Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1201
Revised: December 10, 2009
Accepted: December 17, 2009
Published online: March 14, 2010
AIM: To understand the implication of GATA-4 and GATA-5 methylation in gastric carcinogenesis.
METHODS: Methylation status of GATA-4 and GATA-5 CpG islands in human gastric mucosa samples, including normal gastric biopsies from 45 outpatients, gastric dysplasia [low-grade gastric intraepithelial neoplasia (GIN), n = 30; indefinite, n = 77], and 80 paired sporadic gastric carcinomas (SGC) as well as the adjacent non-neoplastic gastric tissues was analyzed by methylation specific polymerase chain reaction (MSP) and confirmed by denatured high performance liquid chromatography (DHPLC). Immunohistochemical staining was used to detect protein expression. The correlation between GATA-4 and GATA-5 methylation and clinicopathological characteristics of patients including Helicobacter pylori (H. pylori) infection was analyzed.
RESULTS: GATA-4 and GATA-5 methylation was frequently observed in SGCs (53.8% and 61.3%, respectively) and their corresponding normal tissues (41.3% and 46.3%) by MSP. The result of MSP was consistent with that of DHPLC. Loss of both GATA-4 and GATA-5 proteins was associated with their methylation in SGCs (P = 0.01). Moreover, a high frequency of GATA-4 and GATA-5 methylation was found in both gastric low-grade GIN (57.1% and 69.0%) and indefinite for dysplasia (42.9% and 46.7%), respectively. However, GATA-4 and GATA-5 methylation was detected only in 4/32 (12.5%) and 3/39 (7.7%) of normal gastric biopsies. GATA-4 methylation in both normal gastric mucosa and low-grade GIN was also significantly associated with H. pylori infection (P = 0.023 and 0.027, two-sides).
CONCLUSION: Epigenetic inactivation of GATA-4 (and GATA-5) by methylation of CpG islands is an early frequent event during gastric carcinogenesis and is significantly correlated with H. pylori infection.
-
Citation: Wen XZ, Akiyama Y, Pan KF, Liu ZJ, Lu ZM, Zhou J, Gu LK, Dong CX, Zhu BD, Ji JF, You WC, Deng DJ. Methylation of
GATA-4 andGATA-5 and development of sporadic gastric carcinomas. World J Gastroenterol 2010; 16(10): 1201-1208 - URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1201
GATA proteins comprise a small family of transcriptional factors defined by a highly conserved DNA-binding domain that interacts specifically with DNA cis-elements. Six distinct vertebrate GATA proteins have been characterized and classified into two subfamilies based on their structural and expression patterns. GATA-1, -2 and -3 are important in the development and differentiation of the hematopoietic cell lineage, while GATA-4, -5 and -6 guide development and differentiation in the endoderm-derived organs, and specification of proper gut embryogenesis[1-3]. GATA-4 deficient cells exhibit an intrinsic defect in gastric epithelial cell differentiation to parietal cells in mice[4]. Overexpression of GATA-5 induced TFF1 expression[5]. Thus, GATA-4, -5 and -6 may play a critical role in the regulation of stomach-specific gene expression and cancer development. GATA-6 may function as an oncogene since it is often upregulated in proliferating progenitor cells[6-8]. In contrast, GATA-4 and GATA-5 may function as tumor suppressors to drive endodermal-specific differentiation because they are potential upregulators of the differentiation-related genes in the endoderm-derived organs[9].
The endoderm gives rise to the lining epithelium of the gastrointestinal tract. Loss of GATA-4 and GATA-5 might affect gastrointestinal epithelial cell differentiation, leading to tumorigenesis of these cells. In fact, there is growing evidence linking loss of GATA-4 and GATA-5 expression to many malignancies, such as primary esophageal, colorectal, gastric and pulmonary cancers[5,10-12]. Moreover, in addition to genetic inactivation, epigenetic changes such as methylation of CpG islands may play a major role in the loss of GATA-4 and GATA-5 function[5,10,12]. Since methylation of GATA-4 and GATA-5 is a frequent event in gastric cancer cell lines[5], it is interesting to know that methylation silences of GATA-4 and GATA-5 during gastric carcinogenesis.
