Published online Dec 14, 2009. doi: 10.3748/wjg.15.5827
Revised: October 6, 2009
Accepted: October 13, 2009
Published online: December 14, 2009
AIM: To evaluate the use of three-dimensional imaging of pancreatic carcinoma using multidetector computed tomography (CT) in a prospective study.
METHODS: Ten patients with suspected pancreatic tumors were examined prospectively using multidetector CT (Somatom Sensation 16, Siemens, Erlangen, Germany). The images were evaluated for the presence of a pancreatic carcinoma and invasion of the peripancreatic vessels and surrounding organs. Using the isotropic CT data sets, a three-dimensional image was created with automatic vascular analysis and semi-automatic segmentation of the organs and pancreatic tumor by a radiologist. The CT examinations and the three-dimensional images were presented to the surgeon directly before and during the patient’s operation using the Medical Imaging Interaction Toolkit-based software “ReLiver”. Immediately after surgery, the value of the two images was judged by the surgeon. The operation and the histological results served as the gold standard.
RESULTS: Nine patients had a pancreatic carcinoma (all pT3), and one patient had a serous cystadenoma. One tumor infiltrated the superior mesenteric vein. The infiltration was correctly evaluated. All carcinomas were resectable. In comparison to the CT image with axial and coronal reconstructions, the three-dimensional image was judged by the surgeons as better for operation planning and consistently described as useful.
CONCLUSION: A 3D-image of the pancreas represents an invaluable aid to the surgeon. However, the 3D-software must be further developed in order to be integrated into daily clinical routine.
- Citation: Klauß M, Schöbinger M, Wolf I, Werner J, Meinzer HP, Kauczor HU, Grenacher L. Value of three-dimensional reconstructions in pancreatic carcinoma using multidetector CT: Initial results. World J Gastroenterol 2009; 15(46): 5827-5832
- URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5827.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5827
Pancreatic carcinomas are the fourth most frequent cause of death from cancer worldwide. The prognosis remains poor with a relative 5-year survival rate of about 5%. The only procedure resulting in significantly longer survival is R0 resection with adjuvant chemotherapy[1].
Computed tomography (CT) is considered the method of choice for the detection and preoperative staging of pancreatic carcinomas[1-6]. Currently, 16- and 64-row spiral CTs are increasingly becoming available, and these offer improved local resolution over older models. The high-resolution data sets gained from these also offer the option of three-dimensional image post-processing[7,8].
In planning an operation, the location of the pancreatic tumor relative to the surroundings, surgically relevant vessels and adjacent organs is of utmost importance to the surgeon. The option of being able to assess the tumor volume in relation to the pancreatic tissue can also represent further valuable information.
In liver surgery, three-dimensional imaging is increasingly being used for the interactive planning of surgery in complex partial liver resections and living donors. Special procedures are used which - in addition to visualization - permit volumetric assessment of the primary data. Systems which can be used to gain additional information from the layer data include HepaVision2 (MeVis GmbH, Bremen, Germany)[9], LiverLive (Navidez Ltd., Ljublana, Slovenia), and Medical Imaging Interaction Toolkit (MITK) ReLiver (Deutsches Krebsforschungszentrum, Heidelberg, Germany).
The prerequisite for such procedures is the pre-processing step of segmentation, during which interesting regions of the image are marked on the section images. In a previous study, we have already shown that three-dimensional imaging of pancreatic tumors with semi-automatic segmentation is possible analogously to hepatic imaging[10].
The aim of this study is to test in a prospective, clinically controlled study, whether the three-dimensional images of the pancreas and the surrounding structures gained using the CT data sets are useful to the surgeon in planning operations and for intraoperative orientation in patients with pancreatic carcinoma.
Between March 2006 and August 2006, ten patients were included in the study with suspected operable pancreatic carcinoma. The criterion for inclusion in the study was urgent clinical suspicion of a pancreatic carcinoma. The study had the approval of the local ethics committee.
All patients underwent 16-row multislice CT (Somatom Sensation 16, Siemens, Erlangen, Germany), using the hydro technique with administration of Ultravist 370® (Schering, Berlin, Germany).
The operative findings and the histological results obtained during the operation served as the gold standard.
The evaluation was carried out by two experienced radiologists using a standardized evaluation form. This covered the location, size, and peripancreatic extent of the tumor. The resectability of the carcinoma was also assessed.
