Published online Feb 28, 2009. doi: 10.3748/wjg.15.936
Revised: January 20, 2009
Accepted: January 27, 2009
Published online: February 28, 2009
AIM: To evaluate esophageal mucosal defense mechanisms at an epithelial level to establish if pantoprazole treatment can induce ultrastructural healing and improvement in the proliferation activity of the esophageal epithelium in gastroesophageal reflux disease (GERD).
METHODS: This was a single-blinded study for pH-monitoring, and histological, ultrastructural and MIB1 immunostaining evaluation. Fifty eight patients with GERD were enrolled and underwent 24 h pH-monitoring and endoscopy. Patients were treated for 12 and 24 mo with pantoprazole. Esophageal specimens were taken for histological and ultrastructural evaluation, before and after the treatment.
RESULTS: With transmission electron microscopy, all patients with GERD showed ultrastructural signs of damage with dilation of intercellular spaces (DIS). After 3 mo of therapy the mean DIS values showed a significant reduction and the mean MIB1-LI values of GERD showed an increase in cell proliferation. A further 3 mo of therapy significantly increased cell proliferation only in the erosive esophagitis (ERD) group.
CONCLUSION: Three months of pantoprazole therapy induced ultrastructural healing of mucosal damage in 89% and 93% of ERD and non-erosion patients, respectively. Moreover, long-term pantoprazole treatment may be helpful in increasing the capability for esophageal cell proliferation in GERD, particularly in ERD patients.
- Citation: Calabrese C, Treré D, Liguori G, Gabusi V, Vici M, Cenacchi G, Derenzini M, Febo GD. Esophageal cell proliferation in gastroesophageal reflux disease: Clinical-morphological data before and after pantoprazole. World J Gastroenterol 2009; 15(8): 936-941
- URL: https://www.wjgnet.com/1007-9327/full/v15/i8/936.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.936
Gastroesophageal reflux disease (GERD) is represented by a broad spectrum of endoscopic and histological features ranging from endoscopically and histologically normal mucosa to severe endoscopic erosive esophagitis (ERD) accompanied by extensive histological abnormalities[12]. Acid and bile exposure times in GERD patients who are endoscopically negative (NERD) and in subjects with ERD greatly overlap, as demonstrated by 24-h monitoring methodologies and ultrastructural alterations[3–5]. This strongly implies that there must be other factors that are important in defining the degree of gross and microscopic changes within the esophageal mucosa under the impact of the aggressive milieu of the gastroesophageal refluxate. Esophageal mucosal defense mechanisms, balancing luminal aggressive factors, operate at three overlapping levels: the pre-epithelial, epithelial and post-epithelial[6–9]. Because the aggressive factors of gastroesophageal reflux always act at the luminal perimeter of the esophageal mucosa, pre-epithelial defense remains a vanguard of mucosal protection.
The importance of the squamous epithelium biopsy in NERD diagnosis has been reviewed in consideration of the recognition of new histological parameters such as intercellular space dilations[10–13].
Recent data about cell kinetics of the esophageal mucosa have shown that in patients affected by GERD, the proliferation rate of the esophageal epithelium was inferior to that of normal subjects. Ki67-LI gives an accurate estimate of the growth fraction and is reduced in esophageal mucosa exposed to chronic acid. In particular, patients with GERD have a decrease in MIB1 immunostaining of 50% and 25% in NERD and ERD compared to normal subjects[14].
The primary goals of GERD therapy are enduring symptom relief, protection from long-term complications and improved subjective well-being. Proton pump inhibitors (PPIs), which provide powerful gastric acid control[1516], are the treatment of choice in this regard[1718].
The aim of this study was to evaluate the esophageal mucosal defense mechanisms at the epithelial level. Transmission electron microscopy (TEM) and MIB1 antibodies were used to establish if pantoprazole treatment could induce ultrastructural healing and an improvement in the proliferation activity of the esophageal epithelium in patients with erosive or non-erosive GERD.
This study was single-blinded for pH-monitoring and histological, ultrastructural and MIB1 immunostaining evaluations. Patients gave written informed consent to participate in the study, which was approved by the local research committee.
