Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1171
Revised: January 22, 2010
Accepted: January 29, 2010
Published online: March 14, 2010
The finding of lipid accumulation in the liver, so-called hepatic steatosis or non-alcoholic fatty liver disease, is a common condition frequently found in healthy subjects. Its prevalence, in fact, has been estimated by magnetic resonance studies to be about 35% in the general population and 75% in obese persons. Nevertheless, its presence generates liver damage only in a small percentage of subjects not affected by other liver diseases. It should be defined as a “co-factor” capable of affecting severity and progression, and also therapeutic perspectives, of liver diseases to which it is associated. Herein we will evaluate the impact of hepatic steatosis and obesity on the most common liver diseases: chronic viral hepatitis C and B, and alcoholic liver disease.
- Citation: Persico M, Iolascon A. Steatosis as a co-factor in chronic liver diseases. World J Gastroenterol 2010; 16(10): 1171-1176
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1171.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1171
Lipid accumulation in the liver, so-called hepatic steatosis or non-alcoholic fatty liver disease (NAFLD), is a common condition frequently found in healthy subjects not affected by any other liver disease and not drinking alcohol. Hepatic steatosis prevalence has been estimated by magnetic resonance studies to be 35% in the general population and to be 75% in obese persons, and these figures seem to be increasing over time[1,2]. Nevertheless, its presence generates liver damage in only a small percentage of subjects not affected by other liver diseases. In fact, in only 2% of the general population does hepatic steatosis constitute a real hepatic disease: non-alcoholic steatohepatitis (NASH) with deranged aminotransferases and fibrosis.
Steatosis is also frequently found in liver specimens of patients who have undergone liver biopsy for all the hepatic diseases [hepatitis C virus (HCV), hepatitis B virus (HBV), alcoholism, hemochromatosis etc.] and, in some of them (12%), its presence represents an associated disease: steatohepatitis[3]. Moreover, it is well known that a fatty liver is less protected from the mechanisms of inflammation and fibrosis[4].
Data reported in the literature suggest that hepatic steatosis can have different influences on a liver affected by other diseases. Therefore, it cannot always be considered as a “benign” condition and simply ignored. On the contrary, it has to be recognized as a “co-factor” capable of affecting the gravity and progression, and also therapeutic perspectives, of liver diseases.
Herein we will evaluate the impact of hepatic steatosis and obesity on the most common liver diseases: chronic viral hepatitis C and B, and alcoholic liver disease.
Hepatic steatosis is present in about 50% of liver specimens taken from subjects affected by chronic hepatitis C[5]. This prevalence is higher than that reported in the general population from magnetic resonance studies, and there are findings that suggest a possible direct role of the HCV on its development[6]. This hypothesis is supported by a large number of studies that link steatosis to both host characteristics [i.e. body mass index (BMI), waist circumference (WC), insulin resistance (IR)] and viral factors (genotype, viral RNA load).
Several risk factors for liver disease progression are known for both chronic hepatitis C (CHC) and NAFLD. These factors are: high BMI, type 2 diabetes mellitus, older age and alcohol consumption and these can also affect the presence and the gravity of hepatic steatosis[7]. In fact, overweight patients with HCV have more steatosis than lean subjects with CHC and this data has statistical relevance independently from the HCV genotype[8].
It is well known that the presence of steatosis in the liver of CHC patients seems to be related to the presence of virus itself, with a distinctive genotype specificity[8]. Six different HCV genotypes were identified according to Simmonds et al[9], characterized by different epidemiological and clinical peculiarities. In particular, the genotypes 1 and 3, the most prevalent in the western world, were found related to steatosis[6]. HCV virus has, in fact, demonstrated a direct steatogenic effect in cell cultures and transgenic mice[10,11]. This seems to be confirmed in human studies: there is considerable evidence that in HCV-infected subjects (particularly genotype 3-infected), the grade of hepatic steatosis seems to be related to viral load[12]. Moreover, there has been evaluation of steatosis disappearing in response to antiviral therapy and its recurrence in the case of relapse with virus reappearance in the liver[13]. However, “non-3 genotypes” (particularly genotype 1b) also show a distinct association with steatosis, which has encouraged some authors to coin a new definition: virus-associated steatohepatitis (VASH)[14,15].
