Published online Dec 21, 2009. doi: 10.3748/wjg.15.5992
Revised: November 2, 2009
Accepted: November 9, 2009
Published online: December 21, 2009
AIM: To investigate the effect of severe acute pancreatitis (SAP) on pharmacokinetics of Da-Cheng-Qi Decoction (DCQD) components in rats.
METHODS: Rats were divided into SAP group and sham-operation group as a control group (n = 6). Rhein, chrysophanol, rheochrysidin, magnolol, hesperidin and naringin in DCQD were quantified in rat serum by high performance liquid chromatography tandem mass spectrometry for studying their pharmacokinetics.
RESULTS: Early absorption of each DCQD component was tended to degrade in SAP group after treatment with DCQD by gavage. The Cmax (chrysophanol, P = 0.0059; rheochrysidin, P = 0.0288; magnolol, P = 0.0487; hesperidin, P = 0.0277; naringin, P = 0.0023) and AUC (rhein, P = 0.0186; chrysophanol, P = 0.0013; magnolol, P = 0.001; hesperidin, P = 0.0081; naringin, P = 0.0272) of DCQD component were obviously lower in SAP group than in control group. The T1/2α of chrysophanol and rheochrysidin (P = 0.0467 and 0.0005, respectively) and Tmax of chrysophanol and rheochrysidin (P = 0.0101 and 0.0037, respectively) lasted longer in SAP group than in control group.
CONCLUSION: SAP can significantly impact the absorption of DCQD components in rats and their pharmacokinetic parameters.
- Citation: Gong HL, Tang WF, Yu Q, Xiang J, Xia Q, Chen GY, Huang X, Liang MZ. Effect of severe acute pancreatitis on pharmacokinetics of Da-Cheng-Qi Decoction components. World J Gastroenterol 2009; 15(47): 5992-5999
- URL: https://www.wjgnet.com/1007-9327/full/v15/i47/5992.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5992
Acute pancreatitis, occurring suddenly and usually resolving after a few days of treatment, may become life-threatening if severe complications take place. Fulminant acute pancreatitis is more dangerous[1]. Severe acute pancreatitis (SAP), characterized by intricate mechanism, variant symptoms, grave prognosis and multiple complications, seriously threatens the life of patients and brings a heavy burden to the society, families and economy. Each year, about 210 000 patients with acute pancreatitis in the United States are admitted to hospitals[2]. Additionally, neither standard treatment nor other medications are available for SAP patients at present[3]. SAP, similar to Yangming Fushi syndrome (YMFSS) according to the traditional Chinese medicine, has been treated with purgative herbals throughout China for more than three decades[4-6].
Da-Cheng-Qi Decoction (DCQD), a famous preparation of traditional Chinese medicine used in treatment of digestive diseases, is composed of Dahuang (Caulis Fibraureae), Houpu (Cortex Magnoliae Officinalis), Zhishi (immature bitter orange) and Mangxiao (Natrii Sulphas). It has been reported that DCQD can restore gastrointestinal function by facilitating motility, relieving enteroparalysis and evacuating “dry stool”[7], prevent bacterial translocation and counteract with endotoxin, regulate Ca2+-Mg2+-ATPase in the pancreatic acinar cells[8]. SAP can be treated with Chinese herbal decoctions based on the above mechanism. However, no studies are available on the pharmacokinetics of such decoctions in acute pancreatitis. According to the theory “syndrome and treatment pharmacokinetics”, YMFSS should influence the pharmacokinetics of DCQD[9], but it has not been proved experimentally up to date.
Thus, we quantified the DCQD components absorbed in rats with SAP characterized by YMFSS and studied the influence of SAP on the pharmacokinetics of DCQD components[10].
Male clean-grade, healthy Sprague-Dawley rats, weighing 320 ± 25 g, at the age of 90 ± 5 d, were used in this study. The rats were handled according to the University Guidelines and the Animal Ethics Committee Guidelines of the Animal Facility of the West China Hospital, maintained in air-conditioned animal quarters at 22 ± 2°C with a relative humidity of 65% ± 10%, acclimatized to the facilities for 10 d, and then fasted for 24 h with free access to water prior to experiments.