We enrolled 58 patients (26 male; mean age 45.22 ± 12.92; range 23-72) with typical symptoms of GERD (heartburn and/or regurgitation) with at least a 1-year history of GERD (with a frequency of more than twice a week). These were consecutive patients who agreed to undergo both esophageal pH-monitoring and endoscopy. The frequency and intensity of symptoms and their impact on the patients’ quality of life were registered using a structured and validated questionnaire for the diagnosis of GERD[19], and patients with a score higher than 3.1 were considered positive.
Patients with esophageal or gastric malignancy or histologically-proven Barrett esophagus, gastric or duodenal ulcer, previous esophageal or gastric surgery were excluded. Patients taking antisecretory or prokinetic drugs were asked to stop any medication at least 30 and 15 d before the study, respectively. Antacid or alginate preparations were suggested in case of frequent and intolerable symptoms.
The control group consisted of 9 healthy voluntary subjects (mean age 38.2 ± 17.6 years, range 24-60 years; 4 male), and were defined according to the following parameters: absence of typical symptoms or atypical manifestations of GERD, normal 24-h esophageal pH monitoring, endoscopic and histological features, and ultrastructural parameters.
All patients underwent 24-h esophageal pH-monitoring. During the test, meal times and compositions were standardized. The reflux parameters were assessed according to Johnson-DeMeester[20]. The percentage of time with pH < 4.0 over 24 h was evaluated and was considered abnormal if pH < 4.0 was present for more than 6% of the total 24-h period. In the week after 24-h pH monitoring, all the patients underwent upper-gastrointestinal endoscopy to assess the presence or absence of erosive esophagitis.
Patients underwent upper gastrointestinal endoscopy (videogastroscope Olympus GIF 140) after sedation by i.v. administration of midazolam (2.5 mg) to assess the presence or absence of esophagitis. The Los Angeles classification was used to grade the esophagitis[21]. During endoscopy, eight biopsies were taken as follows: two biopsies from each of the four quadrants, 5 cm above the squamo-columnar junction (SCJ), from macroscopically intact (non-eroded) esophageal mucosa. The SCJ (or Z-line) was defined as the border between gastric glandular and esophageal squamous epithelium, and roughly corresponded to the proximal edge of the gastric folds.
Of the 8 specimens taken, 6 were oriented to appropriate cellulose acetate supports (Endofilters Bioptica, Milan, Italy), fixed in 10% buffered formalin and embedded in paraffin, for processing by hematoxylin-eosin and MIB1 evaluation. Two specimens from each patient were used for processing by TEM according to our methodology[11]. Of 58 patients enrolled, 30 patients were affected by NERD (11 male; mean age 49.33 ± 10.19; range 36-61) and 28 by ERD (13 male; mean age 43.93 ± 12.98; range 23-72).
Serial sections of 4 &mgr;m were cut from each paraffin block and stained with hematoxylin-eosin. For each case, whole longitudinally-sectioned samples were examined. Esophagitis was identified and graded according to the Ismail-Beigi et al[1] classification.
MIB1 immunostaining was assessed using anti-Ki-67 monoclonal antibodies (MoAbs) (clone MIB-1; BioGenex Laboratories, San Ramon, CA, USA). Before immunostaining, antigen retrieval was effected by heating the slides, which were fully immersed in 10 mmol/L sodium citrate buffer (pH 6.0) for 20 min in an autoclave. After cooling to room temperature, the slides were incubated with primary MoAbs overnight at a dilution of 1:100. The immunostaining reaction was then developed according to SABC (stretavidin-biotin-peroxidase pre-formed complex) protocol and highlighted using a peroxidase/DAB enzymatic reaction. Sections were finally counterstained with hematoxylin[22].
Quantitative analysis of MIB1 immunostaining was performed on a contiguous field, visualized on the color monitor of a Pentium III PC equipped with a 3 CCD (charge-couple device) color video camera (KY F55B, JVC, Pinebrook, NJ, USA) and connected to a light microscope (Leitz DIAPLAN). For each case, whole longitudinally-sectioned samples were examined. Samples that did not contain at least 1000 cells were excluded. Quantitative evaluation was only carried out on portions of the epithelium in between vertically-sectioned stromal papillae and corresponding to 100 &mgr;m from the basal layer. The MIB1 label index (MIB1-LI) was defined as the ratio of MIB1 positive nuclei to the total number of epithelial cells, and was expressed as a percentage.