Even if the recent literature reports steatosis as “viral” only in genotype 3-infected patients and as “metabolic” (due to host characteristics) in non-3 genotype-infected patients, it should be more accurate to talk about a combination of these two factors. In fact, some studies observed that in non-3 genotype-infected patients who achieved sustained virological response (SVR) there was a reduction or a total disappearance of liver steatosis. In particular, one study reported a reduction of steatosis in about 46% of such patients and its disappearance in 29%. Moreover, in the same study the genotype 3 patients markedly reduced their liver steatosis after weight loss, confirming that host factors could also be actively involved in generating steatosis in these patients[16]. To complete this topic, we should also consider the difficulty in obtaining an accurate evaluation of alcohol consumption history in these patients, as documented by the discordant data with regard to the contribution of alcohol intake to steatosis development in HCV patients[17-19].
The physiopathological mechanisms associated with steatosis and obesity leading to liver disease worsening are still under debate. Nevertheless, some plausible assumptions have been made and they seem to be supported by experimental evidence from cellular and animal models. Some of these involve oxidative stress, subsinusoidal stellate cell activation, higher apoptosis susceptibility and altered response to cellular damage. Furthermore, fibrogenesis could be exacerbated by other components of metabolic syndrome (MS) such as hyperinsulinemia and hyperglycemia.
Oxidative stress: Fatty liver seems to be more susceptible to the damage induced by such factors that lead to an increase in liver production of oxidant substances. In particular, on the basis of the immune response to HCV, it was postulated that an increase in oxidative stress produces an intensified lipoperoxidation and inflammatory cytokine production leading to programmed cell death, i.e. apoptosis[20].
Apoptosis: Apoptosis has a central role in liver disease progression and steatosis[21]. In CHC, a significant increment of this cellular mechanism together with stimulation of fibrosis and inflammatory activity can be observed in liver specimens which also show moderate or severe steatosis, thus suggesting a similar role of apoptosis to that suggested by the histological presentation in NASH[22]. This mechanism might involve a synergistic effect of apoptosis acting together with other intrinsic HCV factors of liver damage and thus leading to the worsening of fibrosis progression.
Steatohepatitis: 6%[23] to 18%[24] of HCV patients with steatosis have an associated steatohepatitis observed in liver specimens. This could be a direct consequence of oxidative stress and lipoperoxidation[25]. Nevertheless, HCV interference in the production of inflammatory cytokines such as tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), interleukin-1 (IL-1) and interleukin-6 (IL-6) might, per se, explain the worsening of steatosis and fibrosis in both “viral” and “non viral” steatosis[26,27]. However, these mechanisms might act in synergism to worsen fibrosis. Indeed, it has been demonstrated that there is a significant reduction of both steatosis and portal fibrosis after weight loss by diet[28] or bariatric surgery[29] in HCV patients.
Insulin resistance: In the field of this research, it has recently been greatly debated as to whether HCV virus exerts its effects on the liver (and on the organism) by involving the intracellular molecular cascade that follows the activation of insulin receptor after its binding with insulin[30]. HCV virus might interfere with this cascade in a genotype-specific manner[31-33]. However, this interference (regardless of genotype) could lead to hepatic steatosis (“viral” at least) and worsening of liver fibrosis by direct stimulation resulting from the action of hyperinsulinemia on hepatic stellate sub-sinusoidal cells, with an increase on extracellular matrix production[34].