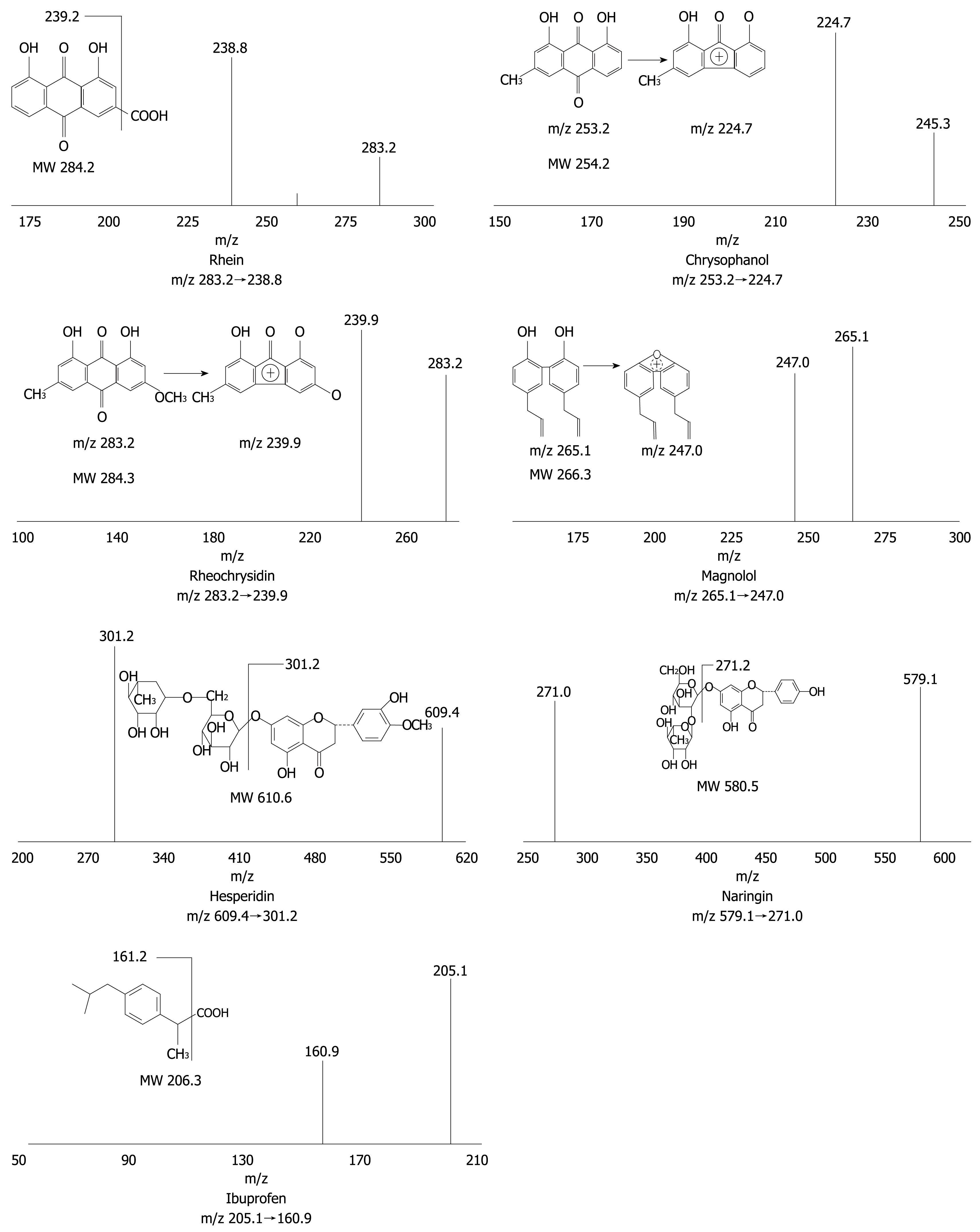
The structures of rhein, chrysophanol, rheochrysidin, magnolol, hesperidin, naringin and ibuprofen (internal standards) are presented in Figure 1. Reference standards for these components of DCQD and the internal standard (IS) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Methanol with a chromatographic grade was obtained from Tedia Company Inc. (USA). Acetic acid and ethyl acetate were bought from Chongqing Chemistry Co. Ltd. (Chongqing, China). Ammonium acetate, sodium hydroxide and hydrochloric acid (analysis grade) were purchased from Chengdu Kelong Chemical Reagent Factory (Chengdu, China). All aqueous solutions and buffers were prepared with deionized water from a Millipore RiosTM-16 water purifier (Millipore, Billerica, MA, USA)[11].
High performance liquid chromatography tandem mass spectrometry (HPLC-TMS) system, consisted of a SIL-HTc autosampler and a LC-10ADvp pump, was provided by Shimadzu (Kyoto, Japan). API3000 triple-quadrupole LC-MS system was purchased from Applied Biosystems (Foster City, CA, USA). The system was controlled with Analyst 1.4.2 software. Separation was performed on a YMC-Pack ODS-A C18 column (5 μm, 150 mm × 4.6 mm, YMC, Kyoto, Japan) and a C18 guard column (5 μm, 4.0 mm × 2.0 mm, Phenomenex Inc., Torrance, CA, USA). The mobile phase is consisted of methanol-water (92:8, v/v) at a flow rate of 0.3 mL/min. The column was maintained at ambient temperature and the injection volume was 80 μL.
A mass spectrometer was operated using an electrospray source configured to the negative ion mode and quantification was performed by multiple reaction monitoring (MRM). Production mass spectra of the analytes are shown in Figure 1 where [M-H]- of each analyte was selected as the precursor ion, and the most abundant or specific fragment ion was selected as the production in MRM acquisition. Instrumental parameters were optimized for each analyte by infusing the corresponding standard solution at a flow rate of 5 μL/min, using a syringe pump integrated into the API 3000 mass spectrometer. Nitrogen was used as a curtain, and auxiliary gas and air were used as a nebulizer gas. Electrospray conditions for the 6 major DCQD components and IS were curtain gas (6.0 L/min), ion-spray voltage (-4500 V), nebulizer gas (6.0 L/min), auxiliary gas (7.0 L/min), turbo temperature (4°C), respectively. Optimized mass spectrometry parameters for each DCQD compound and IS are listed in Table 1[11].