The specimens were rinsed in buffer, post-fixed in 1% buffered osmium tetroxide, and dehydrated through a graded alcohol series. They were then infiltrated through propylene oxide and embedded in an epoxy resin. Blocks were trimmed and ultra-thin sections on copper grids were post-stained with uranyl acetate and lead citrate. Each specimen was analyzed by TEM (Philips 510) and then photographed at an accelerating voltage of 80 kV. Photographs of at least 10 significant fields, each obtained with a negative containing an internal scale marker, were magnified at 3500 ×. Ten photomicrographs were obtained from each patient’s biopsy specimens observed by TEM. Photographs with internal scale marker were digitized and then each field was valued using Endox System software. At least 10 randomly selected perpendicular transects to adjacent membranes were drawn and measured in each image for a total of 100 measurements in each case. Each transect was drawn at a distance no closer than 1 &mgr;m. A mean score of dilations in the intercellular space (DIS) of 0.74 &mgr;m was considered a cut-off score for damage[11].
After endoscopy patients were treated with pantoprazole 40 mg/d for 12 wk. After this period they underwent endoscopy and another series of biopsies were taken and processed, as previously described. At the end of these 12 wk, 50% of patients with ERD (n = 14) and 50% of patients with NERD (n = 15) were randomized, using a computer generated list, to receive an additional 12 wk of therapy. After this second period of therapy, the treated patients underwent endoscopy and a final series of biopsies were taken and processed, as previously described. No therapy with pantoprazole was administered in healthy controls.
The measurements obtained by the above-mentioned method were used to calculate mean DIS scores for each patient and all cases as a whole. The Student’s t-test was performed for both independent variables. The Mann-Whitney U test was performed to compare cell kinetic data in each group of subjects. The paired-sample t-test was performed to compare the means of DIS and MIB1-LI values before and after therapy for each group of patients studied. P < 0.05 was considered statistically significant. Data were analyzed with SPSS software (SPSS, Chicago, IL).
The percentage time of esophageal pH < 4 ranged from 8.7% to 12.8% among all patients, with a mean ± SD value of 10.3% ± 1.8% and a median value of 10%. The percentage time of esophageal pH < 4 for the two groups of patients (NERD and ERD) was 10% ± 1.1% and 10.5% ± 1.3%, respectively. No significant differences were found between the two groups.
Among 28 patients affected by ERD, 22 had a normal histological pattern in specimens of endoscopic mucosa, and 6 had mild esophagitis. In the NERD group, 29 patients showed a normal pattern and only one had histological signs of esophagitis (mild).
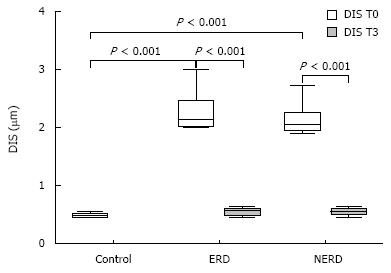
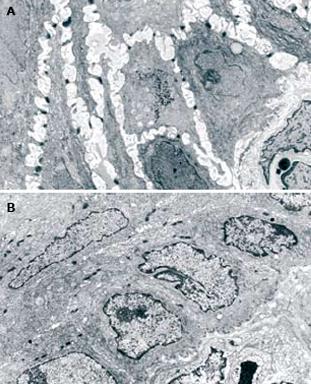
Using TEM, all patients with GERD, with or without erosions, showed ultrastructural signs of damage defined by the presence of DIS (cut-off of DIS > 0.74 &mgr;m) at baseline (T0)[11]. DIS ranged from 0.28 to 7.83 &mgr;m among all subjects, with a mean ± SD value of 1.83 ± 0.33 &mgr;m and a median value of 2.27 &mgr;m. The mean values of DIS in the three groups of subjects (normal, NERD and ERD) were 0.48 ± 0.09, 2.11 ± 0.22 and 2.27 ± 0.48 &mgr;m, respectively. The difference between normal and. patient groups was significant (P < 0.001), while no significant difference was found in the mean value of DIS between the two GERD groups (NERD vs ERD). After 3 mo of therapy, the mean DIS values were 0.55 ± 0.11 and 0.58 ± 0.13 in NERD and ERD patients, respectively. A paired-sample t-test conducted to compare the mean DIS values measured in each patient at T0 and T3, showed a significant reduction of intercellular spaces both in ERD and NERD patients (P < 0.001) (Figure 1). Figure 2 shows TEM photomicrographs of the suprabasal layer of esophageal mucosa before (A) and after (B) pantoprazole treatment. The intercellular spaces clearly recovered after therapy.
