Published online Feb 28, 2009. doi: 10.3748/wjg.15.927
Revised: May 28, 2008
Accepted: June 5, 2008
Published online: February 28, 2009
AIM: To compare thromboembolism rates between hospitalized patients with a diagnosis of ulcerative colitis and other hospitalized patients at high risk for thromboembolism. To compare thromboembolism rates between patients with ulcerative colitis undergoing a colorectal operation and other patients undergoing colorectal operations.
METHODS: Data from the National Hospital Discharge Survey was used to compare thromboembolism rates between (1) hospitalized patients with a discharge diagnosis of ulcerative colitis and those with diverticulitis or acute respiratory failure, and (2) hospitalized patients with a discharge diagnosis of ulcerative colitis who underwent colectomy and those with diverticulitis or colorectal cancer who underwent colorectal operations.
RESULTS: Patients diagnosed with ulcerative colitis had similar or higher rates of combined venous thromboembolism (2.03%) than their counterparts with diverticulitis (0.76%) or respiratory failure (1.99%), despite the overall greater prevalence of thromboembolic risk factors in the latter groups. Discharged patients with colitis that were treated surgically did not have significantly different rates of venous or arterial thromboembolism than those with surgery for diverticulitis or colorectal cancer.
CONCLUSION: Patients with ulcerative colitis who do not undergo an operation during their hospitalization have similar or higher rates of thromboembolism than other medical patients who are considered to be high risk for thromboembolism.
- Citation: Wang JY, Terdiman JP, Vittinghoff E, Minichiello T, Varma MG. Hospitalized ulcerative colitis patients have an elevated risk of thromboembolic events. World J Gastroenterol 2009; 15(8): 927-935
- URL: https://www.wjgnet.com/1007-9327/full/v15/i8/927.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.927
Thromboembolic events are a preventable cause of morbidity and mortality in hospitalized patients. In community-based studies, the incidence of thromboembolism in the general population was as high as 1 in 1000[1], depending on age and other risk factors. Hospitalized patients are at increased risk for these events given their acute illness and prolonged immobility.
People with inflammatory bowel disease (IBD) are considered to be at higher risk for thromboembolism than the average population, but the extent of this risk in hospitalized patients with ulcerative colitis (UC) is not well described. In a population-based study, the incidence rate ratio of deep venous thrombosis and pulmonary embolism in people with ulcerative colitis was 3.04[2]. Published venous thromboembolism rates among series of clinic-based patients with ulcerative colitis range from 1.3% to 6.2%[34]. In one review of 7199 patients with IBD (half of whom had UC), over the course of 11 years, 1.3% had a thromboembolic event. The mortality of those who had thromboembolism was 25% during the acute thrombotic event[4]. This emphasizes the importance of understanding the risk for thromboembolism in this group of patients and the need to identify those who would benefit from pharmacologic prophylaxis.
The purpose of our study is to describe the prevalence of thromboembolic events among hospitalized patients with UC, and to compare this to the rate in other hospitalized patients who are considered to be at high risk for thromboembolism.
We examined the National Hospital Discharge Survey (NHDS) from years 1979 through 2003[5]. This dataset is compiled by the National Center for Health Statistics and is a probability sample of discharges from short-stay hospitals (average length of stay under 30 d). These data are available on compact disc. The current sampling plan is three-staged. Geographic areas are the primary sampling units, with hospitals selected from within these areas. A sample of discharges from each hospital is then selected by systematic random sampling. Each observation has an associated sample weight that is used to calculate the number of discharges represented by a single observation. Diagnosis and procedure-related information extracted from hospital discharge summaries is coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM)[6]. Data available for each entry includes demographic information and a maximum of seven ICD-9 CM diagnosis codes and four ICD-9 CM procedure codes. The ICD-9 CM codes are provided in the dataset in the same order as found on the patient discharge summary from which data is abstracted, although it is not confirmed that the first listed code is necessarily the diagnosis on admission. Additionally, if a myocardial event has occurred during the hospitalization, this is automatically listed as the first ICD-9 CM code regardless of the diagnosis on admission.
These de-identified data are publicly available, therefore, the study was acknowledged to be exempt from review by our institutional review board.
Cases included observations of all ages with an ICD-9 CM code for UC (ICD-9 CM code 556, 556.0-556.9). An ICD-9 CM code for UC was either the first diagnosis (possible primary diagnosis) or any of the other six possible diagnoses, as is true for all other diagnoses examined in this study. Any observation with a code for UC was placed in the appropriate non-surgical or surgical UC group, regardless of the presence of other diagnoses. For observations in which the code for UC was not in the first position, common first-listed diagnoses included gastroenterological conditions such as colitis or gastrointestinal bleeding or abdominal pain; anemia; and volume depletion, all of which could be directly related to UC. Common non-GI first-listed diagnoses included myocardial infarction, stroke, and pneumonia.