Patients were examined using 16-row spiral CT (Somatom Sensation 16, Siemens, Erlangen, Germany) using the hydro technique. This involves distension of the stomach and duodenum using 1.5 L of still water, intestinal paralysis through intravenous administration of 40 mg N-butylscopolaminium bromide (Buscopan®) and positioning the patient on the right side at an angle of 30°[4,11].
Firstly, a native spiral CT of the abdomen was carried out. Then an examination was carried out in the arterial phase (delay 8 s) and in the portal venous phase (delay 35 s) using the Combined Application Reduced Exposure bolus technique with 120 mL contrast medium (Ultravist® 370, Schering AG, Berlin, 5 mL/s) (Table 1).
| Native CT | Arterial phase | Portal venous phase | |
| Anode voltage (kV) | 120 | 120 | 120 |
| Anode current (mAs) | 140 | 170 | 185 |
| Detector collimation | 1.5 (slice: 6 mm) | 0.75 (slice: 3 mm) | 0.75 (slice: 3 mm) |
| Table feed (mm) | 24 | 12 | 12 |
| Reconstructed slice thickness (mm) | 6 | 2/1 | 1.3/3; 2.1/0.5 |
| Delay (s) | 8 | 35 |
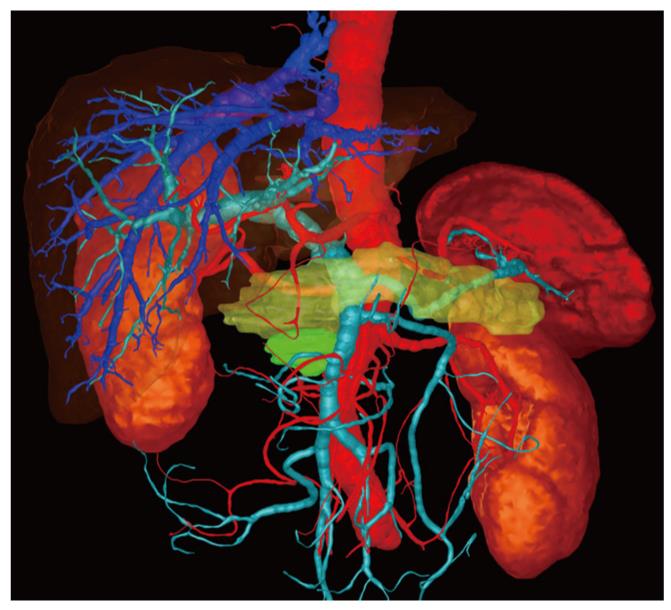
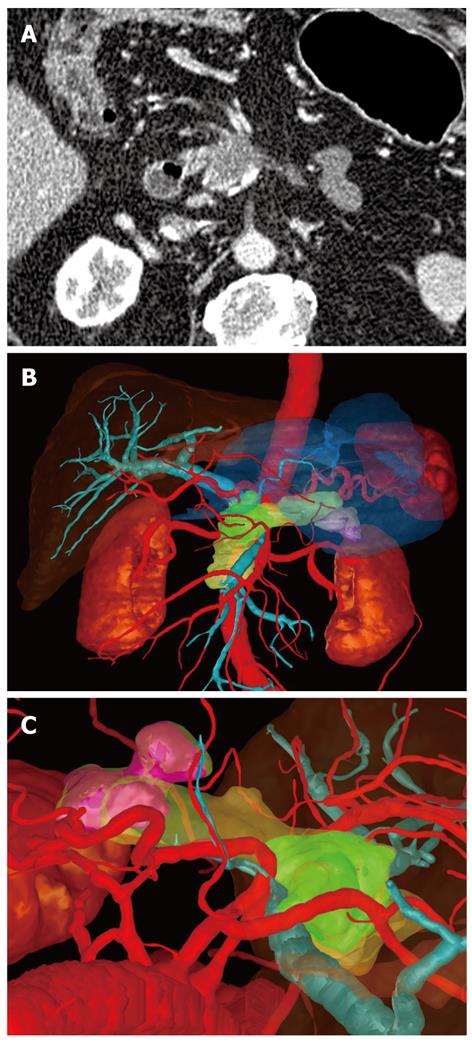
The data sets obtained from CT were transmitted via an internal data connection (Chili, Heidelberg, Germany) to the Department of Medical and Biological Informatics at the German Cancer Research Center. There, the vascular structures were transferred to a semantic model in which the vascular branches could be individually colored, shown, or removed from view. All segmentations were carried out by a radiologist using the software program MITK ReLiver. During the process, the liver, kidneys, duodenum, stomach, pancreas, and pancreatic tumor were segmented using interactive segmenting tools in the section images. It was found to be particularly time-saving to use a combination of an interactive regional growth procedure and a form-based interpolation procedure[12]. On this basis, it was possible to reduce the interaction time to a necessary minimum. Subsequently, the data from the vascular trees, the individually segmented organs and the tumor were fused into a three-dimensional scene.