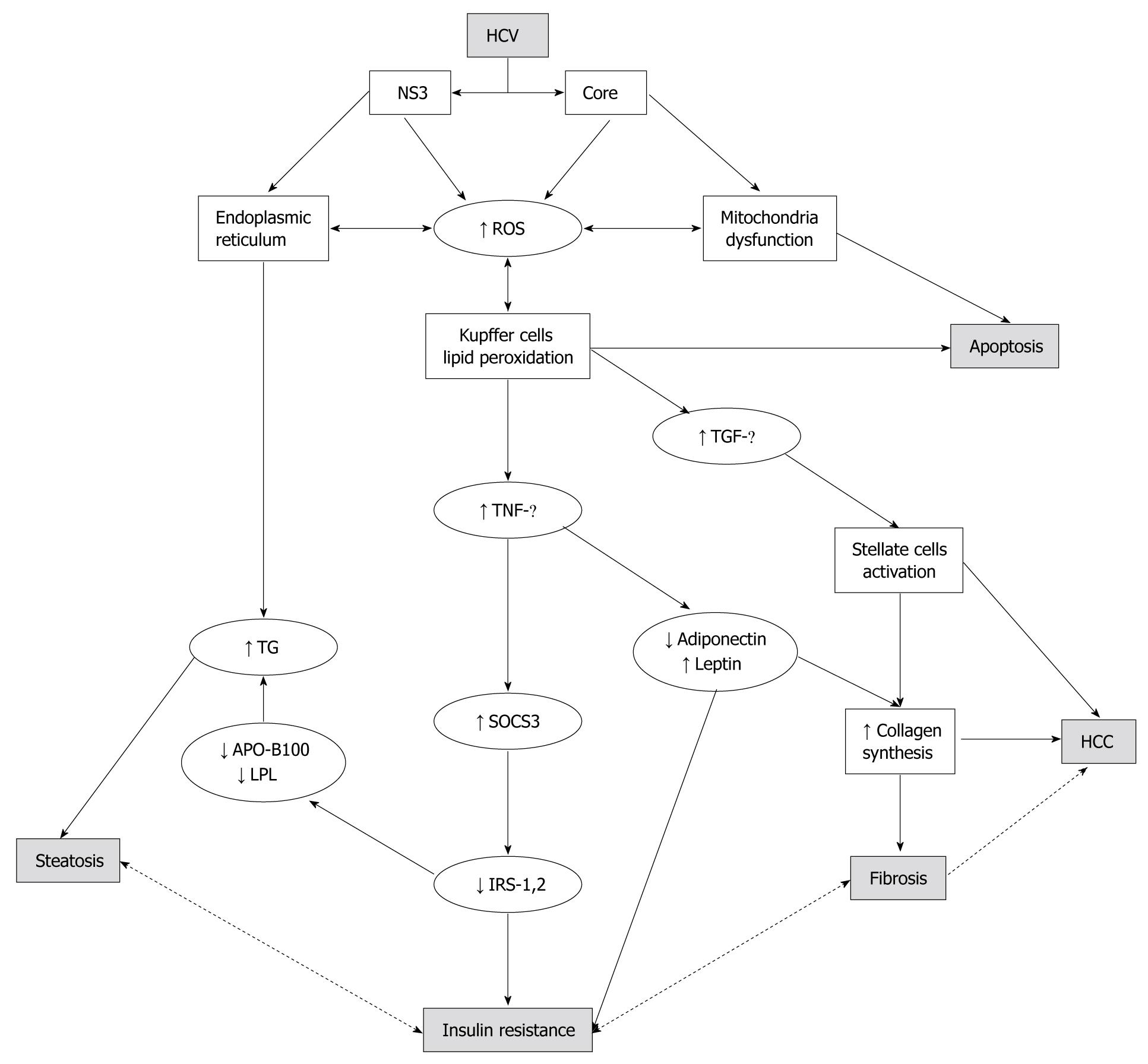
The supposed insulin resistance mechanism involves the reduction of expression of insulin receptor substrates 1 and 2 (IRS1 and 2), which are crucial proteins in the post-receptorial cascade of insulin[35]. The decrease of IRS1 and IRS2 seems to be mediated by a direct over-expression of another intracellular protein: suppressor of cytokine signaling 3 (SOCS3)[31-33]. This over-expression has been revealed only in genotype 1b patients and it is associated with metabolic syndrome and no response to antiviral therapy[32]. Recently, this evidence in vivo was reproduced in vitro. In fact, it was demonstrated that HepG2 cells infected with genotype 1 positive sera had higher SOCS3 levels than those infected with genotype 2 positive sera and that this was associated with a lower IRS1 expression[36]. Therefore, there is accumulating evidence of a direct “metabolic” effect of the HCV virus on a large number of molecular pathways that lead to hepatic steatosis, IR, MS, and liver fibrosis[37,38]. TNF-α, an inflammatory cytokine produced by hepatic stellate cells and adipose tissue, is increased in HCV patients with steatosis[26] and its serum increase, together with the decrease of the adipocytokine, adiponectin, was also demonstrated as being involved in the pathogenesis of NAFLD and IR[27,39]. The imbalance between TNF-α and adiponectin serum levels in HCV/steatosis patients seems to be genotype-specific and correlated with the severity of liver steatosis[26] (Figure 1). These findings, taken together, suggest a definite association between IR and steatosis/steatohepatitis with the HCV virus as the “third player”. However, it is still unclear if steatosis represents the first hit to cytokine production leading to IR or the other way around; IR via cytokine inflammatory pathways leading to hepatic steatosis. Recent evidence, discriminating between “systemic” and “hepatic” IR, showed that in young, lean, insulin-resistant subjects there was a low prevalence of liver steatosis and no cytokine/adipocytokine changes. This suggests that steatosis and cytokines interact without assuming a primary and independent role in the early stage of IR[40]. On the other hand, other authors support the idea that hyperinsulinemia is likely to be the consequence rather than the cause of a fatty liver, as suggested by the fact that fatty liver is associated with both hepatic insulin resistance and impaired insulin clearance[41,42].
From the observations reported above we can confirm that in CHC, hepatic steatosis has to be considered as an important co-factor in the worsening of liver disease. This co-factor should be inhibited by firstly correcting its possible causes, such as being overweight or obese. This suggestion is supported by data from the literature showing that, in patients with MS or IR, a small weight loss (about 4%-5%), in the same way as it acts on blood pressure and glycemic control[41], can induce steatosis reduction even if BMI is not restored to normal levels[28,42]. These factors can influence the response to antiviral treatment: it has in fact been demonstrated that the absence or the presence of lower levels of steatosis are positive predictors of achieving SVR in HCV patients[16,43,44].