The non-surgical comparison groups included all observations with a code for diverticulitis (ICD-9 CM code 562.11 or 562.13) or acute respiratory failure (ICD-9 CM code 518.81, 518.82, 518.84). The observations with acute respiratory failure that also had a procedure code for intubation (ICD-9 CM procedure code 960.4 or 960.5) were excluded as this identified a group of patients who were potentially more sick and had greater risk factors for thrombosis, including prolonged immobility. Observations with diverticulitis were selected as a comparison group because they also had an inflammatory colorectal condition, although the process is localized as opposed to systemic as in UC. Observations with respiratory failure were selected as another comparison group because these are non-surgical patients considered to be at high risk for venous thromboembolic events[7] and are recommended to receive routine thromboembolic prophylaxis when admitted to the hospital. The surgical groups included any observations with UC, diverticulitis, or colorectal cancer (ICD-9 CM code 153, 153.0-153.9, 154, 154.0, 154.1), which also had a procedure code for any colorectal operation (ICD-9 CM procedure code 457, 457.1-457.9, 458, 484, 484.1, 484.9, 485, 486, 486.2-486.9, 461.0, 461.1, 461.3).
We examined both venous and arterial thrombo-embolic events. The two ICD-9 CM codes for pulmonary embolism were 415.1 and 415.19. For deep venous thrombosis, the ICD-9 CM codes included were 451.1, 451.11, 451.19, 451.2, 451.8, 451.81, 451.83, 451.84, 451.89, 451.9, 453.2, 453.8, and 453.9. There were no pregnancy-related thromboembolic events, which carry their own ICD-9 CM codes, among any of the observations examined. Portal vein thrombosis (ICD-9 CM code 452) and renal vein thrombosis (ICD-9 CM code 453.3) were included. There is no code exclusive to mesenteric vein thrombosis, so while this is a described entity in those with UC, it was not examined. Arterial events included aortic or large artery thromboemboli (ICD-9 CM code 444, 444.0, 444.1, 444.2, 444.8, 444.9) and cerebral embolic events (i.e. strokes) (ICD-9 CM code 434.0, 434.1, 434.9).
Covariates examined included age, gender, obesity (ICD-9 CM code 278), atrial fibrillation (ICD-9 CM code 427.31), and prior history of venous thromboembolism (ICD-9 CM code V125.1), which were factors available in this dataset that may affect the risk of thromboembolism or result in a patient being on anticoagulant medications[89].
For analyses of the six groups, weights provided with the NHDS dataset were used to estimate population means and proportions. Thus, we first applied probability weights to the sample to calculate the number of discharges represented by each observation in the dataset, and then calculated our prevalence rates. These six groups are, therefore, referred to as discharges, grouped by the various conditions previously mentioned. Information on sampling strata and primary sampling units is not provided with the NHDS dataset, so confidence intervals and P-values based on the linearization methods appropriate for complex surveys could not be computed. Instead, standard errors and confidence intervals for estimated proportions were approximated using the methods and constants provided in the NHDS documentation[5]. Approximate two-sided Z-tests were then used to evaluate differences in rates between discharges with UC and each comparison group, under the assumption of negligible correlation between the group-specific estimates. Differences in gender and prevalence of atrial fibrillation, obesity, and history of venous thromboembolic events were also analyzed using the Z-test. We also calculated the mean age of the observations in each group.
We then performed three further sets of analyses. In the first, we compared event rates among the non-surgical discharges with UC, diverticulitis, or acute respiratory failure. In the second, we compared event rates among surgical discharges with UC, diverticulitis, or colorectal cancer. Finally, we compared event rates between surgical discharges with UC and non-surgical discharges with UC.
Logistic regression was performed to assess the degree to which between-group differences in event rates might be explained by potential confounders including age, gender, obesity, and atrial fibrillation. Weighted odds-ratios for the association of patient group with event risk can be validly estimated using these data under the working assumption of independence, as in the standard linearization procedures for logistic regression analysis of complex survey data. We defined these regression analyses as exploratory analyses because no valid confidence intervals or P-values can be computed because of the lack of dataset-specific information on sampling stratum and primary sampling units. Despite this limitation, some information is gained by comparing the unadjusted and adjusted odds ratios in cases where the rate difference corresponding to the unadjusted odds ratio is statistically significant. If the adjusted odds ratio is farther from the null value of 1.0 than the unadjusted odds ratio, then it is plausible that the adjusted odds ratio is also statistically significant.