Primary sporadic gastric carcinoma (SGC) and the corresponding normal gastric samples: Fresh-frozen surgical SGC and their corresponding adjacent non-neoplastic “normal” samples were from the Tissue Bank at Beijing Cancer Hospital (n = 80; 62 males and 18 females, aged 35-81 years, average age 58.5 years). The clinical and histological information for each case was also collected according to approved institutional guidelines. The 1997 UICC-TNM criteria were used for classification of gastric cancers.
Gastric dysplasia samples: Biopsies of gastric dysplasia lesions [low-grade noninvasive gastric intraepithelial neoplasia (GIN), n = 30; indefinite for dysplasia, n = 77] (51 males and 56 female, aged 41-65 years, average age 51.7 years) were collected from patients without malignant disease enrolled in a gastro-endoscopic survey in Linqu County, a rural area in Shandong Province, China which has one of the world’s highest rates of gastric cancer[13]. Histopathological diagnosis of each case was made by three senior pathologists at the Department of Pathology in Beijing Cancer Hospital, according to the Padova international classification[14]. Information on Helicobacter pylori (H. pylori) infection by the 13C-urea breath test was also collected[15].
Gastric biopsies from patients with and without chronic gastritis: Gastric mucosa biopsies were collected from outpatients undergoing gastro-endoscopic examination at Beijing Cancer Hospital. Of 45 gastric biopsies used in the present study, 18 patients were diagnosed with superficial chronic gastritis and 27 without obvious pathological changes (43 males and 2 female, aged 19-47 years, average age 30.7 years).
Informed consent was obtained from all subjects and the institutional review committee approved this study.
H. pylori in the normal or corresponding normal gastric samples was analyzed with a H. pylori-specific 23S rRNA-polymerase chain reaction (PCR) assay as described[16].
DNA extraction, bisulfite treatment, and MSP were performed as described previously[17,18]. The MSP primer sequences for GATA-4 and GATA-5 were as follows: GATA-4M sense, 5′-GTATAGTTTCGTAGTTTGCGTTTAGC-3′; GATA-4M antisense, 5′-AACTCGCGACTCGAATCCCCG-3′; GATA-4U sense, 5′-TTTGTATAGTTTTGTAGTTTGTGTTTAGT-3′; GATA-4U antisense, 5′-CCCAACTCACAACTCAAATCCCCA-3′; GATA-5M sense, 5′-AGTTCGTTTTTAGGTTAGTTTTCGGC-3′; GATA-5M antisense, 5′-CCAATACAACTAAACGAACGAACCG-3′; and GATA-5U sense, 5′-TGGAGTTTGTTTTTAGGTTAGTTTTTGGT-3′; GATA-5U antisense, 5′-CAAACCAATACAACTAAACAAACAAACCA-3′ as described by Gou et al[12].
The MSP products for GATA-4 and GATA-5 were subcloned with the pEASY-T1 simple clone system (TransGen Biotech Company, Beijing, China), and sequenced on an ABI PRISM 3730 DNA Analyzer.
Both methylated and unmethylated GATA-4 CpG islands were amplified by a universal primer set without CpG (GATA-4uni sense 5'-GGAGATTTTAGAGTTTGGAT-3' and antisense 5'-CTCCCACTAACTACCTCTCT-3') under thermal cycle conditions [95°C for 15 min → (95°C for 30 s → 52.8°C for 30 s → 72°C for 50 s) × 40 cycles → 72°C for 10 min]. The 385-bp PCR product of the methylated and unmethylated GATA-4 were separated by DHPLC at a partial denaturing temperature of 57.1°C and detected by a fluorescence (FL)-detector. The proportion of the methylated GATA-4 in the testing samples was calculated according to the ratio of the peak area for the methylated GATA-4 to the total peak area for both the methylated and unmethylated GATA-4. Genomic DNA of the HCT116 cell line was used as a GATA-4 methylation positive control.
