C | 19 | 3 | Resection |
| 8 | 80 | M | Alc | 70 | 1 | Resection |
| 9 | 73 | F | C | 30 | 3 | Resection |
| 10 | 78 | M | C | 28 | 3 | Resection |
| 11 | 60 | M | Alc+C | 50 | 1 | Resection |
| 12 | 49 | F | Alc | 30 | 3 | Resection |
| 13 | 67 | F | C | 70 | - | TAE |
| 14 | 74 | F | Alc | 90 | - | TAE |
| 15 | 71 | M | Alc | 40 | - | TAE |
| 16 | 67 | M | C | 45 | - | TAE |
Resection was performed in 12 patients as a single stage operation, ranging from 1 to 6 d from the time of presentation. Seven of these patients had persistent anemia and hemodynamic instability despite aggressive resuscitation and underwent emergency surgery. There were 3 anatomic resections of 2 segments and 4 non-anatomic resections. Four patients stabilized after the initial resuscitative manoeuvres, and surgery was performed from 1-3 d later; the procedures performed in this group were 1 left hepatectomy and 3 wedge resections. One patient required emergency laparotomy for hemorrhagic shock before the investigations were completed and the diagnosis was known; he was found to have multifocal HCC, and a palliative resection of segment 2 and segment 3 was performed to stop the bleeding. This was the only patient with multifocal HCC and/or Child-Pugh C cirrhosis who underwent surgical resection in Child C status and with multifocal and bilobar HCC; he died shortly after the operation from coagulopathy and liver failure. One patient required reoperation for bleeding on day 2 after the initial operation.
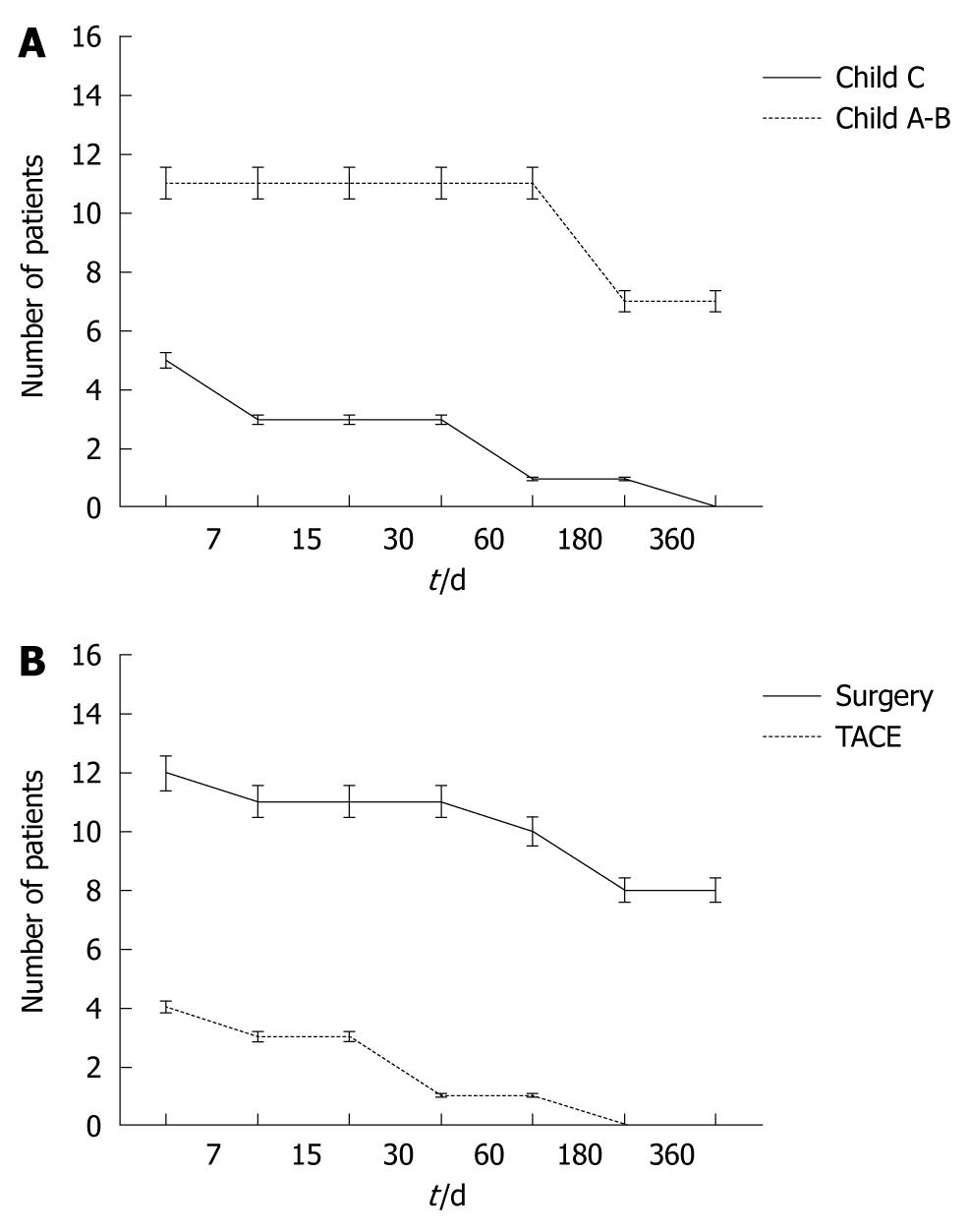
