Published online Feb 28, 2009. doi: 10.3748/wjg.15.983
Revised: January 20, 2009
Accepted: January 27, 2009
Published online: February 28, 2009
AIM: To study the characteristics of mismatch repair gene mutation of Chinese hereditary non-polyposis colorectal cancer (HNPCC) and hMLH1 gene promoter methylation, and to improve the screening strategy and explore the pertinent test methods.
METHODS: A systematic analysis of 30 probands from HNPCC families in the north of China was performed by immunohistochemistry, microsatellite instability (MSI), gene mutation and methylation detection.
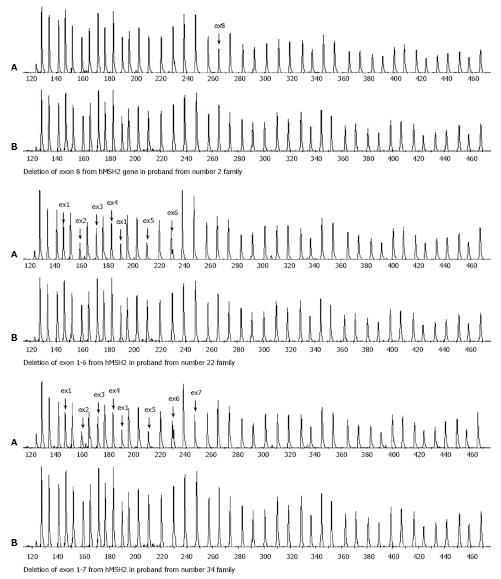
RESULTS: High frequency microsatellite instability occurred in 25 probands (83.3%) of HNPCC family. Loss of hMLH1 and hMSH2 protein expression accounted for 88% of all microsatellite instability. Pathogenic mutation occurred in 14 samples and 3 novel mutational sites were discovered. Deletion of exons 1-6, 1-7 and 8 of hMSH2 was detected in 3 samples and no large fragment deletion was found in hMLH1. Of the 30 probands, hMLH1 gene promoter methylation occurred in 3 probands. The rate of gene micromutation detection combined with large fragment deletion detection was 46.7%-56.7%. The rate of the two methods in combination with methylation detection was 63.3%.
CONCLUSION: Scientific and rational detection strategy can improve the detection rate of HNPCC. Based on traditional molecular genetics and combined with epigenetics, multiple detection methods can accurately diagnose HNPCC.
- Citation: Sheng JQ, Zhang H, Ji M, Fu L, Mu H, Zhang MZ, Huang JS, Han M, Li AQ, Wei Z, Sun ZQ, Wu ZT, Xia CH, Li SR. Genetic diagnosis strategy of hereditary non-polyposis colorectal cancer. World J Gastroenterol 2009; 15(8): 983-989
- URL: https://www.wjgnet.com/1007-9327/full/v15/i8/983.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.983
Hereditary non-polyposis colorectal cancer (HNPCC) is a dominant autosomal genetic syndrome caused by germ-line mutation of mismatch repair gene[1], accounting for 5%-10% of all colorectal cancer[23]. Genetic linkage analysis and genetics show that about 80% of HNPCC are associated with germ-line mutation of hMLH1 and hMSH2[4–6]. Most of these mutations are micromutation (also known as a point mutation), small fragment insertion or deletion, etc[78]. Large fragment deletion in hMLH1 and hMSH2 (especially hMSH2) gene is another way of germ-line mutation[9]. In addition, recent epigenetic studies indicate that CpG island methylation in hMLH1 gene promoter region is also a mechanism underlying gene inactivation and tumorigenesis[10]. Therefore, systematic and comprehensive detection analysis of 30 samples from HNPCC families in the north of China was performed using various methods.
Between 2000 and 2002, 30 probands from HNPCC families were collected and registered by General Hospital of Beijing Military Command and other hospital in Henan, Hebei and Shandong Provinces, and Inner Mongolia Autonomous Region. All study methods were approved by the Ethics Committee of General Hospital of Beijing Military Commad and the 30 probands from HNPCC families gave written consent to participate in the study. Twenty-one families were in line with Bethesda Guideline (BG) I, 7 families Bethesda Guideline III and 2 families Bethesda Guideline IV, respectively[11]. With probands as the core, HNPCC genealogical tree of at least two generations was drawn. Pedigree analysis was performed. Family member files including the age when the tumor was diagnosed, the relation to proband, tumor type and location, etc (including parenteral cancer) were established.