| Spiked amount (ng/mL) | Intra-day | Inter-day | Extract | ||||
| RSD | Ac | RSD | Ac | Recovery | RSD | ||
| Rhein | 39.1 | 3.76 | 104.60 | 3.53 | 107.94 | 104.60 | 3.76 |
| 156 | 5.55 | 100.32 | 5.52 | 106.87 | 100.32 | 5.55 | |
| 625 | 5.31 | 101.07 | 5.56 | 105.36 | 101.07 | 5.31 | |
| 3750 | 4.24 | 101.24 | 6.23 | 98.37 | 101.24 | 4.24 | |
| Chrysophanol | 6.1 | 2.37 | 99.4 | 4.46 | 98.67 | 99.39 | 2.36 |
| 24.4 | 4.38 | 104.17 | 4.88 | 101.14 | 104.17 | 4.37 | |
| 97.7 | 1.64 | 96.66 | 3.33 | 98.09 | 96.66 | 1.64 | |
| 586 | 3.78 | 103.98 | 4.44 | 101.13 | 103.98 | 3.78 | |
| Rheochrysidin | 39.1 | 5.69 | 97.70 | 5.72 | 100.78 | 97.69 | 5.68 |
| 156 | 3.33 | 99.89 | 4.13 | 101.14 | 99.89 | 3.33 | |
| 625 | 3.22 | 105.92 | 3.73 | 104.37 | 105.92 | 3.22 | |
| 3750 | 4.75 | 103.07 | 4.84 | 103.49 | 103.07 | 4.75 | |
| Magnolol | 6.1 | 5.51 | 102.13 | 5.07 | 106.33 | 102.13 | 5.51 |
| 24.4 | 2.39 | 104.78 | 5.79 | 99.86 | 104.78 | 2.39 | |
| 97.7 | 5.51 | 107.81 | 4.92 | 106.23 | 102.47 | 5.51 | |
| 586 | 3.77 | 105.97 | 5.33 | 105.29 | 105.97 | 3.77 | |
| Hesperidin | 6.1 | 4.12 | 95.96 | 4.82 | 98.49 | 95.96 | 4.12 |
| 24.4 | 5.83 | 99.52 | 5.10 | 100.29 | 99.52 | 5.83 | |
| 97.7 | 4.21 | 100.14 | 3.95 | 99.72 | 100.16 | 4.21 | |
| 586 | 2.77 | 97.13 | 4.91 | 98.52 | 97.13 | 2.76 | |
| Naringin | 6.1 | 2.66 | 95.66 | 4.26 | 99.29 | 95.67 | 2.657 |
| 24.4 | 2.82 | 102.46 | 3.95 | 103.37 | 102.45 | 2.82 | |
| 97.7 | 3.81 | 100.78 | 3.96 | 100.66 | 100.78 | 3.81 | |
| 586 | 5.24 | 98.27 | 5.59 | 99.32 | 98.27 | 5.24 | |
Six calibration standards were prepared by spiking 200 μL of blank plasma with 100 μL of each working solution to obtain the plasma concentrations for rhein and rheochrysidin (5000, 3750, 2500, 1250, 625, 312.5, 156, 78.13, 39.1 and 19.53 ng/mL), and for chrysophanol, naringin, hesperidin and magnolol (879, 586, 390, 195, 97.7, 48.8, 24.4, 12.2, 6.1 and 3.1 ng/mL). Quality control (QC) samples were prepared to obtain plasma concentrations for rhein and rheochrysidin (3750, 625, 156 and 39.1 ng/mL) and for chrysophanol, naringin, hesperidin and magnolol (586, 97.7, 24.4 and 6.1 ng/mL). The spiked plasma samples (standard and QC samples) were pretreated and detected in each analytical batch along with the unknown samples[11].
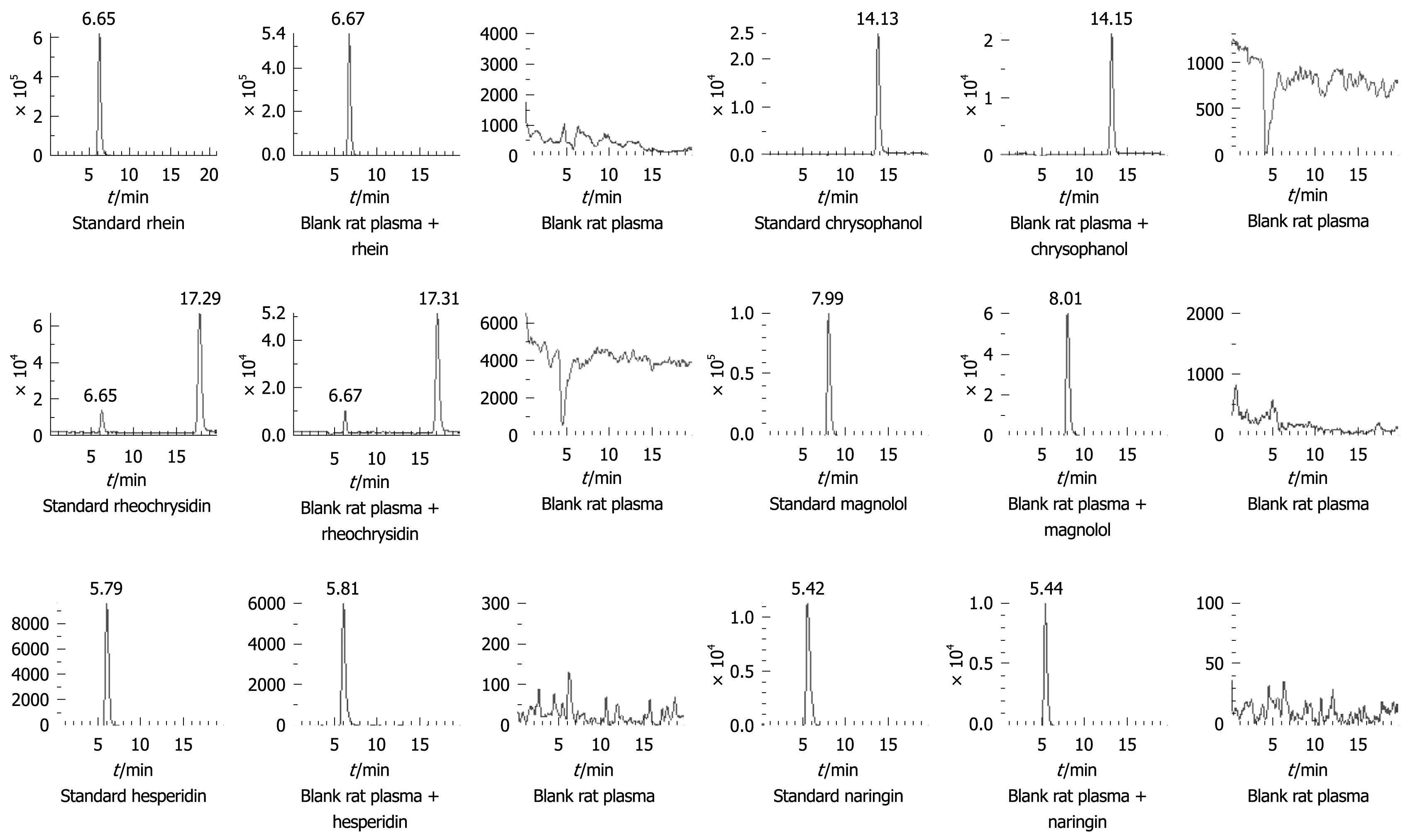
Blank and spiked rat plasma chromatograms were compared to evaluate the selected method (Figure 2). Calibration curves were plotted from the peak area ratio of each analyte to IS vs plasma concentrations using a 1/c2 weighted linear least-squares regression model. The lower limit of quantification was set at the concentration of the lowest non-zero calibration standard (S/N ≥ 10:1) that could be measured with an acceptable accuracy and precision (≤ 20% for both parameters). Intra- and inter-day precisions were determined by assessing the measured results of QC samples at low, medium