Published online Feb 28, 2009. doi: 10.3748/wjg.15.973
Revised: December 11, 2008
Accepted: December 18, 2008
Published online: February 28, 2009
AIM: To investigate the protective effects of electroacupuncture (EA) pretreatment on acetylsalicylic acid (ASA)-induced ulceration in rats.
METHODS: We randomly divided 72 rats into three groups including control (administered with distilled water), ASA group (administered 100 mg/kg ASA) and EA group (administered EA + 100 mg/kg ASA). Each rat was fasted for 18 to 24 h before experimentation, and lesion scores, gastric acidity, cyclooxygenase (COX)-1 and -2 mRNA levels, and total nitric oxide (NO) concentration were measured.
RESULTS: The lesion scores of the EA group were significantly lower than those of the ASA group. Gastric acidity of the ASA and EA groups was reduced compared to the control group. COX-1 and -2 mRNA levels were significantly increased in the EA group as compared to the control and ASA groups, and NO levels were also significantly increased in the EA group as compared to the ASA group.
CONCLUSION: These results suggest that EA-mediated protection against ASA-induced ulceration in rats may occur via gastric defense components.
- Citation: Hwang HS, Han KJ, Ryu YH, Yang EJ, Kim YS, Jeong SY, Lee YS, Lee MS, Sung Tae K, Sun-Mi C. Protective effects of electroacupuncture on acetylsalicylic acid-induced acute gastritis in rats. World J Gastroenterol 2009; 15(8): 973-977
- URL: https://www.wjgnet.com/1007-9327/full/v15/i8/973.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.973
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used as anti-inflammatory and analgesic agents[12]. Unfortunately, they often cause gastrointestinal injury in gastric lesions by inhibiting COX; however, the detailed mechanism remains unclear[3]. Thus, effective strategies are required to protect the gastrointestinal mucosa. Acupuncture has been used to treat a wide variety of conditions, including fever, inflammation, infection and, especially, stomach diseases[45]. Therefore, we have investigated the protective effects of acupuncture against NSAID-induced ulceration in a rat model. Oxidization of arginine by nitric oxide synthase (NOS) creates the volatile gas NO, which has numerous physiologic properties, including regulation of inflammation and a role in gastric mucosal defense[67].
Acupuncture has been used to treat different gastrointestinal disorders in East Asian countries. ST-36 (stomach-36) is the common point for the treatment of gastric symptoms, such as nausea and vomiting, suggesting that acupuncture at ST-36 may stimulate gastrointestinal motility in humans[8]. A previous study has demonstrated that electroacupuncture (EA) at ST-36 inhibits acid secretion in conscious dogs and that naloxone reversed the EA-induced inhibition of acid secretion[9]. Other studies reported that EA stimulation at ST-36 increases gastric acid secretion in rats, and sectioning of the sciatic nerve blocked the acupuncture induced acid secretion[10]. Many studies reported improvement of gastrointestinal symptoms, but the mechanism of the beneficial effects of acupuncture remains to be investigated.
In this study, we investigated whether pretreatment with EA could protect against 100 mg/kg ASA-induced ulceration in rats by regulating acidity, COX mRNA and NO levels of gastric defensive components.
Seventy-two adult male Sprague-Dawley rats (Orient Bio Inc., Korea) weighing 170-210 g were included in this study. The rats were randomly divided into three groups: control (fasted 18-24 h and orally administered 1 mL DW), ASA (fasted 18-24 h and orally administered 100 mg/kg ASA) and EA (fasted 18-24 h, treated with EA for 30 min, and then immediately orally administered 100 mg/kg ASA). Rats were housed at 22 ± 1°C and 55% ± 10% humidity on a 12 h light/12 h dark cycle with free access to food and water. All experimental procedures were approved by the Laboratory Animal Center at the Korea Institute of Oriental Medicine. After the rats were allowed to adjust to their new environment for three to four days, they were kept in cages with raised mesh bottoms and deprived of food, but allowed free access to tap water for 18 to 24 h.