Given the assumed relationship between HCV and steatosis as specified above, we could surmise that chronic hepatitis B (CHB) has an analogous association with steatosis. Only a small number of studies regarding this argument have been published and some of these report steatosis prevalence in CHB[45,46] while others report a direct comparison between CHC and CHB for the prevalence of MS, obesity and steatosis[47,48]. These studies report a steatosis prevalence in CHB patients similar to that of the general population. The presence of steatosis correlates with BMI and MS diagnostic criteria (waist circumference, high blood pressure and dyslipidemia), but not with viral genotype or viral load. Moreover, steatosis does not correlate with fibrosis[45]. Accordingly, these data indicate that the association between steatosis and HCV is specific, whereas this is not the case in HBV-infected patients. Nevertheless, it seems to be appropriate to hypothesize that in HBV-related chronic hepatitis, steatosis is probably also a co-factor of liver disease worsening, particularly if it is characterized as related to a conspicuous entity and/or in a subject with MS and/or if it translates to the real clinical condition of associated steatohepatitis[49].
The typical histological pattern of alcoholic liver disease (defined as drinking more than 40 g a day in males and 30 g in females) is a steatohepatitis[50]. In fact, a diagnosis of NASH is established when, simultaneous with clinical and histological observations of a hepatic liver disease that is quite indistinguishable from an alcohol-related disease, there is a total assurance of alcoholic abstinence[51]. Therefore, it is clear that there is a close association between steatosis and alcoholic liver disease, so close that steatosis cannot be considered as a simple co-factor but as part of the histological damage presentation in this liver disease. Nevertheless, nothing prevents us from supposing that obesity might also increase the severity of steatosis in patients with alcoholic liver disease. Indeed, this hypothesis has been confirmed by some literature findings which indicated that being overweight was associated with extent of steatosis[52] and that it was a risk factor for progression to cirrhosis and HCC in alcoholic liver disease[53,54]. Moreover, obesity and steatosis seem to use the same metabolic mechanisms to produce a worsening of liver damage; increased lipoperoxidation and pro-inflammatory cytokine production have been noted in obese alcoholics[55,56]. On these grounds, steatosis seems to have a double role: as a constantly present histological finding and as a possible co-factor responsible for worsening of histological damage. Thus, correct management of these patients should also include adequate dietetic counseling.