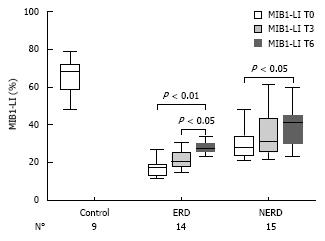
At baseline (T0), MIB1-LI ranged from 12% to 78.8% among all patients, with a mean ± SD of 32.2% ± 16.3%. The mean MIB1-LI value of the healthy voluntary controls was 65.9% ± 9.6%. In 58 GERD patients, 30 with NERD and 28 with ERD, the mean values of MIB1-LI were 31.3% ± 8.8% and 22.3% ± 7.9%, respectively, with a significant difference between the two groups (P < 0.001). In all three groups, proliferating cells were located mainly in the basal zone (100 &mgr;m from the basal layer), with no differences in their architectural distribution towards the mucosa.
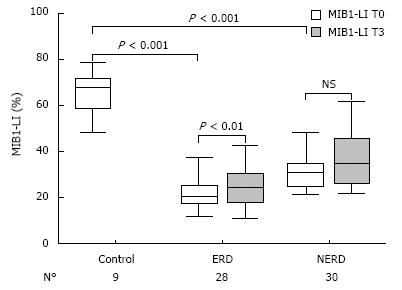
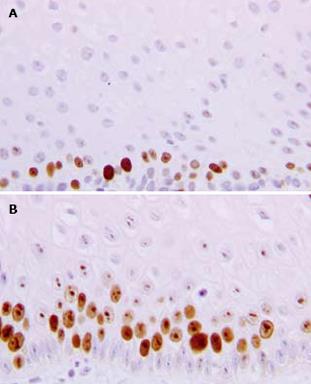
After 3 mo of therapy (T3), the mean MIB1-LI values of NERD and ERD were 37.1% ± 13.2% (range: 22-61.7) and 25.4% ± 10.6% (range: 11-58), respectively (Figure 3). The paired-sample t-test, comparing MIB1-LI values measured in each patient at T0 and T3, showed a significant increase in cell proliferation in ERD (P = 0.006), but not in NERD patients (P = 0.78). In Figure 4, the MIB1-immunostained sections of biopsies taken from the same ERD patient are shown at baseline and after 3 mo of therapy, respectively. A greater number of MIB1-positive cells are clearly visible after therapy.
After 6 mo of therapy (T6), in the 14 ERD and 15 NERD randomized patients, the mean MIB1-LI values of NERD and ERD were 33.3% ± 9.6% and 28.2% ± 5.9%, respectively (Figure 5). Both in NERD and ERD patients, a paired-sample t-test for MIB1-LI showed a significant increase in cell proliferation after 6 mo of therapy compared to the baseline (P < 0.01), while a significant difference for MIB1-LI was achieved between 3 and 6 mo of therapy only in the ERD group (P < 0.05) (Figure 5).
Evidence is accumulating that erosive endoscopic changes within the esophageal mucosa in patients with GERD accompanied by reflux esophagitis result from a disequilibrium between aggressive factors and protective mechanisms[67].
Because aggressive factors always operate on the luminal side of the esophageal mucosa, the esophageal pre-epithelial barrier, represented by a mucus-buffer layer covering the epithelium, plays a role in mucosal protection[6–8].