We examined 8182 observations with UC, 89.2% of whom had no concurrent colorectal operation during the admission. This group was compared with 25 138 observations with diverticulitis who were not treated surgically and 50 905 observations with respiratory failure. The 880 observations with UC who were treated surgically were compared with 6860 observations with surgically treated diverticulitis and 20 336 observations with surgically treated colorectal cancer. The estimated population totals after application of the sampling weights and the characteristics of the non-surgical and the surgical groups are presented in Table 1.
| Non-surgical discharges | Surgical discharges | |||||
| Ulcerative colitis | Diverticulitis | Acute respiratory failure | Ulcerative colitis | Diverticulitis | Colorectal cancer | |
| N | 7302 | 25 138 | 50 905 | 880 | 6860 | 20 336 |
| Estimated population totals1 | 974 206 | 3 805 999 | 6 296 383 | 127 327 | 944 275 | 2 910 807 |
| Age (mean) | 49.8 | 67.7 | 62.3 | 47.6 | 62.2 | 69.6 |
| Male (%) | 46 | 35a | 48 | 54 | 43 | 49 |
| Length of stay (mean) | 7.1d | 6.6d | 11.7d | 16.9d | 13.1d | 13.9d |
| Atrial fibrillation (%) | 2.9 | 4.5a | 11.3c | 2.3 | 3.2 | 5.1e |
| Obesity (%) | 1.3 | 2.5a | 2.2c | 1.1 | 2.3 | 1.5 |
| History of VTE (%) | 0.13 | 0.01 | 0.11 | 0 | 0.06 | 0.18 |
| Mortality (%) | 1.6 | 1.6 | 23.6 | 2.5 | 4.0 | 3.7 |
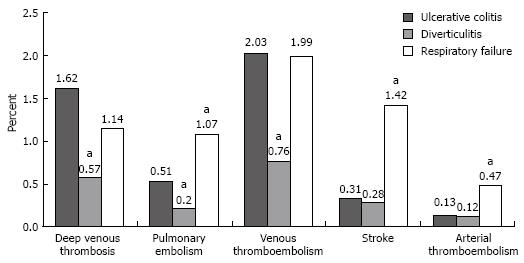
Discharges with UC who did not have surgery had a deep venous thrombosis rate of 1.62% (95% CI, 1.01%-2.23%), a pulmonary embolism rate of 0.51% (95% CI 0.25-0.78%), and a combined venous thromboembolic event rate of 2.03% (95% CI, 1.36%-2.69%; Figure 1). The rates of deep venous thrombosis (P = 0.01), pulmonary embolism (P = 0.03), and combined venous thromboembolic events (P = 0.004; defined as deep venous thrombosis, pulmonary embolism, portal and renal vein thromboses) among those with UC were significantly higher than those for discharges with diverticulitis (Table 2). Discharges with UC had similar rates of deep venous thrombosis and combined venous thromboembolic events and lower rates of pulmonary embolism (P = 0.002), strokes (P < 0.001), and arterial embolisms (P = 0.01) than discharges with respiratory failure (Table 2). The rates for portal vein thrombosis and renal vein thrombosis were very low in non-surgical discharges with UC (0.05% and 0%, respectively) and not significantly different from rates in the comparison groups.
| Thromboembolic event | Ulcerative colitis vs diverticulitis | Ulcerative colitis vs respiratory failure | ||
| Rate difference, % (95% CI) | P value | Rate difference, % (95% CI) | P value | |
| Deep venous thrombosis | 1.05 (1.69 to 0.41) | 0.01 | 0.48 (-0.18 to 1.14) | 0.2 |
| Pulmonary embolism | 0.31 (0.03 to 0.59) | 0.03 | -0.56 (-0.92 to -0.2) | 0.002 |
| Venous thrombo-embolism | 1.26 (0.56 to 1.97) | 0.004 | 0.04 (-0.72 to 0.8) | 0.9 |
| Stroke | 0.03 (-0.28 to 0.34) | 0.9 | -1.11 (-1.52 to -0.7) | < 0.001 |
| Arterial thrombo-embolism | 0.01 (-0.21 to 0.24) | 0.9 | -0.33 (-0.59 to -0.08) | 0.01 |
In an exploratory analysis, odds ratios for the non-surgical discharge groups were compared before and after adjustment for age, gender, obesity, and atrial fibrillation. For the comparison of UC to diverticulitis, the odds ratios increased after adjustment for potential confounders from 2.69 to 3.05 for combined venous thromboembolic events (CI’s not calculable; see methods). Similarly, the odds ratios for UC compared to acute respiratory failure increased after adjustment, from 1.02 to 1.12 for combined venous thromboembolic events. The increase in odds ratios after adjustment supports the view that the associations observed on univariate analysis are, in fact, significant. If anything, the association is further strengthened (with the odds ratio moving farther away from 1.00) by adjusting for the potential confounders. When comparing odds of arterial embolism, the odds ratio for UC compared to diverticulitis increased from 1.12 to 1.48 and the odds ratio for UC compared to respiratory failure increased from 0.29 to 0.39.