DNA extraction: Tumor and normal tissues were obtained from probands. Tumor tissue was fixed with formalin, embedded in paraffin, cut into 6-&mgr;m thick sections which were stained with 0.1% methylthioninium chloride. The location (more than 80% tumor tissue) most suitable to microdissection was marked and microdissection was done. DNA was extracted from tumor and normal tissues with phenol/chloroform/isoamyl alcohol for microsatellite instability (MSI) and immunohistochemistry detection[12]. DNA was extracted from venous blood for gene mutation and methylation detection.
Microsatellite instability and immunohistochemistry detection: Microsatellite instability detection was performed in 5 microsatellite markers: D2S123, D5S346, BAT-25, BAT-26 and BAT-40[1314]. For hMSH2 and hMLH1, immunohistochemical staining was done with the standard biotin-avidin-peroxidase complex method as previously described[15].
hMSH2 and hMLH1 gene mutation detection: Micromutation detection was performed in 25 samples with high microsatellite instability. PCR was performed to amplify all exons (including intron-exon junction) of hMSH2 and hMLH1. PCR products were sequenced with a DNA automatic sequencer (ABIPRISM 3730XL) to find micromutation in the samples and to determine their mutation type.
Large fragment deletion detection in hMSH2 and hMLH1: Multiplex ligation-dependent probe amplification (MLPA) technique[16] was used to detect large fragment deletion in the samples without micromutation with a hMLH1 and hMSH2 large fragment deletion kit purchased from MRC Holland Company. The major steps of MLPA technique include to probe hybridization with the target sequence and specific ligation, to amplify hMSH2 and hMLH1 by PCR with probes, and to analyze PCR products. The PCR products were applied to a ABIPRISM3730 sequencer containing 6% polyacrylamide gel for electrophoresis. The electrophoresis results were analyzed with GeneMapper 3.0 software. The peak of each exon was compared with that of control sample. If the relative height was reduced by 35%-55%, the fragment was determined to have exon deletion. If the relative height was increased by 30%-55%, the fragment was determined to have exon duplication. If the peak was 0, the fragment was determined to have homozygous deletion.
Methylation detection: hMLH1 gene promoter methylation detection was performed in 30 samples. First, DNA was sulfurized with an EZ DNA methylation-gold kit purchased from ZYMO RESEARCH Company, and then methylation-specific PCR (MSP)[17] was performed. PCR amplification was performed twice for each sample with methylation and non-methylation primers, respectively. PCR products were applied to a 10% non-denaturing polyacrylamide gel for electrophoresis, then stained with ethidium bromide, and observed under an ultraviolet lamp.
Of the 30 families, 21 were in line with BG I, 7 were in line with BG III and 2 were in line BG IV (Table 1). One hundred and forty tumors were found in 106 of the 708 members in these families. Of the 140 tumors, 22 (15.7%, 22/140) were extracolonic cancers. Of the 22 tumors, 7 were gastric cancers which are the most common type of extracolonic cancer. Of the colon cancers, 86 (72.9%, 86/118) were right colon cancers and 32 (27.1%, 32/118) were left colon cancer. One patient had synchronous multiple-primary cancers and 7 had metachronous multiple-primary cancers.