For all patients, the 30 d mortality rate was 25% (n = 4 patients, Figure 1A) and was directly related to underlying liver function. All four patients with Child-Pugh C cirrhosis died in the early post-treatment period – one after resection and three after TAE. The causes of death were coagulopathy (n = 2), recurrent bleeding (n = 1) and liver failure (n = 1). Three additional patients with Child-Pugh A or B cirrhosis and treated with surgical resection died within 6 mo due to liver failure (2 mo, n = 1), myocardial infarction (3 mo, n = 1), liver failure and progression of neoplastic disease (4 mo, n = 1, Figure 1B). Two of these patients had G3 HCC with vascular invasion and none of these patients underwent emergency surgery. They were transfused with 17 units of RPC.
Five patients were treated by emergency surgical resection and 2 patients who were treated with delayed surgical resection, survived more than 24 mo (long-term survival 46%).
Seven patients (43%) treated with resection, (emergency n = 5 and delayed n = 2) survived more than 24 mo. In all of these patients, the tumor grade was G1. Six of these patients were transfused with 27 units of RPC (range: 3-8). Postoperative ascites occurred in 5 patients, while 1 patient developed sub-phrenic abscess with collection drained percutaneously.
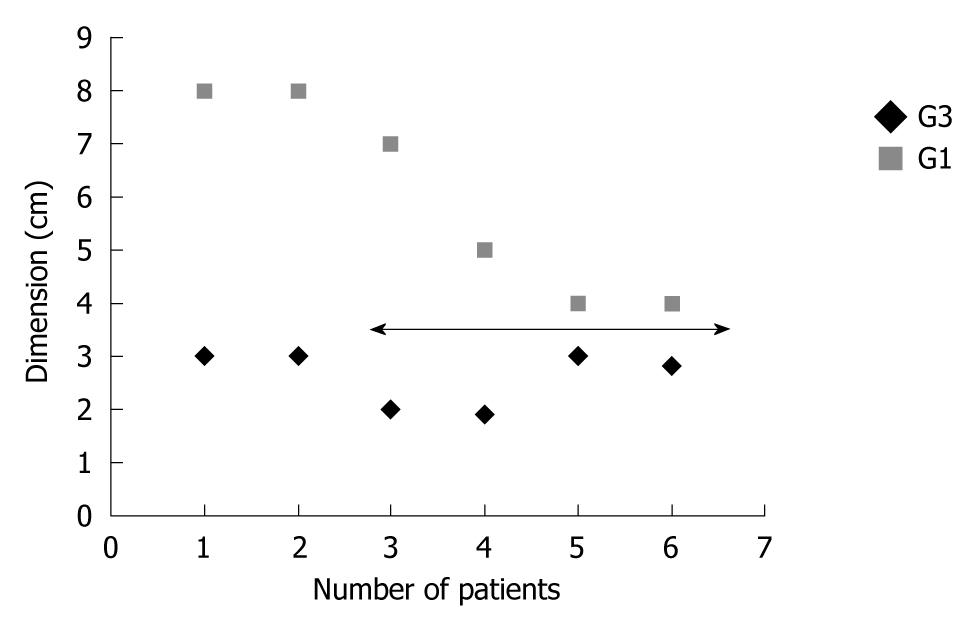
Histological study of the specimens confirmed HCC in all cases. Underlying cirrhosis was present in 11 cases. The average diameter of the HCC was 61 mm (range: 60-90 mm) for the patients who underwent TAE (measured with US or CT) and 43 mm for patients with resected HCC (range: 19-80 mm). The study on the histological specimen showed an average diameter of 60 mm for HCC in G1 status and 26 mm for G3 (Figure 2, P = 0.001).