EA was applied via bilateral electrical stimulation at the ST36 acupuncture point using a pair of bipolar stimulation electrodes in rats under 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane (isoflurane) anesthesia. The electrical stimulation condition was biphasic pulses with 10 Hz frequency, 1 mA current and a duration of 0.1 ms.
Like the EA group, the ASA group was anesthetized for 30 min; during this 30-min period, the EA group also received EA at ST-36 for 30 min before receiving 1 mL ASA (100 mg/kg) under isoflorane anesthesia. The control group was anesthetized for 30 min and administered 1 mL DW. Four hours later, all rats were sacrificed under i.p. pentobarbital (50 mg/kg, HANLIM PHARM. CO., Ltd, Korea) anesthesia to score the lesions of each stomach after inflation with 5 mL phosphate-buffered saline (PBS, pH 7.4). We utilized direct scanning of stomach samples to measure the gastric lesions with public domain image processing and analysis software developed at the National Institutes of Health, USA. The PC version of this program (2.15 MB), known as Scion Image, is freely available on the internet from Scion Corporation (http://www.scioncorp.com, Scion Image for Windows, Release Alpha 4.0.3.2)[11].
Gastric acidity was measured using the pylorus ligation technique. Briefly, the abdomen was incised, and the pylorus was ligated under isoflurane anesthesia. Rats were continuously anesthetized for 30 min. The EA group received EA at ST36 for 30 min under anesthesia. Afterwards, the control group received distilled water, and the ASA and EA groups received ASA (100 mg/kg). Four hours after completion of all procedures, each group of rats was sacrificed under i.p. pentobarbital (50 mg/kg, HANLIM PHARM. CO., Ltd, Korea) anesthesia, and the gastric juices were collected. Gastric juices were centrifuged at 5500 r/min for 10 min and used for the following measurement. Titratable acidity was determined in a 200 &mgr;L aliquot of the gastric juice supernatant with 0.1 mol/L NaOH using phenol red as an indicator. Results were expressed as &mgr;Eq/200 &mgr;L.
Stomach samples for RT-PCR were immediately frozen in liquid nitrogen and stored at -70°C until use. Total RNA was extracted from the stomach samples that were pooled from three rats using RNA Bee (Tel-Test Inc., TX, USA) and isopropanol-chloroform extraction. After isolating the RNA, we performed RNA quantitation using a spectrophotometer (NanoDrop ND-1000, NanoDrop, Texas, USA). Total RNA was reverse transcribed using a RevertAidTM First Strand cDNA Synthesis Kit (Asuragen Inc., Texas, USA) according to the manufacturers’ instructions. β-actin levels were constant in all groups. The resulting cDNAs were amplified by PCR using β-actin, COX-1 or COX-2 specific primers, 10 mol/L dNTP, 10X Taq buffer and Taq. PCR cycling conditions consisted of 45 s at 94°C, 45 s at 59.3°C and 1 min for 72°C for β-actin and for 30 s at 94°C, 30 s at 53.6°C and 45 s at 72°C for COX-1 and -2. The sense and antisense primers were 5'-TGT GATGGTGGGAATGGGTCAG-3' and 5'-TTTGAT GTCACGCACGATTTCC-3' for β-actin (514 bp); 5'-GCCTCGACCACTACCAATGT-3' and 5'-AGG TGGCAT TCACAAACTCC-3' for COX-1 (167 bp); and 5'-TAC CCGGACTGGATTCTACG-3' and 5'-AAGTTGGTG GGCTGTCAATC-3' for COX-2 (214 bp). PCR was conducted for 28 cycles for COX-1 and -2, and 30 cycles for β-actin. Amplified DNA was electrophoresed in a 1.5% agarose gel. Fragment size was assessed by comparison to a 100 bp DNA marker (Solgent, Korea).