| Family | Bethesda guidelines | MSI | Expression of MMR proteins | Micromutation/polymorphism2/large fragment deletion | hMLH1 gene promoter methylation | |
| MSH2 | MLH1 | |||||
| H1 | BG1 | MSI-H | + | + | u | |
| H4 | BG1 | MSI-H | - | + | hMSH2 exon13 IVS13-2 A→C (SA of Exon 14) | u |
| H9 | BG1 | MSI-H | - | + | hMSH2 exon3 c.610G→T (G204stop) | u |
| H17 | BG1 | MSI-H | + | + | hMSH2 exon5 c.899_890insAT1 | u |
| H22 | BG1 | MSI-H | - | + | hMSH2 exon7 IVS7-1G→A (SA of Exon 8)1 | u |
| H10 | BG1 | MSI-H | + | + | hMSH2 exon15 c.2583A→G (Q861Q)12 | u |
| H2 | BG1 | MSI-H | - | + | hMSH2 exon8 deletion | u |
| H5 | BG1 | MSI-H | - | + | u | |
| H11 | BG3 | MSI-H | - | + | u | |
| H23 | BG1 | MSI-H | - | + | hMSH2 exon1-6 deletion | u |
| H25 | BG3 | MSI-H | - | + | u | |
| H13 | BG1 | MSI-H | - | + | hMSH2 exon 7 c.1231 insertion T shift | u |
| H34 | BG4 | MSI-H | - | + | hMSH2 exon1-7 deletion | u |
| H3 | BG1 | MSI-H | + | - | hMLH1 exon18 c.2041G→A (A681T) | u |
| H12 | BG3 | MSI-H | + | - | hMLH1 exon15 IVS15+1 G→A (SD of Exon 15) | u |
| H19 | BG1 | MSI-H | + | - | hMLH1 exon8 c.677G→A (splice site mutation) | u |
| H20 | BG1 | MSI-H | + | - | hMLH1 exon8 c.677G→A (splice site mutation) | u |
| H21 | BG1 | MSI-H | + | - | hMLH1 exon19 c.2141G→A (W714stop) | u |
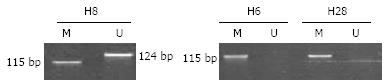
| H28 | BG1 | MSI-H | + | - | hMLH1 exon8 c.655A→G (I219V) | m |
| H29 | BG1 | MSI-H | + | - | hMLH1 exon6 c.503_4insA1 | u |
| H30 | BG1 | MSI-H | + | - | hMLH1 exon9 IVS9+1 G→A (SD of Exon 9) | u |
| H14 | BG1 | MSI-H | + | - | u | |
| H18 | BG1 | MSI-H | + | - | u | |
| H33 | BG3 | MSI-H | + | - | u | |
| H35 | BG3 | MSI-H | + | - | hMLH1 exon17 c.1930 del G | u |
| H8 | BG4 | MSI-L | + | + | m | |
| H6 | BG3 | MSS | + | + | m | |
| H36 | BG1 | MSS | + | + | u | |
| H15 | BG3 | MSS | + | + | u | |
| H27 | BG1 | MSS | + | + | u | |
Microsatellite instability analysis and mismatch repair protein expression in probands are shown in Table 1. Of the 30 samples detected, high frequency microsatellite instability (MSI-H) occurred in 25 samples (83.3%), low frequency microsatellite instability (MSI-L) in one sample (3.3%) and microsatellite stability (MSS) in 4 samples (13.3%). Of the 5 microsatellite loci, MSI-H expression rate was 100% (25/25) and 96% (24/25) in BAT-25 and BAT-26, respectively. Of the 25 samples with MSI-H, loss of hMLH1 or hMSH2 protein expression occurred in 22 (88%), loss of hMLH1 protein expression occurred in 12, and loss of hMSH2 protein expression in 10, respectively. No loss of mismatch repair protein expression was found in the other 8 samples.
Of the 25 samples with MSI-H, pathogenic mutation (Table 1) was detected in 14 (56%). Of the 14 samples, hMLH1 and hMSH2 gene mutation occurred in 9 and 5, respectively. The detection rate of micromutation was 46.7% (14/30). Three novel mutational sites were discovered. Of the 3 novel mutations, a frame shift mutation at c.503_4insA was located in hMLH1, and another frame shift mutation at c.899_890ins AT and a splicing mutation at IVS7-1G→A, SA of Exon 8 were located in hMSH2. A new base replacement (hMSH2, c.2583A→G) was detected in sample H10, which did not cause changes in amino acid sequence. There was no co-segregation phenomenon between this base replacement and disease in its family, suggesting that it is a new change in polymorphism.
Electrophoresis scanning peaks in exons 1-6, 1-7 and 8 of hMSH2 in 3 samples were reduced by over 35% (Figure 1), demonstrating that these exons have deletions which are heterozygotic in nature. Large fragment deletion was not detected in hMLH1. Large fragment deletion in hMSH2 accounted for 37.5% of all hNSH2 pathogenic mutations and 17.6% of total hMLH1 and hMSH2 mutations, respectively. Of the 30 samples, micromutation and large fragment deletion were detected in 17, the detection rate of combined methods was 56.7% (17/30).
hMLH1 gene promoter methylation occurred in 3 of the 30 samples. The detection rate of combined micromutation, large fragment deletion and methylation detection was 63.3% (19/30). MSP electropherogram (Figure 2) showed that hMLH1 gene promoter methylation occurred in 3 samples. Exhaustive methylation occurred in 2 of the 3 samples with their electropherograms displaying M specific fragment but no U deletion, and partial methylation occurred in one of the 3 samples with its electropherogram displaying M and U fragments. Only U specific fragment occurred in the other samples, indicating that no methylation occurs in these samples.