Further evidence from the literature indicates that steatosis, together with obesity and type 2 diabetes mellitus, is a probable risk factor for hepatocellular carcinoma (HCC) development. This has been reported in HCV liver disease in both experimental models[57] and clinical studies[58]. Steatosis seems to play the same role also in alcoholic liver disease: a large multicenter study conducted in 19 000 liver transplant patients indicates that obesity and steatosis are risk factors for HCC developing in alcoholic liver disease patients[59].
From the data analyzed in this brief review, we can conclude that hepatic steatosis, a risk-free, benign condition in healthy subjects, becomes a dangerous co-factor of disease progression when it is present in patients affected by another liver disease. It affects the response to antiviral treatment in HCV patients, and it could also be responsible for the development of HCC, in both HCV and alcoholic patients. Therefore, hepatic steatosis must be considered an important disease co-factor to be taken into account in liver disease patients, in order to make a diagnosis and subsequently to correct the condition.
Peer reviewers: Ekihiro Seki, MD, PhD, Department of Medicine, University of California San Diego, Leichag Biomedical Research Building Rm 349H, 9500 Gilman Drive MC#0702, La Jolla, CA 92093-0702, United States; Astrid van der Velde, PhD, Team Wetenschap, Netherlands Heart Foundation, PO box 300, 2501 CH, The Hague, The Netherlands
S- Editor Wang YR L- Editor Logan S E- Editor Ma WH
| 1. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. |
| 2. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. |
| 3. | Brunt EM, Ramrakhiani S, Cordes BG, Neuschwander-Tetri BA, Janney CG, Bacon BR, Di Bisceglie AM. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Mod Pathol. 2003;16:49-56. |
| 4. | Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463-1466. |
| 5. | Clouston AD, Powell EE. Interaction of non-alcoholic fatty liver disease with other liver diseases. Best Pract Res Clin Gastroenterol. 2002;16:767-781. |
| 6. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. |
| 7. | Ramesh S, Sanyal AJ. Hepatitis C and nonalcoholic fatty liver disease. Semin Liver Dis. 2004;24:399-413. |
| 8. | Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, Jonsson JR. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39:1042-1048. |
| 9. | Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962-973. |
| 10. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. |
| 11. | Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200-1205. |
| 12. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. |
| 13. | Castéra L, Hézode C, Roudot-Thoraval F, Lonjon I, Zafrani ES, Pawlotsky JM, Dhumeaux D. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420-424. |
| 14. | Koike K, Moriya K. Metabolic aspects of hepatitis C viral infection: steatohepatitis resembling but distinct from NASH. J Gastroenterol. 2005;40:329-336. |
| 15. | Masarone M, La Mura V, Bruno S, Gaeta GB, Vecchione R, Carrino F, Moschella F, Torella R, Persico M. Steatohepatitis is associated with diabetes and fibrosis in genotype 1b HCV-related chronic liver disease. J Viral Hepat. 2007;14:714-720. |
| 16. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. |
| 17. | Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, Powell EE. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215-1219. |
| 18. | Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729-736. |
| 19. | Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E, Carlotto A, Bozzola L, Smedile A, Negro F. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004;53:406-412. |
| 20. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. |
| 21. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. |
| 22. | Walsh MJ, Vanags DM, Clouston AD, Richardson MM, Purdie DM, Jonsson JR, Powell EE. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology. 2004;39:1230-1238. |
| 23. | Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729-736. |
| 24. | Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A, Bringman D, Martin LM, Assmann J, Gramlich T. Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. J Clin Gastroenterol. 2004;38:705-709. |