Paraffin sections (4 μm) were dewaxed and rehydrated in xylene and ethanol. For antigen retrieval, the sections were autoclaved in 1.0 mmol/L EDTA for 5 min, followed by immersion in 3% H2O2 methanol for 15 min to block endogenous peroxidase. The sections were then blocked in 15% normal goat serum in phosphate-buffered saline, followed by incubation with polyclonal GATA-4 or GATA-5 antibodies (Sigma-Aldrich, Inc., Missouri, USA) (1:100) overnight at 4°C. Then the sections were incubated with biotinylated goat anti-rabbit IgG working solution for 15 min at room temperature. The standard SP (Streptavidin/peroxidase) process was then performed according to the instructions for the HistostainTM-Plus Kits (Beijng Zhongshan Goldenbridge Biotechnology Company, Beijng, China). Diaminobenzidine was used as a chromogen, followed by counterstaining with hematoxylin. Normal gastric mucosa samples were used as a positive control. PBS was used as a negative control to replace GATA-4/-5 antibody. We regarded GATA-4 and GATA-5 expression as positive when 10% or more cancer cells exhibited GATA-4 and GATA-5 expression.
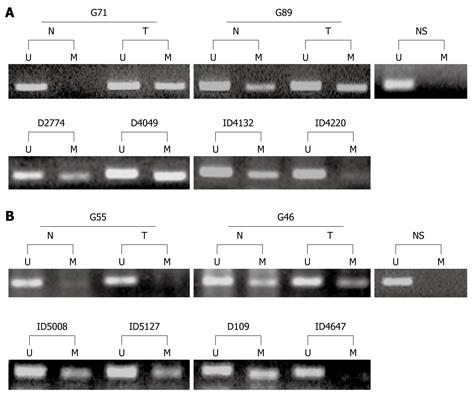
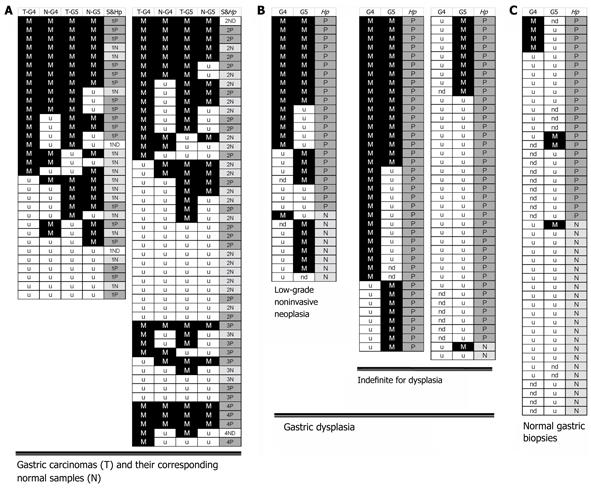
The promoter methylation status of GATA-4 and GATA-5 in eighty SGCs and their corresponding normal samples were analyzed by MSP. GATA-4 and GATA-5 methylation was observed in 43 and 49 SGCs (53.8% and 61.3%), respectively (Figure 1, Figure 2A and Table 1). To our surprise, these genes were also frequently methylated in the corresponding non-neoplastic samples, 33 for GATA-4 (41.3%) and 37 for GATA-5 (46.3%), which were only slightly lower than those in SGCs (Figure 2A and Table 1).