and high concentrations (Table 1). Accuracy was determined as the difference in percentages between the mean and nominal concentrations detected (Table 1). Extraction recoveries of the 6 analytes were determined by comparing the peak areas obtained from rat plasma samples with those from the unextracted standard solutions at the same concentration (Table 1). Bench-top stability of the 6 analytes in rat plasma was determined by assessing the QC samples after stored for 2 and 4 h at room temperature. Freeze-thaw stability was detected after two cycles and long-term stability was determined by assessing the QC samples stored at -30°C for 14 d. QC samples were prepared, injected and reinjected after the samples were maintained in the autosampler at 8°C for 12 d. Stability of the analytes was detected by comparing the measured results with those of freshly prepared samples at the same concentration[11] (Table 2).
| Spiked amount(ng/mL) | Bench-top bias | Long-term bias | Freeze-thaw bias | Extract bias | Autosampler bias | ||||||
| 2 h | 4 h | 7 d | 14 d | 1 | 2 | 8 h | 24 h | 6 h | 12 h | ||
| Rhein | 39.1 | -1.32 | -4.57 | -1.15 | -4.27 | -1.15 | -2.54 | 6.06 | -4.54 | -5.25 | -5.33 |
| 156 | -2.50 | -5.39 | -2.36 | -0.43 | -2.36 | 0.43 | 2.28 | 2.48 | -1.29 | -1.07 | |
| 625 | 5.28 | -1.21 | 1.60 | -2.82 | 1.60 | -2.93 | -0.90 | 3.40 | -0.48 | -0.27 | |
| 3750 | 2.06 | 5.52 | -4.46 | 0.09 | -4.46 | -0.17 | 1.50 | 1.32 | -1.54 | -3.60 | |
| Chrysophanol | 6.1 | -0.67 | 1.00 | 2.42 | 3.54 | 2.42 | 0.90 | 4.73 | 3.19 | 0.73 | 2.36 |
| 24.4 | 5.04 | 4.62 | -2.74 | -2.61 | -2.74 | -6.40 | -2.82 | -4.42 | -3.39 | -3.52 | |
| 97.7 | -0.32 | 5.80 | -5.39 | 0.27 | -5.39 | -1.52 | -2.34 | 0.00 | -3.41 | 0.00 | |
| 568.0 | -0.72 | -2.00 | 2.01 | -2.13 | 2.01 | -2.47 | -2.92 | -0.97 | 0.63 | 2.42 | |
| Rheochrysidin | 6.1 | 1.59 | -2.59 | -4.11 | -3.78 | -4.11 | -5.18 | -3.93 | -6.27 | -6.33 | -5.10 |
| 24.4 | 4.09 | -0.65 | 0.00 | -4.03 | 0.00 | -1.27 | -0.43 | 3.24 | -0.42 | -3.18 | |
| 97.7 | -0.86 | -4.45 | -0.38 | 0.59 | -0.38 | -2.43 | -0.58 | -0.32 | 0.38 | -2.49 | |
| 568.0 | 2.80 | -5.52 | -3.40 | -2.09 | -3.40 | -2.18 | 2.04 | 1.16 | -1.66 | -3.66 | |
| Magnolol | 6.1 | -1.58 | 1.33 | -5.23 | -4.34 | -5.23 | -3.09 | -2.72 | -2.14 | -5.18 | -4.60 |
| 24.4 | 5.76 | 0.84 | 0.42 | -2.49 | 0.42 | 0.28 | -1.95 | 1.53 | 1.25 | -0.83 | |
| 97.7 | 1.82 | 1.17 | 1.48 | -1.10 | 1.48 | 0.90 | 4.23 | 1.47 | -3.21 | 1.35 | |
| 568.0 | -2.02 | -3.03 | -1.60 | -4.43 | -1.60 | -2.21 | 0.23 | -5.01 | -3.82 | -6.14 | |
| Hesperidin | 6.1 | -4.29 | 4.85 | -0.48 | -1.27 | -0.48 | -3.08 | 1.01 | 2.34 | -3.98 | -1.54 |
| 24.4 | -2.03 | -3.11 | 0.41 | 0.00 | 0.41 | -1.36 | 1.46 | 1.46 | -4.21 | 4.76 | |
| 97.7 | 3.42 | -1.69 | 0.11 | 0.39 | 0.11 | 1.37 | 2.67 | 2.33 | 2.70 | 5.12 | |
| 568.0 | -3.00 | -0.81 | -4.73 | -2.25 | -4.73 | -3.55 | 3.75 | 0.62 | -2.76 | 0.23 | |