HCC is the fifth most common tumor in the world and usually occurs in cirrhosis of alcoholic or viral (hepatitis B or C) etiology[1]. HCC is a hypervascular tumor with a high propensity for vascular invasion and the capability to produce growth factors which have a direct effect on endothelial cells to induce neo-angiogenesis. The rupture of HCC is a complication that can occur in 3%-15% of patients, although this has decreased now that screening programs for cirrhotics have been implemented[1,2]. There is a relevant geographical difference between western and oriental countries where a major incidence has been reported in oriental countries[2,6].
The mortality in the acute phase is very high and stands at around 25%-75% of cases[6]. This complication is the third most common cause of death for HCC after neoplastic progression and liver failure; the mortality is higher than that due to bleeding resulting from rupture of esophageal varices[6,7]. Although the incidence of viral cirrhosis is generally predominant, in the northeast area of Italy, as is documented both in the present study and in a recent publication, 50% of HCC occurs in cirrhosis due to alcohol abuse, while the remaining cases can be attributed to hepatitis virus C infection; the incidence of infection related to hepatitis virus B is occasional (Table 1)[8].
The average age reported in the literature varies between 44 and 68 years, with a high prevalence in males (at least 3 times more frequent), confirming the overall impact of HCC[6,9]. In this series, there was a high prevalence in men with respect to women (10 men and 6 women) with an average age of 67 years (range: 42-84 years).
According to the literature, the risk factors for rupture are not well known although rapid growth with necrosis, erosion of vessels, thrombosis of veins in HCC by tumor thrombi or direct invasion individually or combined could be responsible[10,11].
The most common condition for bleeding into the peritoneum is when the HCC is located in one of the free surfaces of the liver. In this case there is no hepatic parenchyma surrounding the tumor and this is free to flood the peritoneum with blood and neoplastic cells. Many intra-parenchymal HCC may spontaneously break, but a deeper position in the center of the liver confines the bleeding around the cancer and the clinical manifestation is only pain without hemoperitoneum. Abnormal clotting and thrombocytopenia typical of cirrhosis may also worsen the hemorrhagic event.
The typical symptoms of spontaneous rupture are epigastric pain associated with clinical signs of shock and peritoneal irritation. This is especially true when the lesion is more peripheral on the liver. Peritoneal irritation due to bleeding is not evident in cases of rupture of a deeper lesion which does not interrupt the liver capsule. In addition to pain and hemorrhagic shock, the risk of peritoneal seeding may worsen the prognosis of these patients. In all of the cases in our study, rupture occurred in a peripheral tumor. A study by Kanematsu et al[12] in 1992, reported that protrusion of the tumor is a relevant prognostic factor of rupture. The peri-operative mortality in cases who undergo urgent surgery is considerably higher than those reported for planned liver resections in patients with cirrhosis and is related to Child status before the rupture[9]. Of course, bleeding and shock are factors which strongly influence the prognosis of these patients, and bleeding “per se” worsens the prognosis, however, decompensated cirrhosis may be fatal in cases of poor liver function. Therefore, the first step of treatment is resuscitation and stabilization of the patient, but achieving hemostasis is the primary goal. When facing a rupture of HCC with underlying cirrhosis it is mandatory to obtain a correct diagnosis in order to achieve the best treatment for each patient. The therapeutic approach may be crucial for prognosis. This choice must take into account the hemodynamic conditions, functional status of the liver and stage of cancer.