| Clinicopathological characteristics | Methylation positive rate (%) | |||
| GATA-4 | GATA-5 | |||
| SGC | N | SGC | N | |
| SGC invasion | ||||
| T1&T2 (n = 20) | 45.0a | 35.0b | 45.0 | 45.0 |
| T3 (n = 50) | 54.0 | 42.0 | 68.0 | 48.0 |
| T4 (n = 10) | 70.0 | 50.0 | 60.0 | 40.0 |
| Metastasis | ||||
| Yes (n = 44) | 52.3 | 31.8 | 61.4 | 36.4 |
| No (n = 36) | 55.6 | 52.8 | 61.1 | 38.9 |
| H. pylori infection | ||||
| Positive (n = 42) | 61.9 | 45.2 | 64.3 | 50.0 |
| Negative (n = 34) | 41.2 | 38.2 | 55.9 | 44.1 |
| Age (yr) | ||||
| ≤ 59 (n = 42) | 45.2 | 33.3 | 57.1 | 28.6 |
| ≥ 60 (n = 38) | 63.2 | 50.0 | 65.8 | 65.8c |
| Sex | ||||
| Male (n = 62) | 51.6 | 41.9 | 61.3 | 51.6 |
| Female (n = 18) | 61.1 | 38.9 | 61.1 | 27.6d |
| Total | ||||
| n = 80 | 53.8 | 41.3 | 61.3 | 46.3e |
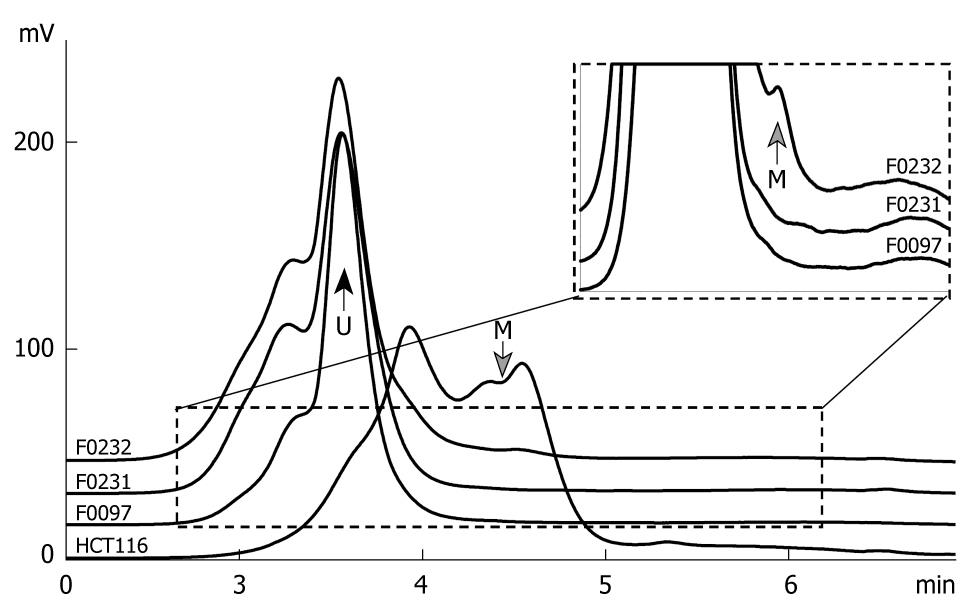
To confirm the results of the MSP assay, we established a DHPLC assay to detect the frequency and proportion of the methylated GATA-4 in 16 paired-SGC representative samples, in which GATA-4 methylation was observed in both SGCs and their corresponding normal tissues by MSP. The methylated and unmethylated GATA-4 was completely separated by DHPLC at a partial denaturing temperature of 57.1°C (Figure 3). GATA-4 methylation was detected in 15 SGCs (93.8%) and 12 adjacent normal samples (75.0%). The proportion of the methylated GATA-4 CpG islands in the DHPLC-positive SGC samples was slightly higher than that in the DHPLC-positive corresponding normal tissues (1.33% ± 0.45% vs 1.10% ± 0.56%, paired t-test, P = 0.054). The DHPLC results corresponded well with the above MSP results.