| Naringin | 6.1 | -1.65 | 2.70 | -1.96 | 1.63 | -1.96 | -4.90 | -1.73 | -0.49 | 2.61 | -2.83 |
| 24.4 | 3.95 | 4.90 | -4.56 | -2.73 | -4.56 | -2.73 | 1.04 | 2.86 | -4.69 | -6.12 | |
| 97.7 | 0.07 | 2.03 | -5.11 | -0.96 | -5.11 | 1.58 | -4.46 | -2.31 | -0.45 | 0.93 | |
| 568.0 | -4.10 | 1.68 | -4.52 | -1.45 | -4.52 | -4.81 | 1.20 | -3.06 | -2.08 | -1.56 | |
Acute pancreatitis was induced in rats. The animals were anesthetized with ethyl ether as previously described[12].
Dahuang, Houpu, Zhishi and Mangxiao were purchased from Chengdu Green Herbal Pharmaceutical Co. Ltd. (Chengdu, China) and authenticated by Professor Yang Song (Department of Pharmacognosy, Sichuan University, China). DCQD was routinely prepared with 6.0 g of Dahuang, 6.0 g of Houpu, 6.0 g of Zhishi and 6.0 g of Mangxiao. For crude drugs, the spray-dried DCQD was reconstituted in water to a concentration of 1 g/mL. The contents of the six DCQD components were measured as previously described[13]. The crude DCQD preparation was administered through the duodenum of rats at a dosage of 20 g/kg. The voucher specimens were kept in our laboratory.
Rats were randomly divided into SAP group and sham-operation group as a control group (n = 6). Rats were given DCQD 2 h after operation. Blood sample (300 μL) was collected into a heparinized eppendorf tube via the tail vein before and after (10, 15, 20, 30, 45 min and 1, 2, 4, 8, 12 h) DCQD was given. After centrifugation at 3000 r/min for 15 min, the plasma samples were stored at -80°C for analysis.
Rats in SAP and control groups were fed with laboratory rodent chow by gavage. Concentration of DCQD components in plasma was measured by HPLC-TMS. Concentration-time curves were plotted for various components from DCQD.
HPLC-TMS for simultaneous determination of the six components has been validated in our laboratory[11,14]. Plasma samples were spiked with the IS (ibuprofen), acidified by HPLC and extracted twice using ethyl acetate. The HPLC-TMS system was operated under MRM modes using electrospray ionization in the negative ion mode.
Data collection, peak integration and calibration were performed with Analyst 1.4.2 software. Calibration curves were plotted according to the peak area ratio of analytes to ISs, and the linear regression between plasma concentration and peak area ratio was weighed by 1/x2. Concentrations of QC and unknown samples were measured by interpolation from the calibration curves. Drug and statistics software programmed by the Chinese Pharmacological Society was used to process the plasma concentration data and compartment model fitting and then all the pharmacokinetic parameters were figured out. The results were expressed as mean ± SD. The pharmacokinetic parameters of each DCQD component were compared with statistical software PEMS3.1 and the difference was compared by sample pairing and t-test. P < 0.05 was considered statistically significant.