| 25. | Zatloukal K, Stumptner C, Fuchsbichler A, Fickert P, Lackner C, Trauner M, Denk H. The keratin cytoskeleton in liver diseases. J Pathol. 2004;204:367-376. |
| 26. | Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349-1357. |
| 27. | Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413-419. |
| 28. | Hickman IJ, Clouston AD, Macdonald GA, Purdie DM, Prins JB, Ash S, Jonsson JR, Powell EE. Effect of weight reduction on liver histology and biochemistry in patients with chronic hepatitis C. Gut. 2002;51:89-94. |
| 29. | Harnois F, Msika S, Sabaté JM, Mechler C, Jouet P, Barge J, Coffin B. Prevalence and predictive factors of non-alcoholic steatohepatitis (NASH) in morbidly obese patients undergoing bariatric surgery. Obes Surg. 2006;16:183-188. |
| 30. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. |
| 31. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. |
| 32. | Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Crocè L, La Mura V, Moschella F, Masutti F. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009-1015. |
| 33. | Persico M, Capasso M, Russo R, Persico E, Crocè L, Tiribelli C, Iolascon A. Elevated expression and polymorphisms of SOCS3 influence patient response to antiviral therapy in chronic hepatitis C. Gut. 2008;57:507-515. |
| 34. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743-1751. |
| 35. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. |
| 36. | Persico M, Russo R, Persico E, Svelto M, Spano D, Andolfo I, La Mura V, Capasso M, Tiribelli C, Torella R. SOCS3 and IRS-1 gene expression differs between genotype 1 and genotype 2 hepatitis C virus-infected HepG2 cells. Clin Chem Lab Med. 2009;47:1217-1225. |
| 38. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. |
| 39. | Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158-1163. |
| 40. | Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587-12594. |
| 41. | Harsha DW, Bray GA. Weight loss and blood pressure control (Pro). Hypertension. 2008;51:1420-1425; discussion 1425. |
| 42. | Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413-419. |
| 43. | Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639-644. |
| 44. | Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, Heaton S, Conrad A, Pockros PJ, McHutchison JG. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484-490. |
| 45. | Peng D, Han Y, Ding H, Wei L. Hepatic steatosis in chronic hepatitis B patients is associated with metabolic factors more than viral factors. J Gastroenterol Hepatol. 2008;23:1082-1088. |
| 46. | Bondini S, Kallman J, Wheeler A, Prakash S, Gramlich T, Jondle DM, Younossi ZM. Impact of non-alcoholic fatty liver disease on chronic hepatitis B. Liver Int. 2007;27:607-611. |
| 47. | Altlparmak E, Koklu S, Yalinkilic M, Yuksel O, Cicek B, Kayacetin E, Sahin T. Viral and host causes of fatty liver in chronic hepatitis B. World J Gastroenterol. 2005;11:3056-3059. |
| 48. | Persico M, Masarone M, La Mura V, Persico E, Moschella F, Svelto M, Bruno S, Torella R. Clinical expression of insulin resistance in hepatitis C and B virus-related chronic hepatitis: differences and similarities. World J Gastroenterol. 2009;15:462-466. |
| 49. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111-117. |
| 50. | Jan CF, Chen CJ, Chiu YH, Chen LS, Wu HM, Huang CC, Yen MF, Chen TH. A population-based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung Community-based Integrated Screening study No. 10). Int J Obes (Lond). 2006;30:794-799. |
| 51. | Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056-1062. |
| 52. | Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol. 2001;35:531-537. |
| 53. | Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108-111. |
| 54. | Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635-638. |
| 55. | Mezey E. Dietary fat and alcoholic liver disease. Hepatology. 1998;28:901-905. |
| 56. | Bunout D, Muñoz C, López M, de la Maza MP, Schlesinger L, Hirsch S, Pettermann M. Interleukin 1 and tumor necrosis factor in obese alcoholics compared with normal-weight patients. Am J Clin Nutr. 1996;63:373-376. |
| 57. | Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365-4370. |
| 58. | Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-3043. |
| 59. | Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150-155. |