These freely rotatable images were presented to the surgeon before and during the operation above the OP field, analogous to the three-dimensional image of the liver[11]. The surgeon also saw the conventional CT images before the operation, which were also available to him in the operating theater on a monitor. After the operation, on a questionnaire, the surgeon assessed the value of the three-dimensional image compared with that of the conventional CT images (Table 2).
| Depiction of the tumor | |
| How well can the tumor localization be assessed? | 1 = excellent; 2 = good; 3 = mediocre; 4 = bad; 5 = very bad |
| How well can vascular invasion be assessed? | |
| How well can organ invasion be assessed? | |
| How comfortable are you with the image? | |
| How well does the imaging material match the operative situation? | Completely; partially; not at all |
| How manageable was the 3D view in comparison with CT? | Less complicated; equally complicated; more complicated |
| Was the operating strategy changed due to the images? | Yes; no |
The questionnaire included comments about representation of the tumor, tumor location, tumor invasion of the vessels and surrounding organs, how well the imaging material matched the operative site, the manageability of the images, a score by which the surgeon could grade how secure he felt looking at the CT or three-dimensional images, and possible changes in the operative strategy.
The individual points on the questionnaire were graded with marks ranging from 1 for excellent to 5 for very bad.
Using the Wilcoxon test, we examined whether the differences between the CT and three-dimensional images were statistically relevant. The P values were calculated using SPSS for Windows XP.
In nine patients, a pylorus-preserving Whipple procedure was carried out. In one patient, explorative laparotomy was performed with placement of a biliodigestive anastomosis and a gastrojejunostomy, since the patient’s advanced age (80 years) made a portal vein resection seem too stressful.
Nine patients had adenocarcinomas of the pancreas and one had a serous cystadenoma. All carcinomas had grown beyond the margins of the organ and invaded the peripancreatic lipid tissue and the duodenum, and were thus staged histologically as T3. In one patient, invasion of the superior mesenteric vein was found intraoperatively.
The three-dimensional images of the pancreatic tumors, as evaluated on the questionnaire, were found to be graded more favorably with regard to assessment of tumor imaging, location, and invasion of the surrounding organs, and to the score indicating how secure the surgeon felt in assessing the images than the conventional CT examination. However, the differences were not statistically significant (P = 0.157, 0.564, 0.157, 0.063, respectively). In evaluating vascular invasion, the results were equally good for both procedures (P = 1.0) (Table 3).
| Patients | Tumor image | Tumor localization | Vascular invasion | Organ invasion | Feel-good score | |||||
| CT | 3D | CT | 3D | CT | 3D | CT | 3D | CT | 3D | |
| Φ | 2.2 | 1.4 | 1.8 | 1.6 | 2.2 | 2.2 | 2.0 | 1.6 | 2.4 | 1.2 |
| P-value | 0.157 | 0.564 | 1.0 | 0.157 | 0.063 | |||||
The three-dimensional imaging correctly and completely matched the operative site in all cases.
The manageability of the three-dimensional image was deemed to be less complicated in four out of five cases and in one case to be equally good.
In one case the surgeon found that the three-dimensional image demonstrated much more clearly than the conventional CT image that there was no invasion of the portal vein (Figure 1). In a second case, extensive invasion of the portal vein by the tumor could be established in both types of image, but the extent of the invasion was demonstrated to the surgeon better in the three-dimensional image, enabling the tumor to be classified as a resectable stage T3 and explorative laparotomy was performed. Intraoperatively, this long-distance invasion was confirmed but no tumor resection was carried out due to the patient’s advanced age; vascular replacement of the portal vein was deemed too stressful (Figure 2).