The gastric mucosa of each rat was removed by scrubbing and then weighed. NO content was determined using the Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical Co, MI, USA). Briefly, samples were homogenized in PBS (pH 7.4), centrifuged at 10 000 r/min for 20 min, and then ultra-filtered using a 30 kDa molecular weight cut-off filter (Millipore Corporation, MA, USA). Nitrate in the prepared samples was converted to nitrite using nitrate reductase and subsequently coupled with N-1-naphthyl-ethylene diamine to give a colored product whose concentration was measured colorimetrically at a wavelength of 540 nm.
All data were expressed as mean ± SE using SPSS for windows (version 11, SPSS, Inc., Chicago, USA). Differences between means were analyzed using one-way analysis of variance (ANOVA) with the Tukey post-hoc test for multiple comparisons as well as Student’s t-test, P < 0.05 was considered significant.
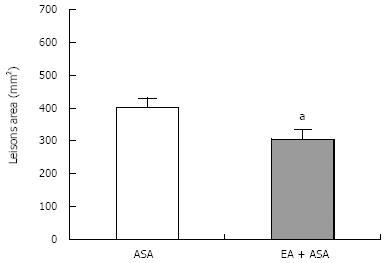
The lesion scores of the EA group (305.55 ± 29.67 mm2, n = 8) were significantly lower (P < 0.05) than those of the ASA group (402.64 ± 28.25 mm2, n = 8), as shown in Figure 1. Thus, EA pretreatment significantly protected against gastric lesions induced by 100 mg/kg ASA.
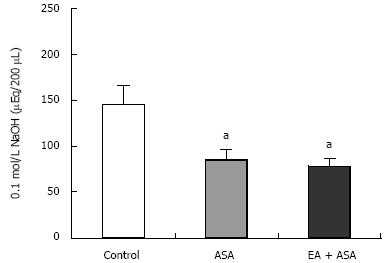
Figure 2 shows the gastric acidity for each group: 145.2 ± 19.9 &mgr;Eq/200 &mgr;L for the control group (n = 5), 84.86 ± 11.03 &mgr;Eq/200 &mgr;L for the ASA group (n = 7), and 78.13 ± 8.01 &mgr;Eq/200 &mgr;L for the EA group (n = 8). The gastric acidity of both the ASA and EA groups was significantly lower than that of the control group (P < 0.05 in both groups).
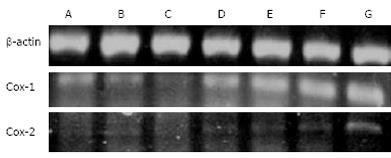
The level of COX-1 mRNA in the ASA group was significantly decreased at one and two hours after administration of 100 mg/kg ASA (Figure 3 columns B and C) and then increased at four hours after administration (Figure 3 column D) compared to the control group (column A). Expression of COX-1 mRNA in the EA group was significantly increased at one, two and four hours after administration of 100 mg/kg ASA (Figure 3, columns E, F, G) compared to the control group. COX-2 mRNA levels were slightly up-regulated in all tested conditions and time points, except for the strong up-regulation observed in the EA group at four hours after administration of 100 mg/kg ASA (Figure 3).
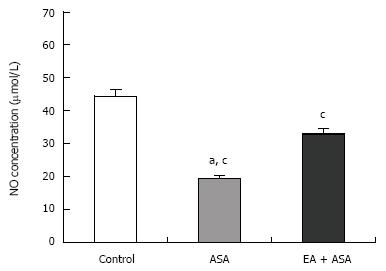
The total nitrate (NO3-) and nitrite (NO2-) concentrations of control, ASA and EA groups were 44.48 ± 2.06 &mgr;mol/L, 19.34 ± 1.05 &mgr;mol/L and 32.96 ± 1.68 &mgr;mol/L, respectively (Figure 4). These results demonstrate that the total NO in the EA group was significantly higher than that of the ASA group, but still significantly lower than that of the control group.
Acupuncture has been used to treat stomach diseases with few adverse effects. In this study, we found that the lesions scores of the EA group were significantly lower than in the ASA group. Although hypotheses abound regarding NSAID-induced gastric damage, the exact mechanism remains unclear.