Although associated with complications such as rebleeding and a mortality rate of around 30%, TAE is the best method to achieve hemostasis without surgery[6,13]. Moreover, when feasible, super-selective TAE is able to preserve liver function and has the dual outcome of being a definitive treatment or a bridge to resectable HCC. As in other studies, the four patients in our study treated with TAE should not be compared with those who were treated with surgical resection. In fact, all four patients had severe cirrhosis or bilobar HCC while the resected patients had favourable location of HCC and good functional reserve. For this reason a comparative study and analysis of the results reported in the literature are very difficult. Our experience confirms that TAE is indicated in cases of poor liver function (Child C) or in cases of multifocal or bilobar HCC. Alternatively, TAE can be used as a bridge to surgical resection[14]. According to previously published reports and analysis of the data from the present study in patients with Child C status who underwent TAE, it is clear that the post-procedure mortality was very high, confirming that poor liver functional reserve determines survival regardless of treatment type[6]. Moreover, it is worth remembering the demerits of TAE which include rebleeding, liver abscess and implanted peritoneal metastases[15].
Histological examination revealed that the average size of the lesions in this study was 43 mm. Substantial differences were seen between the dimensions in relation to grading. In fact, G1 HCC had an average size of 60 mm, while G3 HCC had an average size of 26 mm (P = 0.001). However, all lesions were on the liver surface. We did not find a relationship between severity of hemoperitoneum and size or grading of HCC.
The experienced liver team should evaluate the next treatment steps according to liver functional reserve and extension of HCC. Preoperative assessment of the patient with blood tests is mandatory as is cancer staging using US and radiological imaging.
It was reported by Tanaka et al[6] that rupture of HCC should be differentiated when it occurs in the early phase of cirrhosis rather than in the late phase. The prognosis is strongly determined by the functional status of the liver immediately after bleeding. In fact, 3 patients in the present series were excluded from surgery due to poor liver function and not surprisingly had the worst outcome. In this cohort, functional liver reserve and G3 status influenced outcome, while emergency or delayed surgery and HCC dimensions did not seem to modify the prognosis. Several reports in the literature demonstrate acceptable results even in the case of one stage hepatectomy for ruptured HCC and limited resection is recommended[2,16,17].
Moreover, peritoneal washing with saline solution can help to reduce the chance of spreading peritoneal cancer which may be a complication of TAE[18,19].
In conclusion, in cases of ruptured HCC, any lesion should be considered for surgery if a low-risk curative resection is possible in a Child A-B patient. If necessary and possible, the intervention may be delayed. TAE is palliative procedure indicated when the liver function is compromised or in the case of multifocal-bilobar HCC. The introduction of screening programs should reduce the number of large HCCs which have broken, while we may have some concern about the rupture of smaller poorly differentiated tumors.
Hepatocellular carcinoma (HCC) is a hypervascular tumor that sometimes due to unknown reasons breaks spontaneously regardless of size, grading and state of cirrhosis. Location on the free surface of the liver seems to be a major factor in rupture of HCC. The choice of therapy is closely linked to liver functional reserve, grade of tumor and performance status of the patient.
Larger prospective studies are necessary to assess the relationship between HCC dimensions and grading and the possibility of rupture.
There is an inverse relationship between G1-G3 HCC and dimensions. In the case of ruptured HCC, resection should not be excluded “a priori” but carefully considered since it has the dual purpose of both achieving hemostasis and being a definitive treatment.