Despite all the progress made in diagnosis and surgery, the prognosis for pancreatic carcinomas today is still poor. Surgical resection is the treatment of choice as a curative approach to pancreatic carcinomas. Thus, the main aim of diagnosis consists of correctly evaluating the surgically relevant vessels with regard to possible invasion in order to be able to safely distinguish between resectable and non-resectable pancreatic carcinomas.
Spiral CT is considered to be state of the art in the diagnosis of pancreatic carcinoma and in the evaluation of resectability in most centers[6]. The sensitivity of spiral CT, as cited in the literature, for evaluating resectability in pancreatic carcinoma is between 81% and 96.3%[4,11,13-15].
Due to the particular anatomical relationship between the pancreas and the surrounding vessels, three-dimensional reconstructions are helpful in presenting additional information about this relationship[16,17].
For the surgeon, it is valuable to be able to see the tumor, both by itself and in the context of its surrounding structures, in three dimensions from all sides whereby particular interest is obviously accorded to the presence and extent of contact to the relevant vessels or surrounding organs. In addition, the surgeon can more easily assess the tumor volume in relation to healthy pancreatic parenchyma. With the aid of a freely rotatable, three-dimensional image, he can picture the extent of venous invasion more clearly (even before the operation) than is possible with two-dimensional, axial, coronal and sagittal images.
Thus, the surgeon is provided with as detailed a picture as possible of the field of operation even before the operation takes place. As far as we know, there are no studies so far available assessing the clinical value of three-dimensional image processing of pancreatic tumors. To date, the Harvard Medical School has produced reconstructions of the pancreas and the neighboring vascular systems, but has not clinically evaluated them[18].
Three-dimensional reconstruction already has a firm place in orthopedics and neurosurgery, where only unmoving and fixed anatomical structures are represented without any great range of variation.
The use of this method in visceral surgery is more difficult, since the organs can move and in some cases are shifted and reshaped during respiration and since the rate of anatomical variability is high[10].
In the field of hepatic imaging and in the context of living liver donors and before complex partial liver resections, three-dimensional imaging of the liver, hepatic vessels, and bile ducts has managed to become established in some centers. In this case, in addition to visualization, the volumetry of various liver sections is of interest. Moreover, the three-dimensional reconstruction can be used preoperatively to consider various resection options and to evaluate their technical feasibility with regard to vascular and bile duct anatomy and to the expected liver volume after surgery.
The prerequisite for three-dimensional imaging of the primary data is semiautomatic segmentation of the structures of interest such as pancreas, tumor, stomach, liver, spleen, kidneys and any cysts that might be present. In pancreatic tumors, however, the segmentation process is particularly time-consuming and problematic, since the margins between organ tissue and inflammatory processes or of the tumor and surrounding structures are hard to recognize due to growth beyond the organ borders. In addition, the preparation of data includes all important organs and vascular structures in the abdominal cavity in order to provide the surgeon with a maximum amount of context information so that possible problems in exposure of the pancreas can be included in the surgical planning.
This means that the reconstruction procedure for visualizing pancreatic carcinomas today still requires a great amount of effort both technically and in terms of time required. In addition, an objective and precise localization of the tumor is not possible because segmentation is always conducted according to the subjective interpretation of the radiologist.
An automatic image processing procedure cannot be used for segmenting the tumor since the differences in thickness between organ tissue and tumor are sometimes very slight and cannot be recognized by a computer program. However, there are very promising approaches to segmenting the organs which make a largely automatic procedure seem tangible. Statistical form models learn the mean organ form with its variants from training data. Using even the current models, it can be shown that the work of segmentation can be significantly reduced for the liver[19].
For vessels, no automatic segmentation program exists that can independently mark vascular cross-sections by using the good contrast provided by the various contrast medium phases of the CT examination and the high contrast differences associated with it. Three-dimensional imaging of the vessels, moreover, only depicts the inside of the visualized vessel since the contrast medium that is used for processing the reconstruction only fills the vascular lumen.
Tumor invasion of the relevant vessel can thus only be indirectly visualized, just as with two-dimensional CT images, e.g. on the basis of a sudden difference in vessel caliber or complete vascular occlusion.
In the present study, three-dimensional post-processing of the data sets was highly acclaimed by the surgeons. Analysis of the questionnaire showed that the three-dimensional image was graded better in most points than conventional CT images. Only evaluation of the vessels was graded equally for both procedures.
The so-called “feelgood score” was better for the three-dimensional image than the two-dimensional image in all the cases.