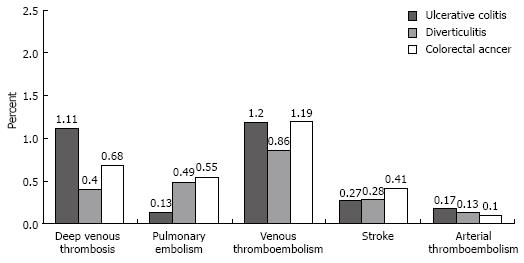
Discharges with UC who underwent an operation for this condition had a deep venous thrombosis rate of 1.11% (95% CI, 0%-2.76%), a pulmonary embolism rate of 0.13% (95% CI, 0%-0.6%), and a combined venous thromboembolism rate of 1.20% (95% CI, 0%-2.89%; Figure 2). The rates of these venous thromboembolic events did not significantly differ among the three surgical groups (Table 3). Discharges with UC had a portal vein thrombosis rate of 0.02% and no occurrence of renal vein thromboses. These event rates were similarly low in the surgical comparison groups. The rates of stroke or other arterial embolic events did not differ among the three surgical groups (Table 3).
| Thromboembolic event | Ulcerative colitis vs diverticulitis | Ulcerative colitis vs respiratory failure | ||
| Rate difference, % (95% CI) | P value | Rate difference, % (95% CI) | P value | |
| Deep venous thrombosis | 0.7 (-0.98 to 2.39) | 0.4 | 0.42 (-1.25 to 2.1) | 0.6 |
| Pulmonary embolism | -0.36 (-0.95 to 0.23) | 0.2 | -0.42 (-0.92 to 0.09) | 0.1 |
| Venous thrombo-embolism | 0.34 (-1.42 to 2.09) | 0.7 | 0.01 (-1.61 to 1.72) | 1.0 |
| Stroke | -0.01 (-0.67 to 0.65) | 1.0 | -0.14 (-0.75 to 0.48) | 0.7 |
| Arterial thrombo-embolism | 0.041 | -2 | 0.061 | -2 |
An exploratory analysis of the role of confounding variables for surgical discharges was also performed. For the comparison of surgical UC and diverticulitis discharges, the odds ratios increased after adjustment for potential confounders from 2.76 to 3.47 for deep venous thrombosis (CI’s not calculable, see methods), from 1.40 to 1.74 for combined venous thromboembolic events, and from 1.28 to 1.93 for arterial embolism. When discharges with UC and surgery were compared with discharges with colorectal cancer and surgery, the odds-ratios increased after adjustment from 1.62 to 2.17 for deep venous thrombosis, from 1.00 to 1.40 for combined venous thromboembolic events, and from 1.62 to 3.95 for arterial embolism. Once again, the increase in odds ratios after adjustment supports the significance of the associations found in the univariate analysis. For stroke, the adjusted odds reversed in both comparison groups such that after adjustment, the discharges with UC had greater odds of stroke than discharges with diverticulitis, changing from 0.97 to 1.60, than discharges with colorectal cancer, changing from 0.67 to 1.50.
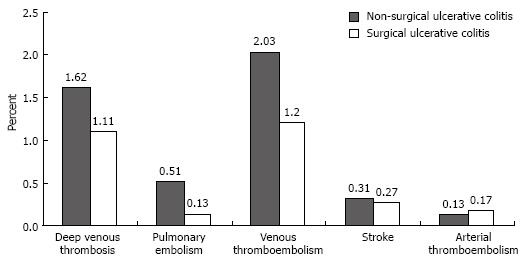
Although the rates of deep venous thrombosis, pulmonary embolism, combined venous thromboembolic events, and stroke were higher in discharges with UC who did not have surgery than in those who did, the differences were not statistically significant (Figure 3).