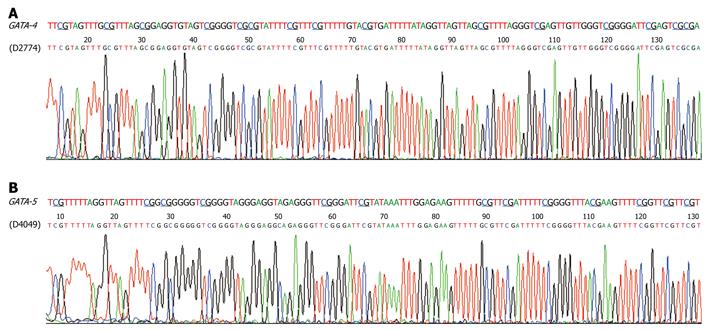
We analyzed the methylation status of these genes in gastric biopsies with low-grade GIN or indefinite for dysplasia in the subjects from Linqu County, a high risk area for stomach cancer in China. Results showed that GATA-4 and GATA-5 were also frequently methylated in these dysplasia lesions, with strong methylated signals in 46 of 98 (46.9%) and 55 of 104 (52.9%), respectively (Figure 2B and Table 2). More GATA-4 and GATA-5 methylation was observed in tissues with GIN (57.1% and 69.0%, respectively) than in indefinite for dysplasia (42.9% and 46.7%), especially for GATA-5 (P < 0.05) (Table 2). GATA-4 and GATA-5 methylation in two representative dysplasia samples were confirmed by bisulfite clone sequencing (Figure 4). In contrast, these genes were rarely methylated in the relatively normal gastric biopsies. Among the 45 gastric biopsies (27 normal and 18 superficial chronic gastritis), GATA-4 and GATA-5 methylation was only 4/32 (12.5%) and 3/39 (7.7%), respectively (Figure 2C), significantly lower than the methylation in gastric dysplasia and SGCs as well as the adjacent normal samples (P < 0.001).
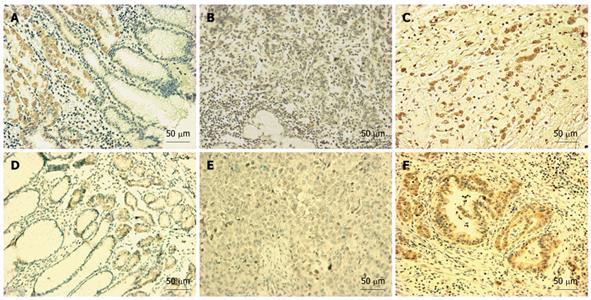
We investigated the expression of GATA-4 and GATA-5 in normal and gastric carcinoma tissues using IHC. These genes were expressed in the gland region of normal gastric mucosa (Figure 5A and D). In contrast, GATA-4 and GATA-5 expression was obviously decreased in many gastric carcinomas (Figure 5B and E, Table 3). A significant inverse relationship was observed between GATA-4 (or GATA-5) methylation and GATA-4 (or GATA-5) protein expression in the gastric samples tested using IHC (Figure 3 and Table 3).
| Protein expression, by IHC | GATA-4 methylation status | GATA-5 methylation status | ||||
| M | U | P-valuea | M | U | P-value | |
| Positive (+ ~ +++) | 7 | 11 | 0.012 | 5 | 9 | 0.011 |
| Negative (- ~ ±) | 11 | 3 | 16 | 3 | ||
As shown in Table 1, an increased trend of GATA-4 methylation was observed in SGCs and the corresponding normal samples with depth of tumor invasion (T1-2, T3, T4), but was not statistically significant. The GATA-5 methylation positive rate in the corresponding normal tissues from elderly and male patients was significantly higher than that from young and female patients, respectively (Table 1).
Moreover, a correlation was observed between GATA-4 (or GATA-5) methylation and H. pylori infection. More GATA-4 and GATA-5 methylation was detected in SGCs and their corresponding normal samples with H. pylori infection than in those without infection, but was not statistically significant (P = 0.071) (Figure 2B). In the gastric dysplasia tissues, particularly in those with low-grade GIN, the GATA-4 methylation positive rate for the patients with H. pylori infection was significantly higher than that in patients without H. pylori infection (15/21 vs 1/7, P = 0.023, Table 2).
In addition, all 4 biopsies with GATA-4 methylation were from H. pylori infected patients (n = 14). No GATA-4 methylation was observed in biopsies from subjects without H. pylori infection (n =18, P = 0.027, two-sides). Of 3 biopsies with GATA-5 methylation, 2 were from H. pylori infected patients (Figure 2C).