Germ-line mutation of mismatch repair gene is the molecular genetic basis of HNPCC pathogenesis. Mismatch repair gene mutation may lead to truncation and lower expression of mismatch repair protein, increasing DNA replication errors and microsatellite instability which results in tumorigenesis[1819]. Mutations in hMSH2 and hMLH1 are most common[4–6]. Therefore, we mainly studied the two genes.
Microsatellite instability detection was performed in 5 microsatellite markers, including D2S123, D5S346, BAT-25, BAT-26 and BAT-40. The MSI-H detection rate was 83.3%, demonstrating that the incidence of MSI-H is high in patients with HNPCC, and microsatellite instability is one of the most important features of HNPCC and reflects mismatch repair gene state at a certain extent. Loss of mismatch repair protein expression occurred in 88% MSI-H samples while no loss of mismatch repair protein expression occurred in MSI-L and MSS samples, indicating that the specificity, sensitivity and consistency of MSI and immunohistochemistry detection are higher in HNPCC families, the detection methods are simple and economic, and the results of combined detection methods may be used as effective screening indicators before genetic testing.
In MSI-H samples, the detection rate of micromutation in hMSH2 and hMLH1 was 56%. Micromutations of hMLH1 occurred in exons 6, 8, 9, 15, 17-19 and accounted for 64.3%. Micromutations of hMSH2 occurred in exons 3, 5, 7 and 13 and accounted for 35.7%. The mutation types included frame shift, non-sense, splicing and missense mutations. By searching the database of International Society for Gastrointestinal Hereditary Tumors (http://www.insight-group.org), we found 3 new mutational sites including a frame shift mutation at c.503_4insA in hMLH1, and a frame shift mutation at c.899_890ins AT, and a splicing mutation at IVS7-1G→A, SA of exon 8 in hMSH2. Since these mutations could lead to protein product truncation, further study is needed in a larger-scale population to confirm whether such mutations only occur in the north of China. It is evident that the mutation spectrum of HNPCC mismatch repair gene in Chinese is broad and multiple.
Grabowski et al[20] reported that large fragment deletion in hMLH1 and hMSH2 accounts for 17% of all pathogenic mutations, which is almost consistent with the results of our study. The three types of large fragment deletion are in line with the findings reported by Nakagawa et al[21]. Large fragment deletion commonly occurs in hMSH2, and should be reckoned with in molecular genetics of HNPCC. Therefore, detection of HNPCC molecular genetics should include large fragment deletion detection.
With the development of epigenetics in recent years, DNA methylation has gradually become a new research focus. In human genome, 5' promoter of 50% genes contains a CpG region, also known as CpG island, with its length > 197 bp. CpG island is in a non-methylation state under normal circumstances. CpG island methylation may lead to loss of gene expression and replication errors[2223]. The promoter methylation of hMLH1 gene is most common in known mismatch repair genes. The detection rate of hMLH1 gene promoter methylation in our study was almost similar to the reported data[24]. Exhaustive and partial methylations were observed in our study, demonstrating that hMLH1 gene promoter methylation may occur in patients with HNPCC, and the methylation level is different in different individuals. A deletion at c.655A→G (1219 V) in hMLH1, loss of hMLH1 protein expression, and hMLH1 gene promoter methylation were found in sample H28, suggesting that further study is needed to explore the protein expression and regulation of HNPCC pathologic mechanism.
An overview of the whole process of detection analysis of the 30 proband samples, microsatellite instability was first performed, then gene micromutation, large fragment deletion and promoter methylation were detected, respectively. The detection rate of gene micromutation was 46.7% (14/30), the detection rate of large fragment deletion detection in combination was increased to 56.7% (17/30). However, the detection rate of three methods in combination was 63.3% (19/30). Almost no comprehensive and systemic detection has been reported both at home and abroad. The detection rate in our study was higher than or similar to reported data[25–27]. Multiple methods in combination may improve the detection efficiency and accuracy of HNPCC and can determine HNPCC families. In order to make early diagnosis and treatment, HNPCC family members should regularly by examined. At the same time, since gene detection is time-consuming and expensive, the cost of various tests and clinical significance should be taken into account according to the actual situation. Therefore, the detection strategy should be made in the following steps. First, families meeting the HNPCC criteria are selected, and then immunohistochemistry and microsatellite instability detection of hMLH1 and hMSH2 are performed. If both of the two detections are negative, mutation detection need not be done. If one of the two detections is positive, micromutations in hMLH1 and hMSH2 should be detected. If micromutation is not detectable, large fragment deletion detection should be considered. MLPA technique can be used in detecting large fragment deletion and is characterized by DNA probe hybridization. PCR technique is rapid, sensitive, specific, reliable and cheap. All the 35 exons of hMLH1 and hMSH2 gene can be detected in the same reaction system with MLPA technique[28]. Epigenetics provides a new idea for the early diagnosis, treatment and prognosis of tumors. Studies indicate that CpG island methylation in the hMLH1 gene promoter region is also a mechanism underlying gene inactivation and tumorigenesis[2930]. Therefore, based on traditional micromutation detection, we should further combine large fragment deletion detection. For samples without micromutation and large fragment deletion, mismatch repair gene promoter methylation detection should be taken into account. Various detection methods in combination can better diagnose HNPCC.