Among discharges with UC who did not have surgery, mortality was higher for those who had a venous thromboembolic event than in those who did not, although the difference was not statistically significant (5.5% vs 1.5%, P = 0.1). Similarly, mortality was higher from arterial events (17.5% vs 1.6%; P value, not calculable due to missing events). Among discharges with UC who did have surgery, mortality was likewise higher in those who had a venous thromboembolism (5% vs 2.5%, P = 0.9) or arterial thromboembolism (0 vs 2.5%; P value, not calculable due to missing events) than those who did not, although the differences were not statistically significant.
In this study, we compared the prevalence rates of venous and arterial thromboembolic events in hospitalized patients with a diagnosis of UC to those of other high-risk hospitalized patients, using a national discharge dataset. The confidence intervals and P-values we calculated in the unadjusted analysis summarize the evidence for or against between-group differences in crude event rates. We found that discharges with UC who did not have surgery had a higher rate of deep venous thrombosis and pulmonary embolism than discharges with diverticulitis treated non-operatively, a group that had a greater prevalence of risk factors for thromboembolism, such as older age and obesity. We also found that compared to discharges with respiratory failure, the discharges with UC who did not have surgery had a similar rate of deep venous thrombosis, but a lower rate of pulmonary embolism. The higher rate of pulmonary embolism in discharges with acute respiratory failure could be related to two factors. It might be due to a causal relationship between pulmonary embolism and acute respiratory failure. On the other hand, the higher rate could result from ascertainment bias, with a higher rate of diagnosis of pulmonary embolism in patients with respiratory failure and pulmonary symptoms because more pulmonary imaging was performed on these patients.
Among surgical discharges with UC, diverticulitis, or colorectal cancer, there were no differences in rates of deep venous thrombosis or pulmonary embolism. We hypothesize that this could be explained by the frequent use of thromboembolic prophylaxis in inpatients undergoing a colorectal operation, which would minimize differences in their risks of thromboembolism. However, this cannot be confirmed, because the dataset does not provide information about individual medications. We also found no difference in thromboembolic event rates between non-surgical and surgical discharges with UC. One may think that postoperative patients would be less mobile than non-surgical patients, an additional risk factor for thromboembolic events. However, the use of thromboembolic prophylaxis may mask any difference between the surgical and non-surgical groups.
The exploratory logistic regression analyses, while not definitive, strongly suggest that the statistically significant higher event rates that we found among both surgical and non-surgical discharges with UC in the unadjusted analysis were not meaningfully confounded by between-group differences in age, gender, obesity, atrial fibrillation, or prior history of venous thromboembolism.
No prior publication has examined the incidence of thromboembolism in patients with UC specifically during hospitalization, although several series have reviewed an institution’s experience with IBD and thromboembolism. One very large single-center study of patients with UC and Crohn’s disease, which reported a 1.3% incidence of thromboembolic events, found that 64% of patients with an event had active disease, 26% had disease controlled by a sulfasalazine or corticosteroid, and 10% were in remission[4]. A population-based study in Manitoba using administrative data demonstrated that patients with Crohn’s disease and those with UC had approximately three times the risk of developing deep venous thrombosis or pulmonary embolism compared to controls from the general population, matched for year of diagnosis, age, gender, and area of residence[2]. This study also attempted to determine whether thromboembolic risk was higher in patients with UC because of frequent hospitalization, but concluded that this was not the case because the rate ratio among only hospitalized patients was similar to that of the entire population. Another study compared the prevalence of venous thromboembolism between outpatients with IBD and matched healthy controls and found that patients with IBD had greater odds of thromboembolism (OR = 3.6; 95% CI, 1.7-7.8; adjusted for operation, injuries, oral contraceptive use, pregnancy, body mass index, and smoking)[3].
Other studies have focused on specific types of venous thromboembolic events in patients with IBD[1011]. In a review of 94 patients who underwent restorative proctocolectomy and had a postoperative computed tomography scan, 45% had portal vein thrombosis[10]. The portal vein thrombosis rate in the patients with UC who underwent colorectal surgery in our study was only 0.02%. Not all of the patients underwent a total colectomy, which is hypothesized as the main risk factor for portal vein thrombosis. In addition, we cannot be certain if the low rate we found is due to the low sensitivity of ICD-9 CM coding for portal vein thrombosis or, more likely, the fact that most patients with UC were not assessed for the occurrence of this condition. A separate study examining superior mesenteric vein thrombosis after colectomy described that 4.8% of the 83 patients who underwent colectomy for IBD developed CT-proven superior mesenteric vein thrombosis[11]. We did not examine mesenteric venous thrombosis because of the unreliability of ICD-9 CM coding for this diagnosis. Arterial thromboembolic events in patients with UC have also been described, with documentation of these events in several case studies and one study of a cohort of IBD patients describing peripheral arterial thrombosis and cerebrovascular accidents in 0.002%[41213]. The magnitude of the risk for arterial events compared with that for the general population is not known.