The acidity of gastric secretions is important for modulating gastric defenses because acid can interfere with restitution, resulting in the conversion of superficial injury to deeper mucosal necrosis[12]. Additionally, because several growth factors (e.g. fibroblast growth factor) that are important for maintenance of mucosal integrity and repair of superficial injury are acid-labile, the presence of acid in the lumen can limit the contributions of these factors to mucosal defense and repair[13]. Decreased acidity in the ASA and EA groups in the present study might have resulted from compensation for the destruction of gastric defense mechanisms by exposure to 100 mg/kg ASA.
The expression of COX-1 mRNA was increased in the EA group, but decreased in the ASA group. The expression of COX-2 mRNA in the EA groups gradually increased over time. This study thus showed that both COX-1 and COX-2 are inducible, which contradicts previous studies[14]. The reduction of gastric blood flow following NSAID administration appears to primarily arise from COX-1 inhibition, but neutrophil adherence following NSAID administration appears to be due to COX-2 suppression[12]. Administration of a selective COX-2 inhibitor, but not a COX-1 inhibitor, has been shown to induce neutrophil adherence[15]. A previous study reported that treatment with a selective COX-2 inhibitor aggravated dextran sulfate sodium-induced inflammation in the colon and that endogenous prostaglandins (PGs) were involved in the mucosal defense against chemo-induced ulceration; this response was produced by COX-1 in the early phase and COX-2 in the late phase[16]. Thus, the presently observed inducible expression of COX-1 in the early phase and COX-2 in the late phase suggests that EA pretreatment might up-regulate the mucosal defense mechanism.
NO in the vascular endothelium mediates various biological actions under physiological conditions[17] and also plays an important role in the modulation of gastric mucosal integrity by interacting with other protective mediators[1819]. Several studies have reported that NO, or NO donors, stimulate PG production in various organs and cells[20–22] and up-regulate acid-induced HCO3- secretion[23]. The total NO concentration in the EA group was significantly increased with respect to that of the ASA group. This increase of total NO likely protected the gastric mucosa against ASA-induced toxicity. Furthermore, EA-induced NO induction may in turn increase production of PG and HCO3-.
In conclusion, EA pretreatment might be effective for gastric defense against ASA-induced ulceration.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used as anti-inflammatory and analgesic agents though they often cause gastrointestinal injury in gastric lesions by inhibiting COX. Thus, effective strategies are required to protect the gastrointestinal mucosa.
In this study, the authors demonstrate that the time-dependent expression of COX could be a potential mechanism for mediating the beneficial effects of acupuncture. The PGs-related acupuncture mechanism should be undertaken, because the endogenous prostaglandins (PGs) are involved in the mucosal defense against chemo-induced ulceration.
The inducible expression of COX-1 in the early phase and COX-2 in the late phase suggests that EA pretreatment might up-regulate the mucosal defense mechanism. The total NO concentration in the EA group was significantly increased with respect to that of the ASA group. The total NO likely protected the gastric mucosa against ASA-induced toxicity. The EA pretreatment might be effective for gastric defense against ASA-induced ulceration.
By understanding the time-dependent expression of COX by EA pretreatment, this study may represent a future strategy for therapeutic intervention in the treatment of patients with gastritis by Nonsteroidal anti-inflammatory drugs.
Acupuncture is a technique of inserting and manipulating fine filiform needles into specific points on the body with the aim of relieving pain and for therapeutic purposes. According to traditional Chinese medical theory, these acupuncture points lie along meridians along which qi, the vital energy, flows.