Recently, we demonstrated that, in patients with GERD, cell proliferation is reduced in esophageal mucosa exposed to chronic acid-peptic insult. In particular, patients with NERD and ERD showed a decrease in cell proliferation to 50% and 75%, respectively, compared to normal subjects[14]. Therefore, esophageal cell proliferation should be taken into consideration as one of the factors involved in the esophageal mucosal defense mechanisms.
In order to better elucidate the relationship between esophageal acid-peptic exposure, ultrastructural alterations of the epithelial lining of the esophagus, and GERD symptoms, our attention was focused on the differences in proliferating activity in NERD and ERD patients.
This study analyzed esophageal-epithelial cell proliferation in patients with gastroesophageal reflux disease with NERD or ERD using immunohistochemical techniques. We investigated whether PPI treatment, reducing the chronic acid-peptic insult, is able to improve esophageal cell proliferation.
After 3 mo, pantoprazole therapy induced, in our subset of patients, ultrastructural healing of mucosal damage in 89% and 93% of ERD and NERD patients, respectively. The ultrastructural healing of the esophageal mucosa was accompanied by a complete resolution of the esophageal symptoms. The patients with an incomplete healing of the DIS after 3 mo of therapy showed persistent symptoms, although these patients became asymptomatic after a further 3 mo of therapy, and showed a complete recovery of the mucosa. These results are similar to our previous study of omeprazole therapy[23].
Our results confirmed that cell proliferation is reduced in esophageal mucosa exposed to chronic acid-peptic insult[14]. In particular, at baseline, the mean MIB1-LI value was 31% and 22% in NERD and ERD patients respectively, compared to 66% in the healthy subjects.
Three months of PPI treatment was able to improve cell proliferation in ERD and NERD patients, though the improvement was only statistically significant in the ERD group. After 6 mo of therapy, we observed a significant further increase in the mean MIB1-LI in both groups of randomized patients. However, even after 6 mo of therapy, the proliferation of the esophageal epithelium in GERD patients did not reach the values of normal subjects.
Two factors regarding the reduced epithelial proliferation activity observed in GERD should be considered. Firstly, the chronic cell damage induced by gastroesophageal reflux could determine a reduction in the proliferation rate of the esophageal epithelium, or a constitutive lower capability for cell proliferation could lead to increased susceptibility to damage induced by gastroesophageal reflux. Our data regarding the cell proliferation rate in the esophageal epithelium after treatment showed that gastroesophageal reflux alone does not induce a decrease in cell proliferation. In fact, we observed that after 3 or 6 mo of PPI treatment, the mean of MIB1-LI in patients with GERD did not reach the mean of that in healthy subjects, although 6 mo of therapy was able to improve the cell proliferation in ERD patients to the same level of NERD patients.
The second factor regards the implied existence of an individual predisposition for the mucosa to react in different ways to acid and pepsin insults. This concept supports the idea that individuals who develop ERD are genetically characterized by a weaker proliferating epithelial cell capability. On the other hand, patients with more efficient epithelial proliferation capacity could have a lower probability of developing macroscopic mucosal lesions when stressed by acid and pepsin. A possible genetic influence in the proliferation capability of the mucosa has a certain appeal.
In conclusion, this is the first demonstration that long-term pantoprazole therapy may induce, in our subset of patients, the ultrastructural healing of mucosal damage both in ERD and in NERD patients. Moreover, long-term pantoprazole treatment could be helpful in increasing the capability for esophageal cell proliferation in GERD, particularly in ERD patients. Further genetic studies are required to better understand the mucosal defense mechanisms and in particular the cellular proliferative activity of esophageal mucosa.
Gastroesophageal reflux disease (GERD) is represented by a broad spectrum of endoscopic and histological features. Esophageal mucosal defense mechanisms, balancing luminal aggressive factors, operate at three overlapping levels: pre-epithelial, epithelial and post-epithelial. The proliferation rate of the esophageal epithelium is inferior in patients affected by GERD compared to normal subjects. Three months of PPI treatment was able to improve cell proliferation in erosive esophagitis (ERD) and NERD patients.
This is the first demonstration that long-term pantoprazole therapy may induce, in our subset of patients, the ultrastructural healing of mucosal damage both in ERD and in NERD patients. Moreover, long-term pantoprazole treatment could be helpful in increasing the capability for esophageal cell proliferation in GERD, particularly in ERD patients.