In the case of advanced liver disease or bilobar multifocal HCC, a non-operative approach such as trans-cutaneous arterial catheter embolization must be attempted. In contrast, preserved liver function and resectable tumor should be considered as clear indications for surgical resection.
This study aimed to assess the treatment and tumor-related variables associated with outcome after treatment of spontaneously ruptured HCC. The paper is interesting.
Peer reviewer: Dr. Bernardo Frider, MD, Professor, Department of Hepatology, Hospital General de Agudos Cosme Argerich, Alte Brown 240, Buenos Aires 1155, Argentina
S- Editor Wang YR L- Editor Webster JR E- Editor Zheng XM
| 2. | Vergara V, Muratore A, Bouzari H, Polastri R, Ferrero A, Galatola G, Capussotti L. Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. 2000;26:770-772. |
| 3. | Recordare A, Bonariol L, Caratozzolo E, Callegari F, Bruno G, Di Paola F, Bassi N. Management of spontaneous bleeding due to hepatocellular carcinoma. Minerva Chir. 2002;57:347-356. |
| 5. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. |
| 6. | Tanaka A, Takeda R, Mukaihara S, Hayakawa K, Shibata T, Itoh K, Nishida N, Nakao K, Fukuda Y, Chiba T. Treatment of ruptured hepatocellular carcinoma. Int J Clin Oncol. 2001;6:291-295. |
| 7. | Ong GB, Chu EP, Yu FY, Lee TC. Spontaneous rupture of hepatocellular carcinoma. Br J Surg. 1965;52:123-129. |
| 8. | Caratozzolo E, Massani M, Recordare A, Bonariol L, Baldessin M, Bassi N. Liver resection in elderly: comparative study between younger and older than 70 years patients. Outcomes and implications for therapy. G Chir. 2007;28:419-424. |
| 9. | Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, Lam CM, Wong J. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19:3725-3732. |
| 10. | Chearanai O, Plengvanit U, Asavanich C, Damrongsak D, Sindhvananda K, Boonyapisit S. Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer. 1983;51:1532-1536. |
| 11. | Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191-198. |
| 12. | Kanematsu M, Imaeda T, Yamawaki Y, Seki M, Goto H, Sone Y, Iinuma G, Mochizuki R, Doi H. Rupture of hepatocellular carcinoma: predictive value of CT findings. AJR Am J Roentgenol. 1992;158:1247-1250. |
| 13. | Shimada R, Imamura H, Makuuchi M, Soeda J, Kobayashi A, Noike T, Miyagawa S, Kawasaki S. Staged hepatectomy after emergency transcatheter arterial embolization for ruptured hepatocellular carcinoma. Surgery. 1998;124:526-535. |
| 14. | Chu F, Morris DL. Single centre experience of liver resection for hepatocellular carcinoma in patients outside transplant criteria. Eur J Surg Oncol. 2006;32:568-572. |
| 15. | Hai L, Yong-Hong P, Yong F, Ren-Feng L. One-stage liver resection for spontaneous rupture of hepatocellular carcinoma. World J Surg. 2005;29:1316-1318. |
| 16. | Yeh CN, Lee WC, Jeng LB, Chen MF, Yu MC. Spontaneous tumour rupture and prognosis in patients with hepatocellular carcinoma. Br J Surg. 2002;89:1125-1129. |
| 17. | Chiappa A, Zbar A, Audisio RA, Paties C, Bertani E, Staudacher C. Emergency liver resection for ruptured hepatocellular carcinoma complicating cirrhosis. Hepatogastroenterology. 1999;46:1145-1150. |
| 18. | Sonoda T, Kanematsu T, Takenaka K, Sugimachi K. Ruptured hepatocellular carcinoma evokes risk of implanted metastases. J Surg Oncol. 1989;41:183-186. |
| 19. | Yeh CN, Chen HM, Chen MF, Chao TC. Peritoneal implanted hepatocellular carcinoma with rupture after TACE presented as acute appendicitis. Hepatogastroenterology. 2002;49:938-940. |