The methodology employed is sound and reproducible. The scientific and innovative contents of this manuscript can reflect the advanced levels of the clinical and basic researches in gastroenterology both at home and abroad.
| 1. | Hall J, Morand EF, Medbak S, Zaman M, Perry L, Goulding NJ, Maddison PJ, O'Hare JP. Abnormal hypothalamic-pituitary-adrenal axis function in rheumatoid arthritis. Effects of nonsteroidal antiinflammatory drugs and water immersion. Arthritis Rheum. 1994;37:1132-1137. |
| 2. | Rashad S, Revell P, Hemingway A, Low F, Rainsford K, Walker F. Effect of non-steroidal anti-inflammatory drugs on the course of osteoarthritis. Lancet. 1989;2:519-522. |
| 3. | Andrews FJ, Malcontenti-Wilson C, O'Brien PE. Effect of nonsteroidal anti-inflammatory drugs on LFA-1 and ICAM-1 expression in gastric mucosa. Am J Physiol. 1994;266:G657-G664. |
| 4. | Pei WF, Xu GS, Sun Y, Zhu SL, Zhang DQ. Protective effect of electroacupuncture and moxibustion on gastric mucosal damage and its relation with nitric oxide in rats. World J Gastroenterol. 2000;6:424-427. |
| 5. | Yan J, Yang RD, He JF, Yi SX, Chang XR, Lin YP. Effect of acupuncture at different meridian acupoints on changes of related factors for rabbit gastric mucosal injury. World J Gastroenterol. 2005;11:6472-6476. |
| 6. | Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512-520. |
| 7. | Konturek SJ, Brzozowski T, Majka J, Pytko-Polonczyk J, Stachura J. Inhibition of nitric oxide synthase delays healing of chronic gastric ulcers. Eur J Pharmacol. 1993;239:215-217. |
| 8. | Takahashi T. Acupuncture for functional gastrointestinal disorders. J Gastroenterol. 2006;41:408-417. |
| 9. | Jin HO, Zhou L, Lee KY, Chang TM, Chey WY. Inhibition of acid secretion by electrical acupuncture is mediated via beta-endorphin and somatostatin. Am J Physiol. 1996;271:G524-G530. |
| 10. | Noguchi E, Hayashi H. Increases in gastric acidity in response to electroacupuncture stimulation of the hindlimb of anesthetized rats. Jpn J Physiol. 1996;46:53-58. |
| 11. | Khan HA. Computer-assisted visualization and quantitation of experimental gastric lesions in rats. J Pharmacol Toxicol Methods. 2004;49:89-95. |
| 12. | Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15:691-703. |
| 13. | Szabo S, Folkman J, Vattay P, Morales RE, Pinkus GS, Kato K. Accelerated healing of duodenal ulcers by oral administration of a mutein of basic fibroblast growth factor in rats. Gastroenterology. 1994;106:1106-1111. |
| 14. | Bhandari P, Bateman AC, Mehta RL, Patel P. Mucosal expression of cyclooxygenase isoforms 1 and 2 is increased with worsening damage to the gastric mucosa. Histopathology. 2005;46:280-286. |
| 15. | Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706-714. |
| 16. | Park YS. [COX-2 inhibitors in inflammatory bowel disease: friends or foes?]. Korean J Gastroenterol. 2007;50:350-355. |
| 17. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. |
| 18. | Pique JM, Whittle BJ, Esplugues JV. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur J Pharmacol. 1989;174:293-296. |
| 19. | Whittle BJ, Lopez-Belmonte J, Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990;99:607-611. |
| 20. | Furukawa O, Kitamura M, Sugamoto S, Takeuchi K. Stimulatory effect of nitric oxide on bicarbonate secretion in bullfrog duodenums in vitro. Digestion. 1999;60:324-331. |
| 21. | Uno H, Arakawa T, Fukuda T, Yu H, Fujiwara Y, Higuchi K, Inoue M, Kobayashi K. Nitric oxide stimulates prostaglandin synthesis in cultured rabbit gastric cells. Prostaglandins. 1997;53:153-162. |
| 22. | Watkins DN, Garlepp MJ, Thompson PJ. Regulation of the inducible cyclo-oxygenase pathway in human cultured airway epithelial (A549) cells by nitric oxide. Br J Pharmacol. 1997;121:1482-1488. |
| 23. | Aihara E, Sasaki Y, Ise F, Kita K, Nomura Y, Takeuchi K. Distinct mechanisms of acid-induced HCO3- secretion in normal and slightly permeable stomachs. Am J Physiol Gastrointest Liver Physiol. 2006;291:G464-G471. |