Epigenetic silencing of GATA-4 and GATA-5 by DNA methylation has been reported in gastrointestinal cancer cell lines and primary carcinomas[5,10,12]. However, the implication of GATA-4 and GATA-5 methylation in the development of gastric cancer is unclear. Hence, we analyzed the methylation status of GATA-4 and GATA-5 CpG islands in gastric tissues from different kinds of lesions. We found that GATA-4 and GATA-5 methylation was detected in 53.8% and 61.3% of SGCs by MSP, respectively. These results were confirmed by the quantitative DHPLC assay. Furthermore, a significant inverse relationship was observed between methylation status and their protein expression in the gastric samples tested using IHC. These results indicate that GATA-4 and GATA-5 may be frequently inactivated in SGCs by DNA methylation. Moreover, the high prevalence of GATA-4 and GATA-5 methylation in the corresponding normal tissues suggests that these aberrant methylations may be a gastric field-effect, a phenomenon which happens within the whole target regions during carcinogenesis[21].
GATA-4 and GATA-5 were also methylated in 46.9% and 52.9%, respectively, of gastric dysplasia, a precancerous lesion of gastric carcinomas. However, both of these genes were seldom methylated in gastric tissues with or without chronic gastritis [4/32 (12.5%) and 3/39 (7.7%), respectively]. These results indicate that GATA-4 and GATA-5 methylation is an early frequent event during the development of gastric carcinomas.
In addition, we found that the GATA-5 methylation positive rate in the corresponding normal tissues from elderly and male patients was significantly higher than that from young and female patients, respectively. These results are consistent with the higher incidence of gastric carcinoma in males than in females, and the increasing prevalence of gastric carcinomas among elderly subjects.
H. pylori infection is the main cause of chronic atrophic gastritis, which may play an important role in gastric carcinogenesis. A number of tumor-related genes such as p16 could be inactivated by DNA methylation in gastric mucosa lesions with H. pylori infection[22,23]. In the present study, we found that more GATA-4 and GATA-5 methylation in gastric samples from patients with H. pylori infection were detected than in those without H. pylori infection, especially for GATA-4 methylation. The GATA-4 methylation positive rate in the low-grade GIN patients with H. pylori infection was significantly higher than that in patients without H. pylori infection (15/21 vs 1/7, P = 0.023). These results suggest that GATA-4 and GATA-5 methylation could be initiated in the precancerous stage by H. pylori infection.
In addition, among gastric tissues with or without chronic gastritis, all 4 biopsies with GATA-4 methylation were from H. pylori infected patients (n = 14), no GATA-4 methylation was observed in biopsies from subjects without H. pylori infection (n =18; P = 0.027, two-sides), and of 3 biopsies with GATA-5 methylation, 2 were from H. pylori infected patients. These results again support the hypothesis that H. pylori infection may contribute to epigenetic inactivation of these genes in gastric mucosa.
In conclusion, GATA-4 and GATA-5 methylation was an early frequent field-effect and significantly correlated with the severity of pathological changes during gastric carcinogenesis. H. pylori infection may contribute to GATA-4 and GATA-5 methylation in the human stomach.
Tumor suppressor genes GATA-4 and GATA-5 are important for development of the stomach during embryogenesis. Epigenetic inactivation of these genes by DNA hypermethylation was previously reported in esophageal and lung cancer.
In the present study, Dr. Wen et al found that aberrant GATA-4 and GATA-5 methylation was also a frequent event in gastric carcinomas and their adjacent tissues. Interestingly, their work demonstrated that epigenetic inactivation of GATA-4 and GATA-5 was observed in about 50% of gastric mucosa samples with epithelial dysplasia, a precancerous lesion in the stomach. However, such a phenomenon was very rare in gastric mucosa biopsies from healthy subjects or patients with chronic gastritis. They also observed that Helicobacter pylori (H. pylori) infection correlated well with GATA-4 and GATA-5 methylation. These results indicate that epigenetic inactivation of GATA-4 and GATA-5 is an early frequent event during gastric carcinogenesis by H. pylori and might be used to screen patients with a high risk of stomach cancer.
The authors have tried to show that methylation of GATA-4 and GATA-5 could be important in the oncogenesis of gastric mucosa in patients coming from a Chinese area where gastric cancer is common. They showed that in cancer and adjacent mucosa methylation of these two antioncogenes is common and apparently related mostly with chronic H. pylori infection.