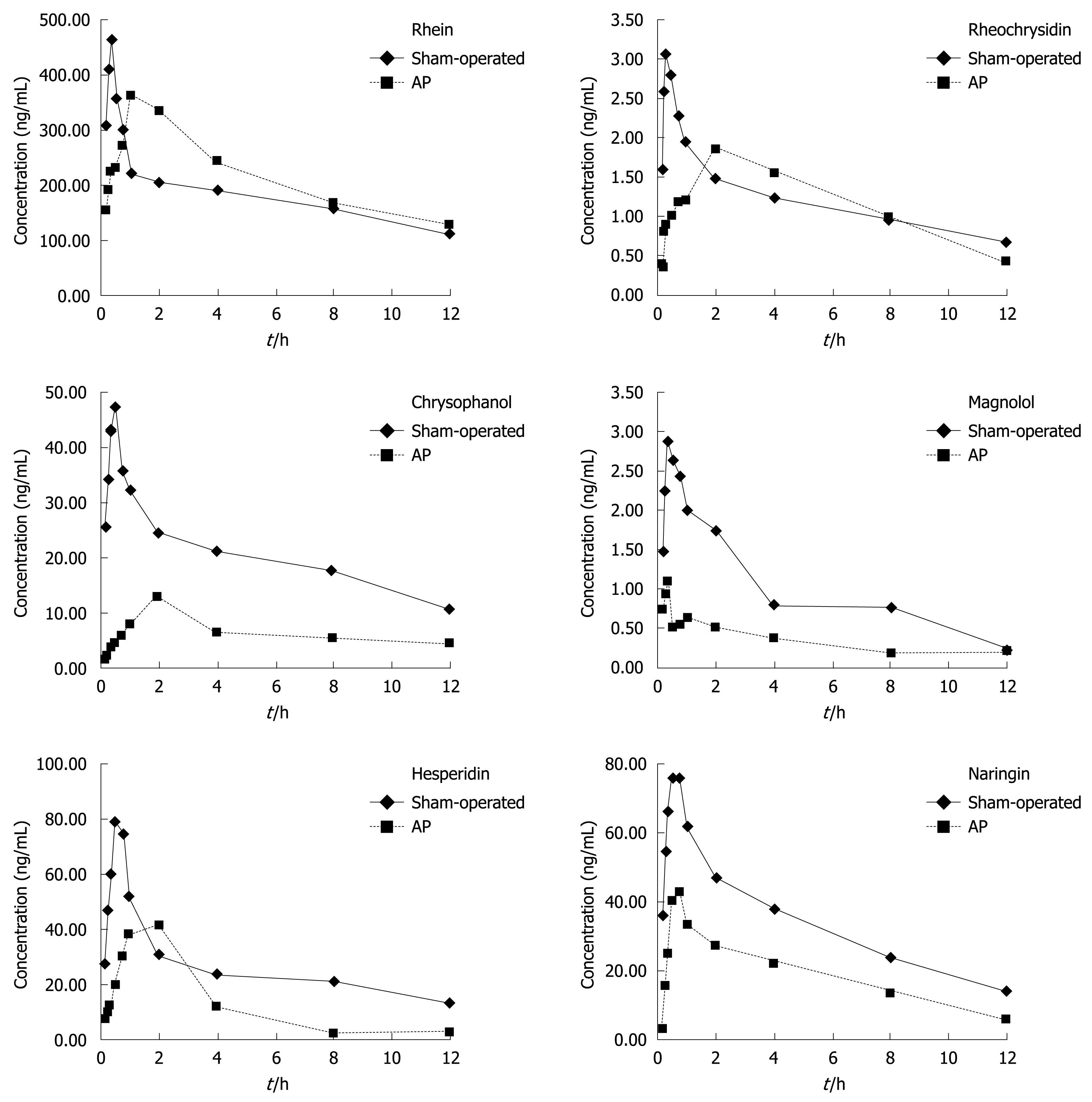
The mean plasma concentration of rhein was obviously higher, the peak time (Tmax) of rhein was significantly shorter while the T1/2α was significantly higher, and the clearance rate (CL/F) and AUC of rhein were obviously lower in SAP group at each time point than in control group within 12 h after treatment with DCQD, suggesting that acute pancreatitis can impact the absorption, distribution and elimination of rhein in rats (Figure 3, Table 3).
| T1/2α (h) | CL/F (L∙h per kg) | AUC(0-∞) (μg/L per hour) | MRT(0-t) (h) | Tmax (h) | Cmax (μg/L) | |
| Rhein | ||||||
| Sham | 0.33 ± 0.13 | 4.03 ± 1.38 | 4720 ± 1514 | 8.86 ± 0.62 | 0.36 ± 0.11 | 510 ± 283 |
| AP | 4.367 ± 2.33 | 0.133 ± 0.06 | 2870 ± 563 | 7.82 ± 3.37 | 1.75 ± 1.25 | 479 ± 126 |
| t | 4.2721 | 6.9106 | 2.8054 | 0.7513 | 2.7242 | 0.2448 |
| P | 0.0016 | 0 | 0.0186 | 0.4698 | 0.0214 | 0.8116 |
| Rheochrysidin | ||||||
| Sham | 0.354 ± 0.302 | 0.356 ± 0.14 | 16.047 ± 6.08 | 4.189 ± 0.463 | 0.569 ± 0.26 | 3.86 ± 1.09 |
| AP | 1.464 ± 0.449 | 0.328 ± 0.109 | 16.63 ± 5.06 | 4.34 ± 0.29 | 1.5 ± 0.55 | 2.58 ± 0.58 |
| t | 5.0247 | 0.3866 | 0.179 | 0.6929 | 3.7597 | 2.5511 |
| P | 0.0005 | 0.7072 | 0.8615 | 0.5041 | 0.0037 | 0.0288 |
| Chrysophanol | ||||||
| Sham | 0.38 ± 0.27 | 24.32 ± 9.65 | 461.3 ± 188.7 | 8.49 ± 0.93 | 0.59 ± 0.24 | 53.02 ± 21.9 |
| AP | 0.89 ± 0.48 | 0.095 ± 0.035 | 115.8 ± 34.89 | 10 ± 5.1 | 1.33 ± 0.52 | 17.59 ± 10.5 |
| t | 2.2683 | 6.0994 | 4.3965 | 0.7088 | 3.165 | 3.4855 |
| P | 0.0467 | 0.0001 | 0.0013 | 0.4947 | 0.0101 | 0.0059 |
The mean plasma concentration of rheochrysidin was significantly higher, the Tmax of rheochrysidin was significantly shorter, and the T1/2α was significantly higher in SAP group at each time point than in control group within 12 h after treatment with DCQD, demonstrating that acute pancreatitis can affect the absorption distribution and excretion of rheochrysidin in rats (Figure 3, Table 3).