This explains why in two cases the surgeon indicated that he found extensive portal vein invasion and exclusion of portal vein invasion to be much clearer for him on the three-dimensional image than on the conventional CT images.
The manageability of the three-dimensional image in comparison to the conventional CT images was deemed less complicated in four out of five cases. In all the cases the findings presented in the three-dimensional image material matched the intraoperative situation completely.
In complex liver surgery, three-dimensional visualization has already been shown to meet with high acclaim by surgeons. Its advantage consists in providing the surgeon with information about vascular anatomy that is indispensable for planning living liver transplantation or in providing additional information about the percentage of residual hepatic tissue following resection.
In their clinical study of the value of three-dimensional data sets before complex liver resections and before living liver donations, Fischer and Lamade found that although three-dimensional presentation did not lead to an improved segment allocation of hepatic tumors in comparison to axial sections, the three-dimensional presentation meant that the position of the tumor within the liver as marked on a liver model was significantly improved. It was shown that the three-dimensional presentation did indeed lead to improved OP representation. Overall, three-dimensional presentation led to approaching a 31% higher precision in tumor localization and improvement in resection recommendations[20,21].
This study also illustrated the disadvantage of this method. The three-dimensional representation of the findings can only be as good as the primary data sets from CT in conjunction with the experience of the radiologist in evaluating CT data. If contact between the tumor and the encircling wall of a vessel can be visualized over a long distance on CT, this will also be visible in the three-dimensional image and can be interpreted in most cases, and in both procedures, as invasion of the relevant vessel. The three-dimensional reconstruction cannot improve on the examination; it can only present the situation in a more plastic form.
A three-dimensional image will thus generally not improve the assessment of the resectability of pancreatic carcinomas. Overall, this new procedure, however, seems to provide a good aid in preoperative planning for the surgeon in surgical therapy of pancreatic carcinoma.
It remains to be seen whether the method, which still involves a great deal of effort, will stand the test of practical daily routine or if the time and technical input necessary for post-processing the image material is too high to be integrated practically into clinical routine or if these factors can be significantly reduced.
One restriction on the present study is the very small case number. The calculated P values showed no significant differences. Thus further studies should be conducted with a larger number of patients.
In summary, it can be said that three-dimensional imaging of pancreatic carcinomas with the surrounding vessels and organs is currently constrained by the great deal of effort involved, but it does primarily provide the surgeon with valuable additional information.
In planning a resection of a pancreatic carcinoma, the location of the pancreatic tumor relative to the surroundings, surgically relevant vessels and adjacent organs is of utmost importance to the surgeon.
The aim is to provide better visualization of pancreatic carcinoma and peripancreatic vessels for the surgeon in the preoperative period.
In complex liver surgery, three-dimensional visualization has already been shown to meet with high acclaim by surgeons, but this study is the first which used this technique in pancreatic carcinoma patients. Its advantage consists in providing the surgeon with information about peripancreatic vascular anatomy that is helpful for planning the resection of a pancreatic carcinoma.
A three-dimensional image of the pancreas represents an additional, valuable aid to the surgeon. However, this method is still time-consuming. The software must be further developed to allow further automation of the segmentation to enable it to be integrated into daily clinical routine.
Segmentation: To circumscribe a freehand region-of-interest on each single CT-slice, where, for example, the tumour or the pancreas is seen.
This is a pilot study of 10 consecutive patients with suspected pancreatic carcinoma subjected to preoperative 3-D spiral CT reconstruction. This is the first such clinical study utilizing this technique.