Hereditary non-polyposis colorectal cancer (HNPCC), which is caused by a germline mutation in the mismatch repair gene or is associated with tumors exhibiting microsatellite instability (MSI), is characterized by increased risk of developing colon cancer and other cancers, such as cancers of endometrium, ovary, stomach, small intestine, hepatobiliary tract, upper urinary tract, brain, and skin. The diagnosis of HNPCC can be made based on the Amsterdam Clinical Criteria or on molecular genetic testing for germline mutations in one of the mismatch repair (MMR) genes.
About 80%of HNPCC are associated with germline mutations in hMLH1 and hMSH2. Most of these mutations are micromutation that is a point mutation, small fragment insertion or deletion, etc. Large fragment deletion in hMLH1 and hMSH2 gene is another way of germline mutation. In addition, recent epigenetic researches indicate that CpG island methylation in the hMLH1 gene promoter region is also a mechanism underlying gene inactivation and tumorigenesis.
They used combined MSI and immunohistochemistry detection, gene mutation detection (i.e. sequencing and large fragment deletion detection) and methylation detection to study the characteristics of MMR gene mutation and hMLH1 gene promoter methylation in Chinese HNPCC patients. The results demonstrate that the combined MSI and immunohistochemistry detection might be used as effective screening indicators before genetic testing. Furthermore, detection of HNPCC molecular genetics should include large fragment deletion detection. For samples without micromutation and large fragment deletion, mismatch repair gene promoter methylation detection should be taken into account.
According to their results, the detection strategy should be made in the following steps. First, families meeting the HNPCC criteria are selected, and then immunohistochemistry and microsatellite instability detection of hMLH1 and hMSH2 are performed. If both of the two detections are negative, mutation detection needs not be done. If one of the two detections is positive, micromutations in hMLH1 and hMSH2 should be detected. If micromutation is not detectable, large fragment deletion detection should be considered. For samples without micromutation and large fragment deletion, mismatch repair gene promoter methylation detection should be taken into consideration. Various detection methods in combination may better diagnose HNPCC.
The authors performed a systematic analysis of various genetic diagnostic methods in Chinese HNPCC patients and assessed their efficiency. The results are interesting and suggest that MSI, immunohistochemistry detection, gene mutation detection and methylation detection in combination may better diagnose HNPCC.