The reason for the increased rate of thromboembolic events in patients with UC remains uncertain, but most likely is related to the interaction between cytokine mediators of chronic inflammation and the coagulation cascade[14]. No study has convincingly demonstrated that patients with IBD have a greater burden of prothrombotic risk factors than the general population, such as factor V Leiden mutations[15], hyperhomocysteinemia[16], antiphospholipid antibodies, or thrombophilia[1718]. However, two studies did identify a higher prevalence of factor V Leiden mutations in patients with IBD who developed thromboembolism than in those who did not[1519].
Does the demonstration that hospitalized patients with UC are at increased risk for thromboembolic events, especially venous thromboembolism, merit a recommendation that these patients receive thromboembolic prophylaxis? To answer this question, one must consider the possible effects of the prophylaxis and balance its risks with the benefits of preventing thromboembolism. The bleeding risks unique to UC patients from pharmacologic heparin therapy can be explored in studies of heparin for treating active UC. Two randomized controlled trials have evaluated unfractionated heparin. In one study of patients with UC, all of whom had rectal bleeding, three of the 12 patients receiving full anticoagulation with heparin developed increased rectal bleeding, one requiring an urgent operation[20]. In the other study, of eight patients with IBD who received a continuous infusion of unfractionated heparin, no major bleeding events occurred[21]. Other trials have examined treatment with low molecular weight heparin. In one randomized trial including only patients with UC, the 16 patients who received full anticoagulant doses of low molecular weight heparin had no episodes of rectal bleeding and only small hematomas at the injection site[22]. In a larger, randomized, controlled trial of 48 patients with UC who received low molecular weight heparin, first at full anticoagulation doses, and later at a dose equivalent to that used for prevention of deep venous thrombosis, there were no complications in the treatment group and one episode of rectal bleeding in the placebo group[23]. Overall, it appears that heparin therapy at doses sufficient to achieve complete anticoagulation is safe among patients with active UC with respect to GI bleeding risks, and that the lower thromboembolic prophylactic doses are likely to not bear any higher risk.
At the same time, the consequences of thromboem-bolism in patients with UC appear to be serious, as one study reported that 25% of IBD patients who developed a thromboembolism died from related events[4]. In our dataset, non-surgical patients with UC who had either a venous or arterial thromboembolism had a mortality rate of 5.5% and 17.5%, respectively, compared to a mortality of 1.5% and 1.6%, respectively in non-surgical patients with UC with no thromboembolic events, though we were unable to determine specifically to what extent thromboembolism contributed to the individual patients’ death. Few studies have specifically examined an inpatient population, so the effects of a thromboembolic event on other important outcomes, such as length of stay and cost, have not been described. In studies of other high risk patient populations, prophylaxis reduces morbidity and mortality in medical patients[24]. Thus, given the higher mortality rate in UC patients with thromboembolism and the low risk of heparin therapy in UC patients, it appears that thromboembolic prophylaxis might be justified. Of course, another important aspect of caring for hospitalized IBD patients is rapid, optimal control of the active disease, as there are studies that suggest that those with active or more severe disease have a greater risk for thromboembolism[342526]. We were unable to determine the severity of disease in the patients in this dataset.
The primary strength of our study was the ability to examine thromboembolic events across a large, diverse population from a representative national sample of hospitalized patients. The NHDS dataset is an excellent resource because it captures a broad spectrum of patients. However, because the dataset is based on ICD-9 CM coding, there is the potential for diagnostic misclassification. While ICD-9 CM coding is generally considered to be highly specific and have a low false-positive rate, coding can also have low sensitivity, resulting in omission of cases[27]. This would imply that our calculated event rates are lower than actual rates, although this misclassification should not differ among the patient groups examined; therefore, our rate comparisons would still be valid. The NHDS dataset has been well-described for examining trends of thromboembolic disease in various risk groups, and conclusions drawn from the relationship of venous thromboembolism to age, race, obesity, and cancer are supported by other studies of those patient populations using different data sources[28–31]. For example, one study examined differences in venous thromboembolic event rates with respect to age in the NHDS dataset. The rate of venous thromboembolism increased in relation to older age, a finding which is supported by other regional population-based studies[30]. In addition, when the NHDS dataset was used to examine stroke as a predictor of venous thromboembolic events, the rates were similar to that in a prospective trial, thus suggesting that the coding for diagnosis of stroke was adequate[32]. Therefore, while the diagnosis and procedure codes in the NHDS have not been validated, findings based on its data reflect those of other data sources.