Peer reviewers: Kiichi Tamada, MD, Department of Gastroenterology, Jichi Medical Shool, 3311-1 Yakushiji, Minamikawachi, Kawachigun, Tochigi 329-0498, Japan; Edward L Bradley III, MD, Professor of Surgery, Department of Clinical Science, Florida State University College of Medicine, 1600 Baywood Way, Sarasota, FL 34231, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
| 1. | Ishiguchi T, Ota T, Naganawa S, Fukatsu H, Itoh S, Ishigaki T. CT and MR imaging of pancreatic cancer. Hepatogastroenterology. 2001;48:923-927. |
| 2. | McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi--detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97-102. |
| 3. | Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB Jr. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764-768. |
| 4. | Richter GM, Wunsch C, Schneider B, Düx M, Klar E, Seelos R, Kauffmann GW. [Hydro-CT in detection and staging of pancreatic carcinoma]. Radiologe. 1998;38:279-286. |
| 5. | Schima W, Ba-Ssalamah A. [Radiologic staging of liver and pancreatic malignancies]. Radiologe. 1999;39:568-577. |
| 6. | Schima W, Ba-Ssalamah A, Plank C, Kulinna-Cosentini C, Prokesch R, Tribl B, Sautner T, Niederle B. [Pancreas. Part II: Tumors]. Radiologe. 2006;46:421-437; quiz 438. |
| 7. | Baum U, Lell M, Nömayr A, Wolf H, Brunner T, Greess H, Bautz W. [Multiplanar spiral CT in the diagnosis of pancreatic tumors]. Radiologe. 1999;39:958-964. |
| 8. | Flohr T, Ohnesorge B, Stierstorfer K, Bruder H, Simon J, Süss C, Wildberger J, Baum U, Lell M, Küttner A. [On the way to isotopic spatial resolution: technical principles and applications of 16-slice CT]. Radiologe. 2005;45:608-617. |
| 9. | Meier S, Schenk A, Mildenberger P, Bourquain H, Pitton M, Thelen M. [Evaluation of a new software tool for the automatic volume calculation of hepatic tumors. First results]. Rofo. 2004;176:234-238. |
| 10. | Grenacher L, Thorn M, Knaebel HP, Vetter M, Hassenpflug P, Kraus T, Meinzer HP, Büchler MW, Kauffmann GW, Richter GM. [The role of 3-D imaging and computer-based postprocessing for surgery of the liver and pancreas]. Rofo. 2005;177:1219-1226. |
| 11. | Richter GM, Simon C, Hoffmann V, DeBernardinis M, Seelos R, Senninger N, Kauffmann GW. [Hydrospiral CT of the pancreas in thin section technique]. Radiologe. 1996;36:397-405. |
| 12. | Kunert T, Heimann T, Schröter A, Schöbinger M, Böttger T, Thorn M, Wolf I, Engelmann U, Meinzer HP. An Interactive System for Volume Segmentation in Computer-Assisted Surgery. In: Galloway RL Jr, editor. Proc. SPIE Vol. 5367, Medical Imaging 2004; Visualization, Image-Guided Procedures, and Display. SPIE The International Society for Optical Engineering, Bellingham, 2004: 799-809. |
| 13. | Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS, Stoker J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438-445. |
| 14. | Klauss M, Mohr A, von Tengg-Kobligk H, Friess H, Singer R, Seidensticker P, Kauczor HU, Richter GM, Kauffmann GW, Grenacher L. A new invasion score for determining the resectability of pancreatic carcinomas with contrast-enhanced multidetector computed tomography. Pancreatology. 2008;8:204-210. |
| 15. | Zeman RK, Cooper C, Zeiberg AS, Kladakis A, Silverman PM, Marshall JL, Evans SR, Stahl T, Buras R, Nauta RJ. TNM staging of pancreatic carcinoma using helical CT. AJR Am J Roentgenol. 1997;169:459-464. |
| 16. | Kalra MK, Maher MM, Mueller PR, Saini S. State-of-the-art imaging of pancreatic neoplasms. Br J Radiol. 2003;76:857-865. |
| 17. | Smith SL, Rajan PS. Imaging of pancreatic adenocarcinoma with emphasis on multidetector CT. Clin Radiol. 2004;59:26-38. |
| 18. | Nakagohri T, Jolesz FA, Okuda S, Asano T, Kenmochi T, Kainuma O, Tokoro Y, Aoyama H, Lorensen WE, Kikinis R. Virtual pancreatoscopy of mucin-producing pancreatic tumors. Comput Aided Surg. 1998;3:264-268. |
| 19. | Heimann T, Wolf I, Meinzer HP. Active shape models for a fully automated 3D segmentation of the liver--an evaluation on clinical data. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2006;9:41-48. |
| 20. | Lamadé W, Glombitza G, Fischer L, Chiu P, Cárdenas CE Sr, Thorn M, Meinzer HP, Grenacher L, Bauer H, Lehnert T. The impact of 3-dimensional reconstructions on operation planning in liver surgery. Arch Surg. 2000;135:1256-1261. |
| 21. | Fischer L, Thorn M, Chiu P, Grenacher L, Meinzer HP, Lamade W. Virtuelle Operationsplanung in der Leberchirurgie. Chir Praxis. 2003;61:459-466. |