Peer reviewer: Roberto Mazzanti, MD, Professor, Chair of Medical Oncology, Department of Internal Medicine, University of Florence, viale Morgagni, 85-50134 Florence, Italy
S- Editor Wang YR L- Editor Webster JR E- Editor Lin YP
| 2. | Laverriere AC, MacNeill C, Mueller C, Poelmann RE, Burch JB, Evans T. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem. 1994;269:23177-23184. |
| 3. | Nishi T, Kubo K, Hasebe M, Maeda M, Futai M. Transcriptional activation of H+/K+-ATPase genes by gastric GATA binding proteins. J Biochem. 1997;121:922-929. |
| 4. | Maeda M, Kubo K, Nishi T, Futai M. Roles of gastric GATA DNA-binding proteins. J Exp Biol. 1996;199:513-520. |
| 5. | Akiyama Y, Watkins N, Suzuki H, Jair KW, van Engeland M, Esteller M, Sakai H, Ren CY, Yuasa Y, Herman JG. GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol Cell Biol. 2003;23:8429-8439. |
| 6. | Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18:2901-2911. |
| 7. | Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048-1060. |
| 8. | Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579-3590. |
| 9. | Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784-789. |
| 10. | Guo M, House MG, Akiyama Y, Qi Y, Capagna D, Harmon J, Baylin SB, Brock MV, Herman JG. Hypermethylation of the GATA gene family in esophageal cancer. Int J Cancer. 2006;119:2078-2083. |
| 11. | Bai Y, Akiyama Y, Nagasaki H, Yagi OK, Kikuchi Y, Saito N, Takeshita K, Iwai T, Yuasa Y. Distinct expression of CDX2 and GATA4/5, development-related genes, in human gastric cancer cell lines. Mol Carcinog. 2000;28:184-188. |
| 12. | Guo M, Akiyama Y, House MG, Hooker CM, Heath E, Gabrielson E, Yang SC, Han Y, Baylin SB, Herman JG. Hypermethylation of the GATA genes in lung cancer. Clin Cancer Res. 2004;10:7917-7924. |
| 13. | Liu F, Pan K, Zhang X, Zhang Y, Zhang L, Ma J, Dong C, Shen L, Li J, Deng D. Genetic variants in cyclooxygenase-2: Expression and risk of gastric cancer and its precursors in a Chinese population. Gastroenterology. 2006;130:1975-1984. |
| 14. | Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167-176. |
| 15. | Zhang L, Shen L, Ma JL, Pan KF, Liu WD, Li J, Xiao SD, Lin SR, Classen M, You WC. Eradication of H pylori infection in a rural population: one-day quadruple therapy versus 7-day triple therapy. World J Gastroenterol. 2006;12:3915-3918. |
| 16. | Liu Z, Shen J, Zhang L, Shen L, Li Q, Zhang B, Zhou J, Gu L, Feng G, Ma J. Prevalence of A2143G mutation of H. pylori-23S rRNA in Chinese subjects with and without clarithromycin use history. BMC Microbiol. 2008;8:81. |
| 17. | Sun Y, Deng D, You WC, Bai H, Zhang L, Zhou J, Shen L, Ma JL, Xie YQ, Li JY. Methylation of p16 CpG islands associated with malignant transformation of gastric dysplasia in a population-based study. Clin Cancer Res. 2004;10:5087-5093. |
| 18. | Wen XZ, Akiyama Y, Baylin SB, Yuasa Y. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene. 2006;25:2666-2673. |
| 19. | Deng D, Deng G, Smith MF, Zhou J, Xin H, Powell SM, Lu Y. Simultaneous detection of CpG methylation and single nucleotide polymorphism by denaturing high performance liquid chromatography. Nucleic Acids Res. 2002;30:E13. |
| 20. | Luo D, Zhang B, Lv L, Xiang S, Liu Y, Ji J, Deng D. Methylation of CpG islands of p16 associated with progression of primary gastric carcinomas. Lab Invest. 2006;86:591-598. |
| 21. | Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci. 2009;39:331-337. |
| 22. | Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989-995. |
| 23. | Dong CX, Deng DJ, Pan KF, Zhang L, Zhang Y, Zhou J, You WC. Promoter methylation of p16 associated with Helicobacter pylori infection in precancerous gastric lesions: a population-based study. Int J Cancer. 2009;124:434-439. |