| 1. | Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomaki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996;2:169-174. |
| 2. | Lynch HT, Smyrk T. Hereditary nonpolyposis colorectal cancer (Lynch syndrome). An updated review. Cancer. 1996;78:1149-1167. |
| 3. | Wagner A, Tops C, Wijnen JT, Zwinderman K, van der Meer C, Kets M, Niermeijer MF, Klijn JG, Tibben A, Vasen HF. Genetic testing in hereditary non-polyposis colorectal cancer families with a MSH2, MLH1, or MSH6 mutation. J Med Genet. 2002;39:833-837. |
| 4. | Nystrom-Lahti M, Wu Y, Moisio AL, Hofstra RM, Osinga J, Mecklin JP, Jarvinen HJ, Leisti J, Buys CH, de la Chapelle A. DNA mismatch repair gene mutations in 55 kindreds with verified or putative hereditary non-polyposis colorectal cancer. Hum Mol Genet. 1996;5:763-769. |
| 5. | Peltomaki P. Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet. 2001;10:735-740. |
| 6. | Peltomaki P, Gao X, Mecklin JP. Genotype and phenotype in hereditary nonpolyposis colon cancer: a study of families with different vs. shared predisposing mutations. Fam Cancer. 2001;1:9-15. |
| 7. | Wagner A, Barrows A, Wijnen JT, van der Klift H, Franken PF, Verkuijlen P, Nakagawa H, Geugien M, Jaghmohan-Changur S, Breukel C. Molecular analysis of hereditary nonpolyposis colorectal cancer in the United States: high mutation detection rate among clinically selected families and characterization of an American founder genomic deletion of the MSH2 gene. Am J Hum Genet. 2003;72:1088-1100. |
| 8. | Wang Y, Friedl W, Lamberti C, Jungck M, Mathiak M, Pagenstecher C, Propping P, Mangold E. Hereditary nonpolyposis colorectal cancer: frequent occurrence of large genomic deletions in MSH2 and MLH1 genes. Int J Cancer. 2003;103:636-641. |
| 9. | Wijnen J, van der Klift H, Vasen H, Khan PM, Menko F, Tops C, Meijers Heijboer H, Lindhout D, Muller P, Fodde R. MSH2 genomic deletions are a frequent cause of HNPCC. Nat Genet. 1998;20:326-328. |
| 11. | Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, Lynch H, Perucho M, Smyrk T, Sobin L. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758-1762. |
| 12. | Chan TL, Zhao W, Leung SY, Yuen ST. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63:4878-4881. |
| 13. | Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-5257. |
| 14. | Yuen ST, Chan TL, Ho JW, Chan AS, Chung LP, Lam PW, Tse CW, Wyllie AH, Leung SY. Germline, somatic and epigenetic events underlying mismatch repair deficiency in colorectal and HNPCC-related cancers. Oncogene. 2002;21:7585-7592. |
| 15. | Sheng JQ, Chan TL, Chan YW, Huang JS, Chen JG, Zhang MZ, Guo XL, Mu H, Chan AS, Li SR. Microsatellite instability and novel mismatch repair gene mutations in northern Chinese population with hereditary non-polyposis colorectal cancer. Chin J Dig Dis. 2006;7:197-205. |
| 16. | Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. |
| 17. | Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821-9826. |
| 18. | Bocker T, Diermann J, Friedl W, Gebert J, Holinski-Feder E, Karner-Hanusch J, von Knebel-Doeberitz M, Koelble K, Moeslein G, Schackert HK. Microsatellite instability analysis: a multicenter study for reliability and quality control. Cancer Res. 1997;57:4739-4743. |
| 19. | Boardman LA. Heritable colorectal cancer syndromes: recognition and preventive management. Gastroenterol Clin North Am. 2002;31:1107-1131. |
| 20. | Grabowski M, Mueller-Koch Y, Grasbon-Frodl E, Koehler U, Keller G, Vogelsang H, Dietmaier W, Kopp R, Siebers U, Schmitt W. Deletions account for 17% of pathogenic germline alterations in MLH1 and MSH2 in hereditary nonpolyposis colorectal cancer (HNPCC) families. Genet Test. 2005;9:138-146. |
| 21. | Nakagawa H, Hampel H, de la Chapelle A. Identification and characterization of genomic rearrangements of MSH2 and MLH1 in Lynch syndrome (HNPCC) by novel techniques. Hum Mutat. 2003;22:258. |
| 23. | Deng G, Chen A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59:2029-2033. |
| 24. | Hawkins N, Norrie M, Cheong K, Mokany E, Ku SL, Meagher A, O’Connor T, Ward R. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376-1387. |
| 25. | Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146-1158. |
| 26. | Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, Stephens P, Edkins S, Tsui WW, Chan AS. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62:6451-6455. |
| 27. | Haung YQ, Yuan Y, Wang YP, Zhu M, Zhang SZ, Zheng S. [Large genomic deletions of mismatch repair genes in Chinese patients with hereditary nonpolyposis colorectal cancer]. Zhonghua Yixue Yichuanxue Zazhi. 2005;22:88-90. |
| 28. | Gille JJ, Hogervorst FB, Pals G, Wijnen JT, van Schooten RJ, Dommering CJ, Meijer GA, Craanen ME, Nederlof PM, de Jong D. Genomic deletions of MSH2 and MLH1 in colorectal cancer families detected by a novel mutation detection approach. Br J Cancer. 2002;87:892-897. |
| 29. | Deng G, Peng E, Gum J, Terdiman J, Sleisenger M, Kim YS. Methylation of hMLH1 promoter correlates with the gene silencing with a region-specific manner in colorectal cancer. Br J Cancer. 2002;86:574-579. |