The mean plasma concentration of chrysophanol was obviously lower, the Tmax of chrysophanol was significantly longer, the Cmax and AUC of chrysophanol were significantly lower, the T1/2α was significantly higher, and the CL/F was lower in SAP group than in control group with no difference in the mean retention time (MRT) between the two groups within 12 h after treatment with DCQD, suggesting that SAP can affect the absorption, distribution, and elimination of chrysophanol in rats (Figure 3, Table 3).
The mean plasma concentration of magnolol was obviously lower, the Cmax and AUC of magnolol were obviously lower while the T1/2α, MRT and Tmax were similar between the two groups within 12 h after treatment with DCQD, suggesting that SAP can significantly affect the bioavailability of magnolol (Figure 3, Table 4).
| T1/2α (h) | AUC(0-∞) (μg/L per hour) | MRT(0-t) (h) | Tmax (h) | Cmax (μg/L) | |
| Magnolol | |||||
| Sham | 1.58 ± 1.06 | 24.89 ± 9.87 | 6.6 ± 1.85 | 0.71 ± 0.25 | 3.47 ± 2.13 |
| AP | 1.11 ± 0.48 | 5.739 ± 1.888 | 9.202 ± 2.27 | 0.428 ± 0.282 | 1.491 ± 0.596 |
| t | 1.0821 | 4.6204 | 2.2156 | 1.8329 | 2.2431 |
| P | 0.3046 | 0.001 | 0.0511 | 0.0967 | 0.0487 |
| Hesperidin | |||||
| Sham | 0.45 ± 0.25 | 479.39 ± 225.94 | 5.62 ± 2.45 | 0.67 ± 0.13 | 89.38 ± 25.02 |
| AP | 0.69 ± 0.36 | 162.98 ± 69.76 | 4.93 ± 2.233 | 1.25 ± 0.59 | 53.7 ± 22.026 |
| t | 1.3413 | 3.2953 | 0.5133 | 2.3141 | 2.5744 |
| P | 0.2095 | 0.0081 | 0.6189 | 0.0432 | 0.0277 |
| Naringin | |||||
| Sham | 1.47 ± 1.57 | 623.24 ± 332.55 | 6.43 ± 2.1 | 0.64 ± 0.24 | 88.23 ± 23.66 |
| AP | 1.1 ± 0.7 | 267.68 ± 53.65 | 7.12 ± 1.96 | 0.83 ± 0.13 | 45.13 ± 9.59 |
| t | 0.5272 | 2.5852 | 0.5659 | 1.732 | 4.0511 |
| P | 0.6095 | 0.0272 | 0.5839 | 0.1139 | 0.0023 |
The mean plasma concentration of hesperidin was obviously higher, the Tmax of hesperidin was significantly longer, the Cmax and AUC of hesperidin were significantly lower in SAP group at each time point in control group within 12 h after treatment with DCQD, showing that acute pancreatitis can impact the absorption and distribution and pharmacokinetics of hesperidin in rats (Figure 3, Table 4).
The mean plasma concentration of naringin and the Cmax and AUC of naringin were obviously lower in SAP group at each time point than in control group within 12 h after treatment with DCQD, revealing that acute pancreatitis can impact the absorption, distribution and bioavailability of naringin in rats (Figure 3, Table 4).
In the present study, the early absorption of each DCQD component tended to degrade in SAP group, the Cmax and AUC of DCQD components such as chrysophanol, magnolol, hesperidin and naringin were obviously lower in SAP group than in control group, suggesting that lack of an effective blood volume and a systematic inflammatory response to organ damage in SAP rats would affect the distribution, metabolism and excretion of DCQD components[15].
No significant difference was found in T1/2α and Tmax of DCQD components such as magnolol and naringin between the two groups, which may be due to the way of modeling experiments. Rats were anaesthetized with ethyl ether and recovered 10 min later with free activity. Two hours after treatment with DCQD, the rats became conscious and maintained normal physiology, indicating that influence of anesthesia on physiology and pharmacokinetics in rats can be ignored[16].
However, the T1/2α and Tmax of rhein, rheochrysidin and chrysophanol were longer in SAP group in control group. In addition, the absorption of DCQD components was greatly affected by variant molecular constitutions and lower pH of SAP rats in vitro.