The NHDS dataset we used enabled us to perform a cross-sectional analysis of the prevalence of thromboembolic events in hospitalized patients. However, a major limitation of our study is that there are covariates known to be associated with thromboembolism which could not be examined because they were not provided in the dataset, such as severity of UC, medications (e.g. steroids, thromboembolic prophylaxis or anticoagulation, oral contraceptives), tobacco use, actual timing of the event, and reason for hospitalization. We cannot distinguish whether the thrombosis was perhaps the reason for admission as opposed to an event that happened during the hospitalization. In addition, we don’t know if UC was active and the reason for admission, or whether it was merely a part of the past medical history for that observation. Fifty percent of the non-surgical UC group did have UC as the first-listed ICD-9 CM code, and many of the other common first-listed diagnoses were gastroenterology-related, but our data is limited in that we cannot verify the effect of active UC on TE risk. Eighty percent of the surgical UC group had UC as the first-listed ICD-9 CM code, which is reasonable because those undergoing an operation during that admission would be likely to have their primary diagnosis be UC. We acknowledge there may be bias in that some observations might have more active disease than others, but this study is a broad examination of trends in TE rates across hospitalized patients with a history of UC and does not attempt to stratify TE risk under specific conditions.
Given that the NHDS samples discharges and not individual persons, we acknowledge the possibility that a person with several admissions for the same problem could be sampled more than once. Another limitation is that the NHDS does not provide the information required for valid calculation of standard errors in a logistic regression. Thus, whether or not the thromboembolic rates would differ between discharges with UC and other high-risk discharges after adjustment for potential confounding cannot be determined. However, the significantly higher thromboembolism rates among non-surgical discharges with UC compared to high-risk discharges with diverticulitis in unadjusted analysis, and the fact that adjustment increased the odds ratios for UC in the logistic model, strongly suggest that observed differences in thromboembolic event rates are independent of potential confounding effects.
In spite of these limitations, our findings suggest that in hospitalized patients with UC, the risk for thromboembolic events is as great or greater than for other hospitalized medical patients who are considered to have a high risk for thromboembolism, and for whom thromboembolic prophylaxis is recommended routinely. These data highlight the importance of further prospective studies to clarify the risks and benefits of thromboembolic prophylaxis in patients with UC and to establish the optimal prophylactic regimen.
People with inflammatory bowel disease (IBD) are considered to be at higher risk for thromboembolism than the average population, with up to three times the incidence rate of events compared with those without IBD. Hospitalized patients may be at an additional increased risk of thromboembolism given their decreased mobility or postoperative state. However, the rate of thromboembolic events in hospitalized IBD patients is not well described, and could have bearings on the degree of prophylaxis recommended for these patients.
Important areas of research surround studies of the prevalence of prothrombotic factors such as homocysteine, activated Protein C resistance, and gene mutations in the susceptibility of IBD patients to thromboembolic events. Larger studies need to be conducted to determine whether the prevalence of such disorders is truly higher in IBD, and thus if detected can help stratify those who need more vigilance for thromboembolic events.
Few studies have examined the rate of thromboembolic events among a nationally representative hospitalized population. We confirmed previous evidence that ulcerative colitis (UC) patients have a higher rate of thromboembolism than other groups of patients, in particular those groups of patients also considered to be at higher risk.
This study emphasizes that among hospitalized patients considered to be at risk for thromboembolic events, those with UC appear to have a higher rate of thromboembolism. This can have a bearing on the degree of prophylaxis for DVT/PE that these patients receive. In addition, if patients are identified to be at higher risk there may be those that benefit to prophylaxis even when not hospitalized.
This manuscript is reasonably well done, but does not add much to our knowledge about thromboembolism in IBD.