Their data are important in the application of therapy in gastroesophageal reflux. It should also be recognized that there are individual differences in the way the mucosa reacts to acid and pepsin insults. This concept supports the idea that individuals who develop ERD are genetically characterized by a weaker proliferating epithelial cell capability. Further studies are needed to evaluate this more fully.
GERD indicates chronic symptoms or mucosal damage produced by abnormal reflux in the esophagus. Esophageal mucosa: esophageal mucosa consists of partially keratinized stratified squamous epithelium with three functional regions: stratum corneum, stratum spinosum, and stratum germinativum. Tissue resistance has three protective components: these are designated as preepithelial, epithelial, and postepithelial defenses.
In this manuscript, the authors reported ultrastructural healing of esophageal mucosal damage with PPI therapy.
| 1. | Ismail-Beigi F, Horton PF, Pope CE 2nd. Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58:163-174. |
| 2. | Johnson LF, Demeester TR, Haggitt RC. Esophageal epithelial response to gastroesophageal reflux. A quantitative study. Am J Dig Dis. 1978;23:498-509. |
| 3. | Champion G, Richter JE, Vaezi MF, Singh S, Alexander R. Duodenogastroesophageal reflux: relationship to pH and importance in Barrett’s esophagus. Gastroenterology. 1994;107:747-754. |
| 4. | Schlesinger PK, Donahue PE, Schmid B, Layden TJ. Limitations of 24-hour intraesophageal pH monitoring in the hospital setting. Gastroenterology. 1985;89:797-804. |
| 5. | Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Carlo S, Zahlane D, Miglioli M, Di Febo G. Effect of omeprazole on symptoms and ultrastructural esophageal damage in acid bile reflux. World J Gastroenterol. 2005;11:1876-1880. |
| 6. | Namiot Z, Sarosiek J, Rourk RM, Hetzel DP, McCallum RW. Human esophageal secretion: mucosal response to luminal acid and pepsin. Gastroenterology. 1994;106:973-981. |
| 7. | Sarosiek J, Namiot Z, Piascik R, Hetzel DP, Rourk RM, Edmunds MC, Daniel TM, McCallum RW. What part do the mucous cells of submucosal mucous glands play the esophageal pre-epithelial barrier? editors. The esophageal mucosa. Elsevier: Amsterdam 1994; 278-290. |
| 8. | Meyers RL, Orlando RC. In vivo bicarbonate secretion by human esophagus. Gastroenterology. 1992;103:1174-1178. |
| 9. | Orlando RC. Esophageal epithelial resistance. J Clin Gastroenterol. 1986;8 Suppl 1:12-16. |
| 10. | Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200-1205. |
| 11. | Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, Scialpi C, Miglioli M, Di Febo G. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525-532. |
| 12. | Zentilin P, Savarino V, Mastracci L, Spaggiari P, Dulbecco P, Ceppa P, Savarino E, Parodi A, Mansi C, Fiocca R. Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group. Am J Gastroenterol. 2005;100:2299-2306. |
| 13. | Dent J. Microscopic esophageal mucosal injury in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2007;5:4-16. |
| 14. | Calabrese C, Trere D, Fabbri A, Cenacchi G, Vici M, Derenzini M, Di Febo G. Endoscopic appearance of GERD: putative role of cell proliferation. Dig Liver Dis. 2007;39:713-719. |
| 15. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. |
| 16. | Miner P Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616-2620. |
| 17. | Rohss K, Lind T, Wilder-Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur J Clin Pharmacol. 2004;60:531-539. |
| 18. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190-200. |
| 19. | Pacini F, Calabrese C, Cipolletta L, Valva MD, Russo A, Savarino V, Vigneri S. Burden of illness in Italian patients with gastro-oesophageal reflux disease. Curr Med Res Opin. 2005;21:495-502. |
| 20. | Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974;62:325-332. |
| 21. | Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85-92. |
| 22. | Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res. 2000;257:231-237. |
| 23. | Calabrese C, Bortolotti M, Fabbri A, Areni A, Cenacchi G, Scialpi C, Miglioli M, Di Febo G. Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol. 2005;100:537-542. |