In summary, SAP can obviously impact the absorption and pharmacokinetic parameters of DCQD containing rhein, chrysophanol, rheochrysidin, magnolol, hesperidin and naringin in rats.
Severe acute pancreatitis (SAP), characterized by intricate mechanism, variant symptoms, grave prognosis and multiple complications, seriously threatens the life of patients and brings a heavy burden to the society, families and economy. Additionally, either standard treatment or other medications for SAP is available at present. In China, clinical and experimental researches on Da-Cheng-Qi Decoction (DCQD) have shown that DCQD is a valid prescription for the treatment of SAP.
SAP, similar to Yangming Fushi Syndrome (YMFSS) according to the traditional Chinese medicine, has been treated with purgative herbals throughout China for more than three decades. However, no studies are available on the pharmacokinetics of DCQD components in rats with acute pancreatitis.
According to the theory “syndrome and treatment pharmacokinetics” in traditional Chinese medicine, YMFSS should influence the pharmacokinetics of DCQD, which has, however, not been proved experimentally up to date. This is the first study to report the effect of acute pancreatitis on the pharmacokinetics of DCQD components in rats.
Acute pancreatitis was found to have certain effects on the pharmacokinetics of DCQD components in rats, showing that DCQD can be used in treatment of SAP.
The authors investigated the effect of acute pancreatitis on the pharmacokinetics of DCQD components in rats, which may contribute to the treatment of SAP.
Peer reviewer: Geng-Tao Liu, Professor, Department of Pharmacology, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
S- Editor Wang YR L- Editor Wang XL E- Editor Zheng XM
| 1. | Haraldsen P, Sun ZW, Börjesson A, Olanders K, Lasson A, Andersson R. Multimodal management - of value in fulminant acute pancreatitis? Pancreatology. 2003;3:14-25. |
| 2. | Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, Shaheen NJ, Sandler RS. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004;126:1448-1453. |
| 3. | Zavoral M, Maratka Z. Acute pancreatitis-etiology, development, diagnosis and treatment. Gastrointest Endosc. 1997;46:386-387. |
| 4. | Katakai M, Akamaru T, Tani T. [An analysis of the frequency of formulations and crude drugs described in Shan-Han-Lun]. Yakushigaku Zasshi. 2002;37:28-35. |
| 5. | Ren YY, Gong HL, Tang WF, Wan MH, Huang X. [Effects of ranitidine on pharmacokinetics of rhein from Dachengqi Decoction in rats after oral administration.]. Zhongxiyi Jiehe Xuebao. 2009;7:868-872. |
| 6. | Tang WF, Wan MH, Zhu L, Chen GY, Xia Q, Huang X. [Immuno-modulatory effect of somatostatin combined with traditional Chinese medicine on severe acute pancreatitis at early stage: a randomized control trial]. Zhongxiyi Jiehe Xuebao. 2005;3:103-107. |
| 7. | Xia Q, Jiang JM, Gong X, Chen GY, Li L, Huang ZW. Experimental study of Tong Xia purgative method in ameliorating lung injury in acute necrotizing pancreatitis. World J Gastroenterol. 2000;6:115-118. |
| 8. | Qiu Y, Li YY, Li SG, Song BG, Zhao GF. Effect of Qingyitang on activity of intracellular Ca2+-Mg2+-ATPase in rats with acute pancreatitis. World J Gastroenterol. 2004;10:100-104. |
| 9. | Huang X, Ren P, Wen AD, Wang LL, Zhang L, Gao F. Pharmacokinetics of traditional Chinese syndrome and recipe: a hypothesis and its verification (I). World J Gastroenterol. 2000;6:384-391. |
| 10. | Tang WF, Wan MH, Huang QR, Zhu ZY, Zhao JL, Wu YZ, Huang X. Effect of Da-Cheng-Qi decoction on the pharmacokinetics of ranitidine in rats. Biomed Chromatogr. 2008;22:851-856. |
| 11. | Yu Q, Xiang J, Tang W, Liang M, Qin Y, Nan F. Simultaneous determination of the 10 major components of Da-Cheng-Qi decoction in dog plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2025-2031. |
| 12. | Ren YY, Gong HL, Tang WF, Wan MH, Zhao JL, Huang X. [Dachengqi Decoction induces pancreatic acinar cell apoptosis in experimental acute pancreatitis in rats]. Zhongxiyi Jiehe Xuebao. 2009;7:651-656. |
| 13. | Tang W, Wan M, Zhu Z, Chen G, Huang X. Simultaneous determination of eight major bioactive compounds in Dachengqi Tang (DT) by high-performance liquid chromatography. Chin Med. 2008;3:5. |
| 14. | Tang WF, Huang X, Yu Q, Qin F, Wan MH, Wang YG, Liang MZ. Determination and pharmacokinetic comparison of rhein in rats after oral dosed with Da-Cheng-Qi decoction and Xiao-Cheng-Qi decoction. Biomed Chromatogr. 2007;21:1186-1190. |
| 15. | Wacke R, Park S, Mundkowski RG, Block N, Kuhn-Thiel A, Drewelow B. The penetration of moxifloxacin into the pancreas of male rats in experimental acute necrotizing pancreatitis. Chemotherapy. 2003;49:167-171. |
| 16. | Mizunuma H, Khorram O, McCann SM. Blockade of stress-induced prolactin release in monosodium glutamate-treated rats. Brain Res Bull. 1983;10:23-26. |