| 1. | Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21:23-29. |
| 2. | Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost. 2001;85:430-434. |
| 3. | Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542-548. |
| 4. | Talbot RW, Heppell J, Dozois RR, Beart RW Jr. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140-145. |
| 6. | Buck CJ, Lockyer KD. Saunders2007ICD-9-CM Volumes 1, 2, & 3. WB Saunders: Louis 2006; . |
| 7. | Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, Janbon C, Leizorovicz A, Olsson CG, Turpie AG. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul Fibrinolysis. 2003;14:341-346. |
| 8. | Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415-3420. |
| 9. | Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A, Dalen JE. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933-938. |
| 10. | Remzi FH, Fazio VW, Oncel M, Baker ME, Church JM, Ooi BS, Connor JT, Preen M, Einstein D. Portal vein thrombi after restorative proctocolectomy. Surgery. 2002;132:655-661; discussion 661-662. |
| 11. | Fichera A, Cicchiello LA, Mendelson DS, Greenstein AJ, Heimann TM. Superior mesenteric vein thrombosis after colectomy for inflammatory bowel disease: a not uncommon cause of postoperative acute abdominal pain. Dis Colon Rectum. 2003;46:643-648. |
| 12. | Novacek G, Haumer M, Schima W, Muller C, Miehsler W, Polterauer P, Vogelsang H. Aortic mural thrombi in patients with inflammatory bowel disease: report of two cases and review of the literature. Inflamm Bowel Dis. 2004;10:430-435. |
| 13. | Schneiderman JH, Sharpe JA, Sutton DM. Cerebral and retinal vascular complications of inflammatory bowel disease. Ann Neurol. 1979;5:331-337. |
| 14. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. |
| 15. | Guedon C, Le Cam-Duchez V, Lalaude O, Menard JF, Lerebours E, Borg JY. Prothrombotic inherited abnormalities other than factor V Leiden mutation do not play a role in venous thrombosis in inflammatory bowel disease. Am J Gastroenterol. 2001;96:1448-1454. |
| 16. | Mahmud N, Molloy A, McPartlin J, Corbally R, Whitehead AS, Scott JM, Weir DG. Increased prevalence of methylenetetrahydrofolate reductase C677T variant in patients with inflammatory bowel disease, and its clinical implications. Gut. 1999;45:389-394. |
| 17. | Aichbichler BW, Petritsch W, Reicht GA, Wenzl HH, Eherer AJ, Hinterleitner TA, Auer-Grumbach P, Krejs GJ. Anti-cardiolipin antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1999;44:852-856. |
| 18. | Koutroubakis IE, Petinaki E, Anagnostopoulou E, Kritikos H, Mouzas IA, Kouroumalis EA, Manousos ON. Anti-cardiolipin and anti-beta2-glycoprotein I antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1998;43:2507-2512. |
| 19. | Attvall E, Frigyesi A, Sternby B. What is the impact of resistance to activated protein C (Leiden mutation to factor V) in inflammatory bowel disease? Int J Colorectal Dis. 2006;21:705-710. |
| 20. | Panes J, Esteve M, Cabre E, Hinojosa J, Andreu M, Sans M, Fernandez-Banares F, Feu F, Gassull MA, Pique JM. Comparison of heparin and steroids in the treatment of moderate and severe ulcerative colitis. Gastroenterology. 2000;119:903-908. |
| 21. | Ang YS, Mahmud N, White B, Byrne M, Kelly A, Lawler M, McDonald GS, Smith OP, Keeling PW. Randomized comparison of unfractionated heparin with corticosteroids in severe active inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1015-1022. |
| 22. | Zezos P, Papaioannou G, Nikolaidis N, Patsiaoura K, Papageorgiou A, Vassiliadis T, Giouleme O, Evgenidis N. Low-molecular-weight heparin (enoxaparin) as adjuvant therapy in the treatment of active ulcerative colitis: a randomized, controlled, comparative study. Aliment Pharmacol Ther. 2006;23:1443-1453. |
| 23. | Bloom S, Kiilerich S, Lassen MR, Forbes A, Leiper K, Langholz E, Irvine EJ, O'Morain C, Lowson D, Orm S. Low molecular weight heparin (tinzaparin) vs placebo in the treatment of mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2004;19:871-878. |
| 24. | Turpie AG. Thrombosis prophylaxis in the acutely ill medical patient: insights from the prophylaxis in MEDical patients with ENOXaparin (MEDENOX) trial. Am J Cardiol. 2000;86:48M-52M. |
| 25. | Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97-101. |
| 26. | Sonoda K, Ikeda S, Mizuta Y, Miyahara Y, Kohno S. Evaluation of venous thromboembolism and coagulation-fibrinolysis markers in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2004;39:948-954. |
| 27. | Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care. 1994;32:81-90. |
| 28. | Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60-68. |
| 29. | Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118:978-980. |
| 30. | Stein PD, Hull RD, Kayali F, Ghali WA, Alshab AK, Olson RE. Venous thromboembolism according to age: the impact of an aging population. Arch Intern Med. 2004;164:2260-2265. |
| 31. | Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Alshab AK, Meyers FA. Venous thromboembolic disease: comparison of the diagnostic process in blacks and whites. Arch Intern Med. 2003;163:1843-1848. |
| 32. | Skaf E, Stein PD, Beemath A, Sanchez J, Bustamante MA, Olson RE. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol. 2005;96:1731-1733. |